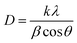Modification of neodymium-doped ZnO hybrid nanoparticles under mild hydrothermal conditions
Behzad
Shahmoradi
*a,
K.
Soga
b,
S.
Ananda
c,
R.
Somashekar
c and
K.
Byrappa
c
aFaculty of Environmental Health Engineering, Kurdistan University of Medical Sciences, Sanandaj 66174, Iran. E-mail: bshahmorady@gmail.com
bDepartment of Materials Science and Technology, Tokyo University of Science, 2641 Yamazaki, 278-8510 Noda, Chiba, Japan
cUniversity of Mysore, Manasagangotri, Mysore 570 006, India
First published on 25th May 2010
Abstract
The morphology and particle size of neodymium-doped ZnO hybrid nanoparticles were tailored through fabrication under mild hydrothermal conditions (T = 150–250 °C, P = autogeneous, t = 18 h) for the first time using two surface modifiers: caprylic acid and n-butylamine. Characterization of these nanoparticles was carried out using powder XRD, FTIR, SEM, zeta-potential analysis and UV-vis spectroscopy. The results revealed that modification of ZnO nanoparticles using neodymium as a dopant and caprylic acid or n-butylamine as a surfactant could change the optical and physical properties of the surface-modified neodymium-doped ZnO hybrid nanoparticles. The work proved the efficiency of caprylic acid and n-butylamine as suitable surfactants for surface modification of neodymium-doped ZnO hybrid nanoparticles.
Introduction
Transition metal oxides with nanostructures have attracted considerable interest in many areas of chemistry, physics, environmental science and material science.1 Zinc oxide is a promising group II–VI oxide semiconductor showing quantum confinement effects at room temperature.2 Zinc oxide nanoparticles are versatile materials that have been used in a variety of applications such as UV absorption, sunscreen lotions, antibacterial treatments,3 catalysts,4 photocatalysts5 and additives in many industrial products. It is technologically an important material due to its wide range of optical and electrical properties as well as semiconductor properties, with a large binding energy (60 meV) and wide band gap (3.37 eV).6Doping methods have been extensively utilized for modifying the electronic structures of ZnO nanoparticles to achieve new or improved catalytic, electro-optical, magnetic, and other chemical and physical properties. Dopants can segregate on the ZnO nanostructure surfaces or they can be incorporated into the lattice, where the dopants can be substitutional or interstitial or both.7 Nonstoichiometric pure ZnO is an n-type semiconductor,8 so doping of ZnO with a suitable dopant can alter the band gap energy and make it more or less efficient in photodegradation of organic and toxic pollutants. However, the doping of nanoparticles with an appropriate metal may not resolve the problems of agglomeration and poor dispersibility of semiconductor nanoparticles, which need a new processing strategy to be overcome. Therefore, caprylic acid and n-butylamine were selected as surface modifiers to resolve these problems. The present authors have modified ZnO nanoparticles with these surfactants because they have low toxicity, low density, low melting point and are eco-friendly.
Most of the ZnO particles have been synthesized by traditional high temperature solid state methods, which are energy consuming and also difficult for controlling the particle properties. ZnO nanoparticles can be prepared on a large scale at low cost by novel solution routes, such as chemical precipitation, sol–gel synthesis, and solvothermal/hydrothermal reaction.9–13 Hydrothermal technique is a promising alternative synthetic method because of the low process temperature and very easy control of particle size. The hydrothermal process has several advantages over other growth processes, such as the use of simple equipment, catalyst-free growth, low cost, large surface area for particles, environmental friendliness and less hazardous.14 The particle properties, such as morphology and size, can be controlled via the hydrothermal process by adjusting the reaction temperature, time and concentration of precursors.
Experimental
Preparation of modified neodymium-doped ZnO nanoparticles
Neodymium-doped ZnO nanoparticles were fabricated under hydrothermal conditions (T = 150–250 °C, P = autogeneous). 1 M of reagent grade ZnO (Loba Chemie, India) was taken as starting material and the dopant (Nd2O3, 2 mol%; 5 mol%) was added. A certain amount of 1 M NaOH was added as mineralizer to the precursors. n-Butylamine and caprylic acid (Sisco Research Lab PVT, Ltd., Mumbai, India) with different concentrations (0.8, 1.0, 1.2, 1.4 and 1.6 M) were added into the above mentioned mixture separately and it was stirred vigorously for a few minutes. The final mixture was then transferred to a Teflon liner (Vfill = 15 ml), which was later placed inside a General-Purpose autoclave designed and fabricated at the University of Mysore. The autoclaves were provided with Teflon liners of 30 ml capacity. Then the assembled autoclave was kept in an oven with a temperature programmer-controller. The temperature was programmed and kept at 150–250 °C for 18 h. After the experimental run, the autoclaves were cooled to room temperature. The resultant product in the Teflon liner was then transferred into a clean beaker and the product was washed with double distilled water. The surplus solution was removed using a syringe and the remnants were centrifuged for 20 min at 3000 rpm. The product was recovered and dried in a hot air oven at 50 °C for a few hours. The dried particles were subjected to a systematic characterization using powder X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, zeta-potential measurement, scanning electron microscopy (SEM) and UV-vis. spectrometry.Characterization of the surface-modified neodymium-doped ZnO nanoparticles
The powder X-ray diffraction patterns were recorded using a Bruker D8 Advance, Germany, with Cu Kα, λ = 1.542 Å, radiation; voltage = 30 kV; current = 15 mA; scan speed ∼5° min−1. The data were collected in the 2θ range 10–80°. The Fourier transform infrared spectra were recorded using FTIR, JASCO-460 PLUS, Japan, at a resolution of 4 cm−1. The optical properties were studied using a UV-vis spectrophotometer (Minispec SL 171, Elico, India). The particle size was calculated using the Scherrer equation. SEM images of the neodymium oxide doped ZnO hybrid nanoparticles were recorded using a Hitachi S-4200, Japan. Zeta-potentials were measured using Zetasizer 2000 instrument (Malvern instruments), UK.Results and discussion
The powder XRD data reveals a highly crystallized wurtzite structure and there are three small new peaks at 12.34, 26.76 and 28.32°, which correspond to neodymium oxide, indicating that the neodymium has been doped into the ZnO nanoparticle structure when caprylic acid and n-butylamine were used as surfactants (Fig. 1). It reveals that the neodymium is doped into the ZnO nanoparticles structure. There is a slight change in the lattice parameters of the a- and c-axes of neodymium-doped ZnO hybrid nanoparticles (ZnO-X, X = 2, 5% neodymium) when compared to pure ZnO. This confirms the existence of neodymium atoms in ZnO hybrid nanoparticles. The ionic radii of the Nd(III) ion (99.5 pm) is bigger than that of Zn(II) (74 pm),15 so the doping of Nd(III) should make the cell parameter bigger than that of pure ZnO and there is gradual change in the volume (Table 1).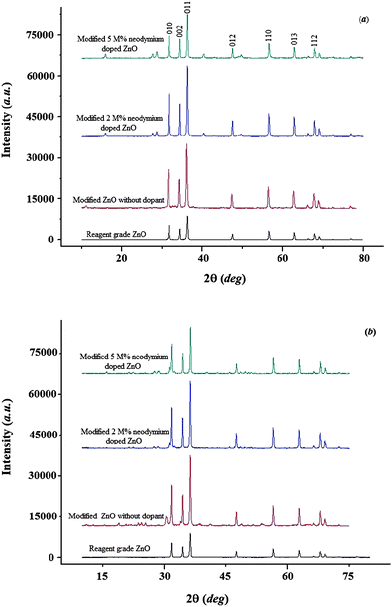 | ||
| Fig. 1 XRD patterns of neodymium-doped ZnO modified with (a) caprylic acid and (b) n-butylamine. | ||
| Catalyst | Surface modifier | a/Å | c/Å | a:c ratio | V/Å3 | Ref. |
|---|---|---|---|---|---|---|
| Pure ZnO | NIL | 3.249 | 5.207 | 0.6239 | 47.601 | [21] |
| 2 mol% Nd3+:ZnO | n-Butylamine | 3.2508 | 5.2090 | 0.6242 | 47.672 | Present work |
| 5 mol% Nd3+: ZnO | n-Butylamine | 3.2542 | 5.2098 | 0.6230 | 47.779 | Present work |
| 2 mol% Nd3+: ZnO | Caprylic acid | 3.2522 | 5.2102 | 0.6242 | 47.724 | Present work |
| 5 mol% Nd3+: ZnO | Caprylic acid | 3.2540 | 5.2128 | 0.6242 | 47.800 | Present work |
The functional groups present in the modified nanoparticles can be studied using FTIR spectroscopy. Fig. 2 shows the FTIR spectra of the reagent grade ZnO, surface-modified undoped ZnO and neodymium-doped ZnO nanoparticles modified with n-butylamine and caprylic acid as surface modifier, respectively. The intensity of the peaks was greater for 1.6 M surface-modified neodymium-doped ZnO hybrid nanoparticles compared to 0.8 M neodymium-doped (2 mol% and 5 mol%) ZnO hybrid nanoparticles. The FTIR spectra of the modified hybrid nanoparticles show the presence of new peaks, implying that the reagents were chemically immobilized on the surface of nanoparticles. Thus, it can be concluded that the neodymium-doped ZnO hybrid nanoparticles, which were synthesized with the above said modifier, have organic coverage on their surfaces, which has changed the surface property of the nanoparticles. The new peaks around 600 and 850 cm−1 could correspond to Nd–O absorption bands. The peaks around 1620 and 3680 cm−1 correspond to the presence of CH3 and N–H stretching bands. In addition, the appearance of new peaks around 3450 cm−1 corresponds to the O–H stretching band (Fig. 2I). The absorption peaks around 1200 to 1500 cm−1 belong to the carboxyl group and 2950 cm−1 belongs to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H stretching band, which is because of the modification with caprylic acid.16 As Fig. 2II shows, the ZnO nanoparticles have been covered by caprylic acid, which makes them organic–inorganic hybrid nanoparticles.17
O and O–H stretching band, which is because of the modification with caprylic acid.16 As Fig. 2II shows, the ZnO nanoparticles have been covered by caprylic acid, which makes them organic–inorganic hybrid nanoparticles.17
 | ||
| Fig. 2 FTIR spectra of a) reagent grade ZnO, b) modified undoped ZnO hybrid nanoparticles, c) 2 mol% neodymium-doped ZnO hybrid nanoparticles modified with 0.8 M surfactant, d) 2 mol% neodymium-doped ZnO hybrid nanoparticles modified with 1.6 M surfactant, e) 5 mol% neodymium-doped ZnO hybrid nanoparticles modified with 0.8 M surfactant and f) 5 mol% neodymium-doped ZnO hybrid nanoparticles modified with 1.6 M surfactant: I) n-butylamine and II) caprylic acid. | ||
Fig. 3 shows characteristic SEM images of 5 mol% neodymium-doped ZnO hybrid nanoparticles modified with 10 M a) and b) n-butylamine and c) and d) 10 M caprylic acid. These images show the effect of applied surfactant and dopants on the tailoring of morphology, agglomeration and particle size of the nanoparticles fabricated. The agglomeration was less when a higher concentration of the surface modifier was used. The surface modification has led to the controlling of growth direction; also particle size and agglomeration have been significantly reduced. It was found that the surface modifier could not only affect the dispersibility of the neodymium-doped ZnO hybrid nanoparticles synthesized, but also change their morphology and the size of the particles. The morphology of the particles are like small prisms.
 | ||
| Fig. 3 SEM images of 5 mol% neodymium-doped ZnO modified with 1.0 M a) and b) n-butylamine; c) and d) caprylic acid. | ||
The average size of the nanoparticles could be determined by applying the Scherrer equation to the half intensity width of the 010, 002, and 011 peaks, i.e.
| Particle | Surface modifier | β/° | θ/° | D/nm |
|---|---|---|---|---|
| 2 mol% Nd3+:ZnO | n-Butylamine | 0.235 | 34.54 | 5.2 |
| 5 mol% Nd3+:ZnO | n-Butylamine | 0.235 | 34.58 | 7.1 |
| 2 mol% Nd3+:ZnO | Caprylic acid | 0.235 | 34.72 | 7.1 |
| 5 mol% Nd3+:ZnO | Caprylic acid | 0.235 | 34.84 | 7.18 |
Zeta-potential of surface-modified neodymium-doped ZnO hybrid nanoparticles
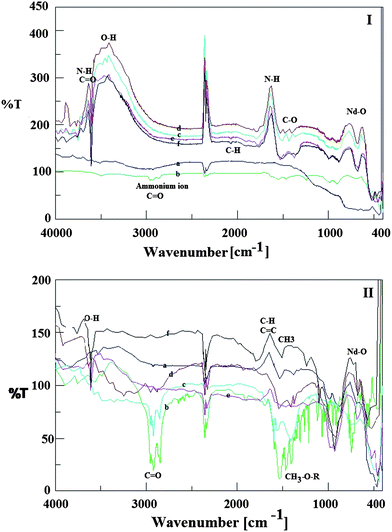
Zeta (ζ) potential measurements were performed for ZnO nanoparticles in order to characterize the surface charge of the nanoparticles and Fig. 4 shows the result as a function of pH. The obtained ζ potential of the nanoparticles was found to decrease with increase of pH as is expected for a surface with an acid–base group. The iso-electric point or point of zero charge (PZC) for ZnO nanoparticles was found to be 4.0 and 4.2 for modification by caprylic acid and n-butylamine, respectively. For small enough nanoparticles, a high ζ potential will confer stability, i.e. the solution or dispersion will resist aggregation. When the potential is low, attraction exceeds repulsion and the dispersion will break and flocculate.18,19 Therefore, colloids with high ζ potential (negative or positive) are electrically stabilized while colloids with low zeta-potentials tend to coagulate or flocculate. Our results indicate that the modified neodymium-doped ZnO hybrid nanoparticles are stable and highly negatively charged at pH > 4.0–4.2 and this negative charge intensity is proportional to the increasing pH. On the other hand, the particles fabricated are positively charged at pH < 4.0. The modified nanoparticles synthesized had low agglomeration, which prevents their flocculation or coagulation tendency.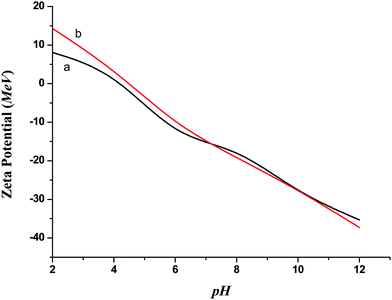 | ||
| Fig. 4 Zeta-potential of ZnO nanoparticles modified with a) caprylic acid and b) n-butylamine as surfactant. | ||
Band gap energy of modified neodymium-doped ZnO hybrid nanoparticles
The effect of doping was studied by UV-vis spectroscopy in the range of 200–600 nm. Fig. 5 shows that pure ZnO had no absorption in the visible light region (λ > 400 nm). The modified neodymium-doped ZnO hybrid nanoparticles using 1 M NaOH as solvent showed a remarkable absorption band shift toward the longer wavelength region, which indicates a decrease of the band gap energy. In addition, according to the band edges of the samples, determined from differentiation of the three curves, the band gap of the three samples were calculated according to the equation Eg = hc/λ, where Eg is the band gap energy (eV), h is Planck's constant, c is the speed of light (m s−1), and λ is the wavelength (nm).20 The Eg of pure ZnO, 2 mol% neodymium-doped ZnO hybrid nanoparticles and 5 mol% neodymium-doped ZnO hybrid nanoparticles were 3.35, 3.27 and 3.22, respectively.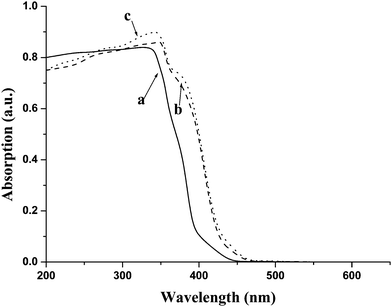 | ||
| Fig. 5 Effect of doping on band gap energy of the modified neodymium-doped ZnO hybrid nanoparticles: a) reagent grade ZnO b) 2 mol% neodymium-doped and c) 5 mol% neodymium-doped ZnO hybrid nanoparticles modified with n-butylamine. | ||
Conclusion
Neodymium-doped ZnO hybrid nanoparticles were successfully modified under mild hydrothermal conditions. Caprylic acid and n-butylamine were used as surface modifiers. Surface modification changed morphology and size of the neodymium-doped ZnO nanoparticles synthesized. In addition, it changed the surface charges and increased stability of the nanoparticles, which is necessary to achieve higher photodegradation efficiency. Doping the ZnO nanoparticles with a suitable metal, such as Nd3+, can reduce the band gap energy and enhance the feasibility of the photodegradation in the visible light. Pure ZnO is photoactive in the near UV areas; so, only 3–4% of solar light is utilized. Therefore, simultaneous surface modification and doping of ZnO nanoparticles not only changes the surface morphology, size and surface charges of the nanoparticles synthesized, but also significantly shifts the band gap energy to the visible region, where utilization of solar light is more comparable to the UV region.References
- N. F. Hamedani and F. Farzaneh, Synthesis of ZnO Nanocrystals with Hexagonal (Wurtzite) Structure in Water Using Microwave Irradiation, J. Sci., Islamic Repub. Iran, 2006, 17(3), 231–234 Search PubMed.
- L. N. Dem'yanets, L. E. Li and T. G. Uvarova, Hydrothermal Synthesis and Cathodoluminescence of ZnO Crystalline Powders and Coatings, J. Cryst. Growth, 2006, 287(1), 23–27 CrossRef CAS.
- L. Zhang, Y. Jiang, Y. Ding, M. Povey and D. York, Investigation into the Antibacterial Behaviour of Suspensions of ZnO Nanoparticles (ZnO Nanofluids), J. Nanopart. Res., 2007, 9(3), 479–489 CrossRef CAS.
- J. Strunk, K. Kähler, X. Xia and M. Muhler, The Surface Chemistry of ZnO Nanoparticles Applied as Heterogeneous Catalysts in Methanol Synthesis, Surf. Sci., 2009, 603(10–12), 1776–1783 CrossRef CAS.
- B. Shahmoradi, I. A. Ibrahim, N. Sakamoto, S. Ananda, T. N. G. Row, K. Soga and K. Byrappa. (2010). In situ Surface Modification of Molybdenum Doped TiO2 Organic-Inorganic Hybrid Nanoparticles under Hydrothermal Conditions and Treatment of Pharmaceutical Effluent. Environmental Technology (in press) Search PubMed.
- G.-C. Yi, C. Wang and W. I. Park, ZnO Nanorods: Synthesis, Characterization and Applications, Semicond. Sci. Technol., 2005, 20(4), S22–S34 CrossRef CAS.
- W. Li, A. I. Frenkel, J. C. Woicik, C. Ni and S. Ismatshah, Dopant Location Identification in Nd3+-doped TiO2 Nanoparticles, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 155315 CrossRef.
- K. Matsubara, P. Fons, K. Iwata, A. Yamada, K. Sakurai, H. Tampo and S. Niki, ZnO Transparent Conducting Films Deposited by Pulsed Laser Deposition for Solar Cell Applications, Thin Solid Films, 2003, 431–432, 369–372 CrossRef CAS.
- L. Wang and M. Muhammed, Synthesis of Zinc Oxide Nanoparticles with Controlled Morphology, J. Mater. Chem., 1999, 9, 2871–2878 RSC.
- A. Shokuhfar, J. Samei, A. E. Kandjani and M. R. Vaezi, Synthesis of ZnO Nanoparticles via Sol–Gel Process Using Triethanolamine as a Novel Surfactant, Defect Diffus. Forum, 2008, 273–276, 626–631 Search PubMed.
- M. Yoshimura and K. Byrappa, Hydrothermal Processing of Materials: Past, Present and Future, J. Mater. Sci., 2008, 43(7), 2085–2103 CrossRef CAS.
- B. Basavalingu, P. Madhusudan, A. S. Dayananda, K. Lal, K. Byrappa and M. Yoshimura, Formation of Filamentous Carbon through Dissociation of Chromium Carbide under Hydrothermal Conditions, J. Mater. Sci., 2008, 43(7), 2153–2157 CrossRef CAS.
- K. Byrappa, A. K. Subramani, K. M. Lokanatha Rai, S. Ananda, M. H. Sunitha, B. Basavalingu and K. Soga, Impregnation of ZnO onto Activated Carbon under Hydrothermal Conditions and its Photocatalytic Properties, J. Mater. Sci., 2006, 41, 1355–1362 CrossRef CAS.
- K. Byrappa, and M. Yoshimura, Handbook of Hydrothermal Technology, Noyes Publications/William Andrew Publishing LLC, USA, 2001 Search PubMed.
- K. Barbalace, http://klbproductions.com/; Periodic Table of Elements - Sorted by Ionic Radius, EnvironmentalChemistry.com, 1995–2010, accessed on-line: 1/25/2010, http://EnvironmentalChemistry.com/yogi/periodic/ionicradius.html.
- D. L. Pavia, G. M. Lampman, G. S. Kriz, Infrared Spectroscopy, in Introduction to Spectroscopy, Thomson Learning, USA, 3rd edn, 2001, 13–101 Search PubMed.
- T. Adschiri, K. Byrappa, Supercritical Hydrothermal Synthesis of Organic-Inorganic Hybrid Nanoparticles, in Nanohybridization of Organic-Inorganic Materials, ed. A. Muramatsu, Springer-Verlag, Germany, 2008 Search PubMed.
- Zeta Potential of Colloids in Water and Waste Water”, ASTM Standard D 4187–82, American Society for Testing and Materials, 1985.
- J. Lyklema, Fundamentals of Interface and Colloid Science.Elsevier, Wageningen, 1995 Search PubMed.
- J. Wang, R. Li, Z. Zhang, W. Sun, X. Wang, R. Xu, Z. Xing and X. Zhang, Degradation of Hazardous Dyes in Wastewater using Nanometer Mixed Crystal TiO2 Powders under Visible Light Irradiation, Water, Air, Soil Pollut., 2008, 189, 225–237 CrossRef CAS.
- L. N. Dem'yanets, D. V. Kostomarov and I. P. Kuz'mina, Chemistry and Kinetics of ZnO Growth from Alkaline Hydrothermal Solutions, Inorg. Mater., 2002, 38(2), 124–131 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |