Selective synthesis of nickel oxide nanowires and length effect on their electrochemical properties†
Huan
Pang
a,
Qingyi
Lu
*a,
Yizhou
Zhang
a,
Yecheng
Li
a and
Feng
Gao
*b
aState Key Laboratory of Coordination Chemistry, Coordination Chemistry Institute, Nanjing National Laboratory of Microstructures, Nanjing University, Nanjing, 210093, P. R. China. E-mail: qylu@nju.edu.cn
bDepartment of Materials Science and Engineering, Nanjing University, Nanjing, 210093, P. R. China. E-mail: fgao@nju.edu.cn
First published on 11th May 2010
Abstract
Nickel oxide nanostructures with different lengths have been easily synthesized by calcining nickel foil after soaking in LiOH solution of different concentrations. The measurements of electrochemical properties of these NiO nanostructures show that different lengths of NiO nanostructures would have different electrochemical properties and the NiO nanowires with the longest length have the largest specific capacitance of 180.00 F g−1.
In recent years, shape-controlled synthesis of low-dimensional nanocrystals has been an interesting research topic for their interesting physical and chemical properties.1–4 Due to fast redox reactions, high specific surface areas and shortened diffusion paths in the solid phase, synthesized nanomaterials have raised an important and hot field in electrochemical capacitors.5–9 Nanowires, one of the one-dimensional (1D) nanostructures, are perfect building blocks for functional nanodevices and represent the smallest dimension for efficient electron and exciton transport.10,11 Commonly, the electrical conductivity is one of the most important aspects of electrode materials which can effectively enhance the utilization of active materials and mitigate the internal resistance of the capacitors.12 Therefore the synthesis of one-dimensional nanostructures to be used in the field of nanoelectrode materials is highly desirable for materials scientists and researchers in materials engineering who study material properties.
Nickel oxide (NiO) is a promising material for its applications in the fields of fuel cell electrodes, heterogeneous catalysis, antiferromagnetic layers, electrochemical capacitors etc.13–16 Valuable functions greatly depend on the structures. So far, NiO nanostructures with different morphologies such as nanoplates, nanotubes, and hollow nanospheres have been obtained.17–19 However, there are few reports about NiO nanowires used in electrochemical capacitors. In this study, we report an easy synthesis route of NiO nanowires with different lengths by calcining Ni foil after soaking in LiOH solutions of different concentrations. The length of NiO nanowires can be adjusted from nanoscale to microscale. These obtained NiO nanowires are on Ni foil substrate and can be directly used as electrodes without any further work, which is economical and effective for general practical use. The electrochemical capacitor property measurements of these NiO nanowires/Ni foil electrodes show that the length of the NiO nanowires has an obvious effect on their electrochemical properties. The NiO nanowires/Ni foil electrode of the longest length has a highest specific capacitance of 180.00 F g−1, more than 10 times those of the shorter ones under the same conditions. The measurement of cyclability shows the longest NiO nanowires/Ni foil electrodes also have long-term electrochemical stability, which would make it possible for such NiO nanowires/Ni foil electrodes to be a promising electrochemical material for energy storage.
The NiO nanowires with different length were fabricated through an easy route as Scheme 1a shows (see ESI† SI1 for the experimental details). Fig. 1 shows XRD patterns of the NiO nanostructures/Ni foils prepared under different conditions. The diffraction peaks at 2θ = 37.6, 43.7, and 63.2 can be indexed as NiO (JCPDS card No. 47-1049) and the diffraction peaks at 2θ = 44.8 and 52.2 can be indexed to Ni (JCPDS card No. 87-0172), which come from Ni foil. These results suggest that NiO crystals have formed on the Ni foil. The mass of the NiO nanocrystals prepared with LiOH concentrations of 30 g L−1, 15 g L−1 and 50 g L−1 are calculated to be 3.97, 3.13, and 3.50 mg, repsectively (see ESI† SI2 for the calculation details).
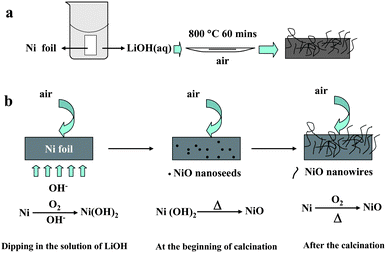 | ||
| Scheme 1 (a) The simple route of the synthesis of NiO/Ni foil nanostructures; (b) the possible mechanism for the formation of NiO nanowires. | ||
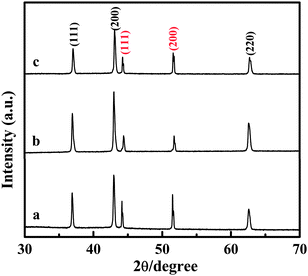 | ||
| Fig. 1 XRD patterns of the as-synthesized NiO nanocrystals/Ni foil. The peaks of Ni foil are marked in red. Ni foils were dipped in the solution of LiOH with concentrations of (a) 30 g L−1; (b) 15 g L−1 and (c) 50 g L−1 before the calcinations at 800 °C. | ||
Fig. 2a and b display SEM images of NiO/Ni prepared with LiOH concentration of 30 g L−1 and it can be seen that the product has a wire-like morphology attached to the substrate. These NiO nanowires criss-cross on a large area of the foil and their diameter and length are about 50 nm and 20 μm, respectively. If the Ni foil wasn't soaked in LiOH solution first, it can be found that there are only irregular particles obtained by calcining pure Ni foil (ESI† Fig. SI3a). It can be deduced that such long NiO nanowires, obtained by calcining LiOH-eroded Ni foil, might grow through a “nanoseed-catalyzing” mechanism, which has been confirmed an effective route for 1D nanostructure.20 As shown in the Scheme 1b, the surface of Ni foil would form a layer of Ni(OH)2 film in the alkaline solution. When the Ni foil was calcined in the air, the Ni(OH)2 film would decompose rapidly and form many NiO nanocrystals. Although NiO crystal is generally a rock-salt with an isotropic crystal growth, the existence of the NiO nanoseeds would break the symmetry of isotropic crystal structures to induce anisotropic growth in the nucleation step and direct the one-dimensional growth of NiO to form NiO nanowires.21
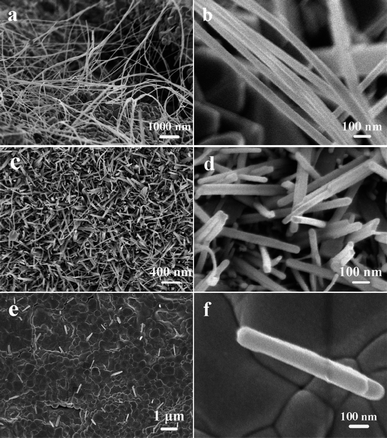 | ||
| Fig. 2 SEM images of the as-synthesized samples. | ||
From the nanoseed-catalyzing growth mechanism, the erosion of Ni foil to form Ni(OH)2 layer is a key step for the growth of the 1D NiO nanostructures. By varying the concentration of LiOH solution (CLiOH), the density of NiO nanoseeds would change, which would lead to the growth of different NiO nanostructures. When the Ni foil was soaked in LiOH solution with a lower concentration of 15 g L−1, just NiO nanorods with shorter length could be obtained as shown in Fig. 2c and d. Compared to nanowires shown in Fig. 2a, these nanorods have length of several hundred nanometres and few long ones can be seen. The phenomenon might be due to the fact that the low CLiOH would produce less seeds on the surface of the Ni foil at the beginning of the calcination, leading to the slower growth rate of 1D NiO nanostructures and resulting in the formation of shorter 1D nanostructures. On the other hand, when the Ni foil was soaked in LiOH solution with a higher concentration of 50 g L−1, a NiO layer composed of NiO nanoparticles formed and covered the surface of Ni foil and occasionally some NiO nanorods can be found as SEM images shown in Fig. 2e and f. The result might be due to that the higher CLiOH would produce too many seeds on the surface of the Ni foil at the beginning of the calcination, forming a layer to cover the surface of Ni foil and hinder NiO to grow out to form 1D nanostructures. Further increasing the concentration of LiOH solution would result in the formation of NiO layer composing of irregular NiO particles by calcining Ni foil after soaked in the LiOH solution with a concentration of 60 g L−1 (as shown in ESI† Fig. SI3b).
By simply controlling the concentration of LiOH solution, different NiO nanostructures including nanowires, nanorods, and nanoparticles have been obtained on the surface of Ni foil. Different nanostructures would show different electrical properties. In this research, we studied electrochemical properties of these NiO nanostructures/Ni foil by directly exploiting NiO nanostructure/Ni as electrodes. Fig. 3a shows the cyclic voltammetric (CV) curves of the three synthesized NiO/Ni foils prepared with LiOH concentrations of 30 g L−1, 15 g L−1 and 50 g L−1 at a scan rate of 50 mV s−1. All the three CVs appear two pairs of redox peaks, which might come from the redox processes of NiO/NiOOH. The CV curve of NiO nanowires/Ni foil (the longest one) shows a rapid current response on potential reversal at each end potential, a representation of a capacitive behavior,9 in which the highest current response reflects the highest specific capacitance. Chronopotentiometry measurements confirm this suggestion. Fig. 3b shows charge–discharging curves of different NiO nanostructures/Ni foil electrodes obtained in potential range of 0–0.55 V in 3% KOH at a charging–discharging current of 0.126 A g−1. The shapes of the charge–discharge curves do not show the characteristics of pure double-layer capacitor, but mainly pseudo-capacitance, which are in agreement with the result of the CV curves. The NiO nanowires/Ni foil electrode has the largest specific capacitance of 180.00 F g−1, and longest discharging time, while those of the two others are 14.69 and 7.46 F g−1, respectively. The chronopotentiometry measurements of the samples at a higher charging–discharging current of 1.25 A g−1 (shown in Fig. SI4a†) also get similar results with the specific capacitances of 166.0, 12.1, and 6.1 F g−1 for the three samples, respectively. On the other hand, as a long cycle life is very important for electrochemical capacitors, the cycle charge/discharge test has also been employed to examine the service life of the NiO nanostructures/Ni foil. Fig. 3c shows the variation of specific capacitance with cycle number at 0.5 mA and reveals that the NiO nanowires/Ni electrode has good cycle properties as an excellent electrode material for electrochemical capacitors and only approximately 1.7% loss of specific capacitance after 400 cycles. At a higher current of 5 mA, the NiO nanowires/Ni electrode still have the good cycle properties and just 6.6% loss of specific capacitance even after 1000 cycles (shown in Fig. SI4b†). To evaluate the contribution of the nickel foil to the measured capacitance, we also measured CV scan and CP scan of pure Ni foil under similar experiment conditions. The results are as shown in Fig. SI5.† The nickel can undergo redox reaction in alkaline solution during a CV scan and contribute the capacitance to the measured capacitance. However, these contributions are very small, which can be ignored. Similar situations have been reported by many researchers in which they used nickel foam sheet or nickel film as the substrate of active materials.22,23 So, the good electrochemical properties of the NiO nanowires/Ni foil should be related to the fact that the NiO nanowires criss-cross on the large area of the surface of Ni foil, which might promote the electron transfer on the electrode. To identify the exact electrical conductivity of the electrodes, we measured Nyquist plots of the NiO nanostructures/Ni foil at room temperature in the frequency range 1–105 Hz under open-circuit conditions, which are shown in Fig. 3d. It can be found that the sequence of the resistances, deduced from the intercept of the arc with the real Zre axis, is the NiO nanowires/Ni foil < NiO nanorods/Ni foil < NiO nanoparticles/Ni foil, indicating that the NiO nanowires/Ni foil has the highest electrical conductivity. The good electrical conductivity can enhance the transfer of electrons and enhance electrochemical behaviors.
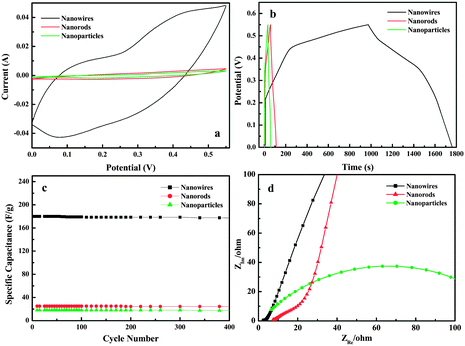 | ||
| Fig. 3 (a) Cyclic voltammetric (CV) curves of the NiO nanostructures/Ni foil within a potential window of 0 to 0.55 V vs. Ag/AgCl at a scan rate of 50 mV s−1; (b) charge–discharge curves in the potential range from 0 to 0.55 V; (c) variation of specific capacitance with cycle number at 0.5 mA; (d) Nyquist plots of the NiO nanostructures/Ni foil in the frequency range 1–105 Hz. | ||
In summary, the NiO nanowires/Ni foil electrodes have been successfully synthesized by “nanoseed-catalyzing” mechanism. By controlling the concentration of LiOH solution, different NiO nanostructures including nanowires, nanorods, and nanoparticles can be obtained on the surface of Ni foil. The measurements of electrochemical properties show that different NiO nanostructures display different electrochemical properties and the long NiO nanowires/Ni foil electrode has good electrochemical reversibility and fine power-storage property. This is a good example for the structured nanomaterial as excellent supercapacitor and for the shape–property relationship study of nanomaterials.
This work is supported by the National Natural Science Foundation of China (Grant No. 20671049, 20721002 and 50772047), the National Basic Research Program of China (Grant No. 2007CB925102), and the Program for New Century Excellent Talents in University.
Notes and references
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS.
- K. B. Zhou, X. Wang, X. M. Sun, Q. Peng and Y. D. Li, J. Catal., 2005, 229, 206 CrossRef CAS.
- Y. W. Zhang, M. E. Grass, S. E. Habas, F. Tao, T. F. Zhang, Y. R. Borodko, P. D. Yang and G. A. Somorjai, Abstracts of Papers, 234th ACS National Meeting, Boston, MA, United States, August 19–23, American Chemical Society, Washington, DC, 2007, INOR-138 Search PubMed.
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS.
- Y. G. Wang and Y. Y. Xia, Electrochim. Acta, 2006, 51, 3223 CrossRef CAS.
- D. D. Zhao, M. W. Xu, W. J. Zhou, J. Zhang and H. L. Li, Electrochim. Acta, 2008, 53, 2699 CrossRef CAS.
- S. L. Xiong, C. Z. Yuan, X. G. Zhang, B. J. Xi and Y. T. Qian, Chem.–Eur. J., 2009, 15, 5320 CrossRef CAS.
- E. E. Kalu, T. T. Nwoga, V. Srinivasan and J. W. Weidner, J. Power Sources, 2001, 92, 163 CrossRef CAS.
- K. W. Nam and K. B. Kim, J. Electrochem. Soc., 2002, 149, A346 CrossRef CAS.
- C. Z. Wu, J. Dai, X. D. Zhang, J. L. Yang and Y. Xie, J. Am. Chem. Soc., 2009, 131, 7218 CrossRef CAS.
- Y. Huang, X. F. Duan, Y. Cui, L. J. Lauhon, K. H. Kim and C. M. Lieber, Science, 2001, 294, 1313 CrossRef CAS.
- M. S. Wu and H. H. Hsieh, Electrochimica. Acta., 2008, 53, 3427 CAS.
- X. F. Song and L. Gao, J. Phys. Chem. C, 2008, 112, 15299 CrossRef CAS.
- J. Park, E. Kang, S. U. Son, H. M. Park, M. K. Lee, J. Kim, K. W. Kim, H. J. Noh, J. H. Park, C. J. Bae, J. G. Park and T. Hyeon, Adv. Mater., 2005, 17, 429 CrossRef.
- X. Wang, L. Li, Y. G. Zhang, S. T. Wang, Z. D. Zhang, L. F. Fei and Y. T. Qian, Cryst. Growth Des., 2006, 6, 2163 CrossRef CAS.
- J. X. Zhu, Z. Gui, Y. Y. Ding, Z. Z. Wang, Y. Hu and M. Q. Zou, J. Phys. Chem. C, 2007, 111, 5622 CrossRef CAS.
- X. M. Ni, Q. B. Zhao, F. Zhou, H. G. Zheng, J. Cheng and B. B. Li, J. Cryst. Growth, 2006, 289, 299 CrossRef CAS.
- G. Malandrino, L. M. S. Perdicaro, I. L. Fragalà, R. L. Nigro, M. Losurdo and G. Bruno, J. Phys. Chem. C, 2007, 111, 3211 CrossRef CAS.
- X. M. Sun, J. F. Liu and Y. D. Li, Chem.–Eur. J., 2006, 12, 2039 CrossRef CAS.
- F. Kim, K. Sohn, J. S. Wu and J. X. Huang, J. Am. Chem. Soc., 2008, 130, 14442 CrossRef CAS.
- Y. N. Xia, P. D. Yang, Y. G. S, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and H. Q. Yan, Adv. Mater., 2003, 15, 365.
- J. Cheng, G. P. Cao and Y. S. Yang, J. Power Sources, 2006, 159, 734 CrossRef CAS.
- K. C. Liu and M. A. Anderson, J. Electrochem. Soc., 1996, 143, 124 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, mass calculation, SEM images of the samples, electrochemical behaviors of the NiO/Ni electrodes at higher current, CV and charge-discharge curves of pure Ni foil. See DOI: 10.1039/c0nr00027b |
| This journal is © The Royal Society of Chemistry 2010 |
