Size-controlled synthesis of Pd nanocrystals using a specific multifunctional peptide†
Chin-Yi
Chiu
ab,
Yujing
Li
ab and
Yu
Huang
*ab
aDepartment of Materials Science and Engineering, University of California, Los Angeles, California 90095, USA. E-mail: yuhuang@seas.ucla.edu; Fax: +1 3102067353; Tel: +1 3107949589
bCalifornia Nanosystems Institute, University of California, Los Angeles, California 90095, USA
First published on 12th May 2010
Abstract
Here we report a peptide-mediated synthesis of Pd NCs in aqueous solution with controllable size in the sub-10 nanometre regime. The specific multifunctional peptide Q7 selected using the phage display technique can bind to the Pd NC surface and act as a stabilizer to mediate Pd crystal nucleation and growth. At the nucleation stage, Q7 bound to and helped stabilize the different-sized small Pd NC nuclei achieved using different concentrations of the external reducing agent, NaBH4. At the growth stage, Q7 played the dual role of binding to and reducing the precursor onto the existing nuclei, which led to the further controllable growth of the Pd NCs. By using the variable sizes of nuclei as seeds, and by introducing different amounts of precursors Pd NCs with tunable sizes from 2.6 to 6.6 nm were achieved with good size distribution.
Palladium (Pd) is an important catalyst for various applications such as hydrogen storage, catalytic converters for automotive emissions, and organic reactions including Suzuki, Heck and Stille coupling.1 The catalytic property of Pd nanocrystals (NCs) depends on their particle size ranging from 1 to 10 nm.2 Systematic studies for synthesizing Pd NCs with different sizes have been explored by using a variety of stabilizers and reducing agents.3 Compared to traditional polyol synthesis, a water-based system has been explored to synthesize metal NCs because it is environmental-friendly and the introduced reducing agent for the reduction of a metal precursor is safe and easy to handle.4 In recent years, biomolecules such as proteins, polypeptides, DNA and RNA have attracted attention for mediating the formation of inorganic NCs in aqueous solution.1e,4d,5 The aqueous-stabilized nanoparticles functionalized with biomolecules provide a variety of bio-related application such as biodetection and in the assembly of hybrid structures.5d Biomolecules can be specifically selected or designed to recognize a chosen target material through biomimetic selection cycles.6 The selected biomolecules can be used as stabilizers or capping agents to mediate the nucleation and crystal growth in the attempt to control the size and shape of the resulting NCs.4d,7 So far, there have been a few reports on Pd NCs synthesis in the sub-10 nm regime in aqueous solution, but only with limited size range or size distribution.2,8
The power of reducing agents has been known to be an important parameter for metal NCs synthesis.4c Herein, we report the size-controlled synthesis of Pd NCs using a multifunctional peptide molecule which can serve both as the stabilizer and a mild reducing agent. Specifically, we used a strong reducing agent, NaBH4, to control the size of Pd nuclei NCs in the range of 2 to 4.5 nm with good size distribution by controlling the nuclei size via concentration modulation of reducing agent; and took advantage of the self-reduction ability of the special peptide molecule to further fine tune the size of Pd NCs over a broad size range from 2.2 to 6.6 nm through the seeding growth approach.9
The phage display (Ph. D.) technique was used to identify peptide sequences that specifically bind to the Pd surface.10 The Pd binding peptide sequence Gln-Gln-Ser-Trp-Pro-Ile-Ser (QQSWPIS, termed as Q7, MW 885.98) was identified after three rounds of biopanning (see details in ESI†). Q7 peptide molecules were then prepared employing the Fmoc solid phase peptide synthesis (SPPS) technique, purified by HPLC method and tested by liquid chromatography mass spectrometry (LC-MS) (see Fig. S1, ESI†).
Three parallel reactions were carried out to confirm the specific binding effect of the selected peptide to Pd NCs synthesis (see details in ESI†). The peptide sequence Ser-Leu-Lys-Leu-Ala-Tyr-Pro (SLKLAYP, termed S7, MW 832.02) appearing in biopanning was used as a nonspecific control. The peptide was also synthesized by SPPS and purified by HPLC. The obtained peptides were dissolved in deionized water with a concentration of 2 mg ml−1. To synthesize Pd NCs, sodium tetrachloropalladate Na2PdCl4 and sodium borohydride NaBH4 were used as precursor and external reducing agent, respectively. Three vials containing 1 mM of Na2PdCl4 aqueous solution mixed with no peptide (blank reaction), 250 μl of S7 peptide solution (negative control reaction) and 250 μl of Q7 peptide solution (positive control reaction) were prepared, respectively. Then, one shot of 100 μl of fresh 50 mM NaBH4 was injected into each vial and the reaction was stirred vigorously at room temperature. Before the introduction of NaBH4, the solution appeared yellow. After injecting the reducing agent, the solutions quickly changed to brown indicating the formation of Pd NCs. Fig. 1 shows the TEM images of the obtained Pd NCs of the blank reaction, negative control reaction and positive control reaction after 10 min. The Pd NCs from the blank reaction (Fig. 1a) had a size of 36 ± 9 nm. In the negative control reaction with the nonspecific S7 peptides, small Pd NC aggregations were found (Fig. 1b) and the Pd NCs were of irregular shape. While the Pd NCs obtained from the positive control solution stabilized by Q7 were well dispersed and showed an NC size of 2.9 ± 0.4 nm with good size distribution (Fig. 1c). Comparing the results of the three reactions, it seemed that both Q7 and S7 were able to deter the Pd NC growth, therefore the NCs found in the positive and negative reactions were smaller than those found in the blank reaction. In contrast to the well dispersed Pd NCs in the positive reactions, the aggregation of small Pd NCs found in the negative control experiment (Fig. 1b) suggested that although S7 peptide might affect the crystal growth of Pd through possibly interfering with the reduction of the precursors or weak binding onto the Pd NC surface, it was not able to stabilize Pd NCs in aqueous solution. These results confirmed that the Q7 peptide was able to specifically bind to and slow down the growth of Pd NCs, as well as stabilize Pd NCs in aqueous solution.
 | ||
| Fig. 1 TEM images of the obtained Pd NCs from the blank reaction without peptide (a), the negative control reaction with a nonspecific peptide (b), and the positive control reaction with Q7 (c) at 10 min. | ||
The Q7 peptide sequence has amino acids containing a hydroxyl group and amide group rich motif similar to the previously reported sequences.10 The interaction between hydroxyl (i.e., serine) residual groups and the metal surface may contribute to the strong binding ability of Q7 to the Pd crystal surface.10 More interestingly, Q7 also contains the amino acid tryptophan (W) which was reported to be able to reduce silver and gold ions.11 Here we found that the Q7 sequence containing W could also reduce the Pd ion precursor, Na2PdCl4. To further elucidate the role of W and Q7 in Pd NCs synthesis and Pd surface binding, we studied the effects of W, Q7 and Gln-Gln-Ser-Pro-Ile-Ser (QQSPIS (Q7 without W residue) termed Q6, MW 699.77) on the Pd NCs growth, respectively. 500 μg ml−1 of W, Q7 and Q6 were incubated in 5 mM Na2PdCl4 solutions, respectively. Fig. 2(a) shows the color evolution of the three incubations containing W, Q7 and Q6. After 4 h of incubation, it was found that the color of the solutions containing W and Q7 changed from light yellow to brown, indicating both W and Q7 performed the same effect on reducing PdCl42− to Pd (Fig. S2† shows TEM images of the incubations), while the color of the solution containing Q6 displayed no change after 4 h (Fig. 2(a)), which confirmed that it was the W residue that has the reducing power while the rest of the residues (i.e. Q6) only contributed to the binding ability of the Q7 sequence. To further elucidate the role of W on Pd binding, Pd NCs were synthesized with the same method described previously with a final concentration of 1 mM Na2PdCl4, 1 mM NaBH4 and 100 μg ml−1 of W, Q7 and Q6 were prepared, respectively. A TEM image of the obtained Pd NCs from W-contained reaction was taken at 1 min of the reaction as shown in Fig. 2(b), which shows severe aggregation of Pd NCs. Pd NC precipitations were found in the solution within 3 min after the introduction of NaBH4, while NCs from Q7- and Q6- stabilized reactions were stable even after a couple of hours (Fig. 2(c) and (d)). It was also found that the Q7-stabilized Pd NCs (Fig. 2(c)) displayed more uniform shape and better size distribution when compared with Q6-stablized Pd NCs, which would grow to ∼10 nm and coalesce (Fig. 2(d)). The results suggest that Q6 peptide sequence which is similar to Q7 peptide sequence but possesses no W residue, exhibited less size control over Pd NCs synthesis, possibly due to its lower binding affinity on the Pd surface. Therefore, we conclude that the W residue in Q7 plays an important dual role in Pd NC synthesis in both the reduction of Na2PdCl4 and the tight binding to Pd surface. We then further explored the dual functionality of Q7 to obtain Pd NCs of tunable sizes with good size distribution.
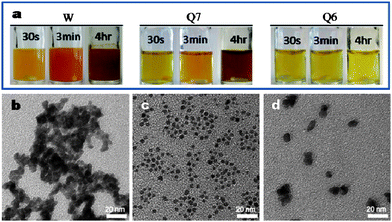 | ||
| Fig. 2 (a) Photo images of color evolution of incubating Na2PdCl4 with W, Q7 and Q6, respectively. (b), (c) and (d) are TEM images of the obtained Pd NCs from the reactions with W, Q7 and Q6, respectively; NaBH4 was used as a reducing agent in the reactions. | ||
It has been established that selected metal-binding molecules can perform self-reduction ability to reduce metal ion precursors without an external reducing agent. However, the incubation time was either long or the size-control of nanoparticles was limited.5d,7b,11,12 Herein, we combined a external reducing agent and the self-reducing power of the Q7 peptide to achieve fine size control of Pd NCs over a broader size range. Different concentrations of external reducing agent, NaBH4, were first explored to control the nucleation stage of Pd NCs synthesis. In short, variable amount of 50 mM of NaBH4 (i.e. 50 μl, 100 μl and 150 μl) were injected into the pre-prepared mixed solution containing 1 mM Na2PdCl4 and 100 μg ml−1 of Q7 peptides, to reach the final NaBH4 concentrations of 0.5 mM, 1 mM and 1.5 mM, respectively. Droplets of the three reaction solutions were taken at 20 s and let dry on TEM grids. As shown in Fig. 3, the Pd NCs formed in the early stage of reactions were well dispersed; and with the decreasing concentration of NaBH4 from 1.5 mM, 1 mM to 0.5 mM, the average size of Pd NCs increased from 2.2 nm, 2.7 nm to 3.0 nm, respectively. It has been shown that homogeneous nanoparticle dispersions are favored by a single, rapid nucleation event.13 Herein the introduction of the strong reducing agent led to the rapid reduction of PdCl42− and generated a burst of Pd atoms which led to Pd crystal growth. The resulting small Pd nuclei or clusters were instantly covered and stabilized by Q7 peptides, which prevented the coalescence of the small nuclei or clusters and therefore helped maintain the narrow size distribution of the Pd NCs. With decreasing concentration of NaBH4, fewer Pd atoms were generated which led to the decreased nuclei numbers and increased Pd NC sizes.13a
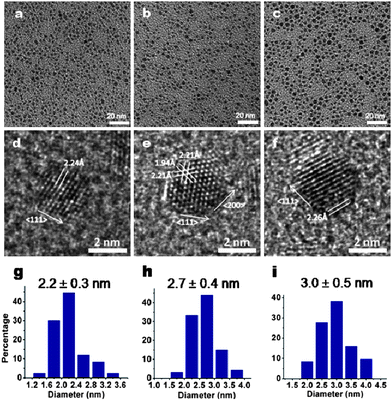 | ||
| Fig. 3 TEM images of Pd NCs obtained at 20 s reaction time with different concentrations of NaBH4: 1.5 mM (a), 1 mM (b) and 0.5 mM (c), corresponding high-resolution TEM images (d–f) and size distributions (g–i). | ||
Interestingly the Pd NCs continued to grow slowly for a long period of time, i.e. ∼1 h, following the introduction of NaBH4, although the Pd NCs obtained at 10 min reaction time showed similar size to that of the nuclei at 20 s (Fig. S3†) suggesting a slower growth process. The reaction triggered by NaBH4 was expected to complete over a course of a few minutes as NaBH4 has been shown to be a strong reducing agent.14 We suggest the succeeding slower growth period of the Pd NCs was a result of the continued reaction made possible by the multifunctional Q7 peptide, and specifically by the W in the Q7 sequence. As shown in Fig. 4, after one hour, the Pd NCs grew from 2.2 nm to 2.6 nm, 2.7 nm to 3.4 nm and 3.0 nm to 4.3 nm from the three reaction solutions following the nucleation event shown in Fig. 3, respectively. Pd NCs obtained after several hours of reaction did not show obvious size change, suggesting the completion of the reaction at 1 h. In addition, no small sized Pd NCs were observed, indicating the NCs generated during the burst nucleation event served as seeds for the subsequent NC growth, where the Q7 peptides continued to reduce Pd directly onto the seeding NCs.15 Comparing Fig. 4 to Fig. 3, it was also noted the increment in size was more pronounced in larger size nuclei which were achieved with a lower concentration of NaBH4. We attributed this effect to the difference in the remaining concentrations of the precursors (Na2PdCl4) after the NaBH4 reduction. For example, after the nucleation event, the reaction with 0.5 mM NaBH4 had most left-over PdCl42− precursor in the solution which led to a faster reduction rate. As a result, in the growth stage of Pd NCs from 20 s to 1 h, a size increase of 3.0 to 4.3 nm was observed. While the reaction solution with 1.5 mM NaBH4, a small increase from 2.2 to 2.6 nm was observed due to the lower amount of remaining precursor available following the reaction of NaBH4. This result also proves at growth stage it is Q7 peptide not NaBH4 used to reduce Pd ions because higher concentration of NaBH4 did not lead to larger-sized Pd NCs. These observations suggest that the size of Pd NCs is controllable by varying the concentration of the reducing agent NaBH4, and is further tunable with the amount of the precursor, Na2PdCl4 in the solution.
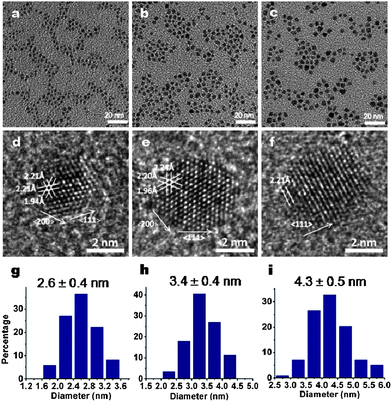 | ||
| Fig. 4 TEM images of Pd NCs obtained at 1 h reaction time with different concentrations of NaBH4: 1.5mM (a), 1mM (b) and 0.5mM (c), and corresponding high-resolution TEM images (d–f) and size distributions (g–i). | ||
Experiments were then carried out to fine tune the size of Pd NCs over the range of 2 to 7 nm by exerting controls over the nucleation and crystal growth of Pd NCs, respectively. Three nuclei (or seeding) solutions were prepared with different concentrations of NaBH4 as described above, which contained Pd NCs of sizes, 2.6 nm, 3.4 nm and 4.3 nm, respectively (Fig. 5(a, e, i)). 5 mM of Na2PdCl4 was used as the growth solution and no external reducing agent was introduced at the NC growth stage. Different volumes of the growth solution, i.e. 50 μl, 100 μl and 200 μl, were introduced into the seeding solutions, respectively, and all reactions were kept vigorously stirring for one hour before the samples were taken for TEM characterization. As shown in Fig. 5, Pd NCs were obtained by tuning the volume of the growth solution introduced to the three seeding solutions. The Q7 peptide reduced the precursor in the growth solution directly onto the Pd NC seeds to create larger Pd NCs. Using this approach, we could exert a precise size control on the obtained Pd NCs to have size varieties from 2.6 nm to 6.6 nm with approximately 1 nm control.
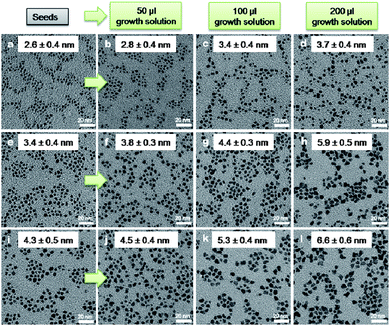 | ||
| Fig. 5 TEM images of size evolutions of Pd NCs by varying the volume of growth solution into different seeding solutions. (a, e and i) represent seeding solutions with Pd NCs of 2.6, 3.4 and 4.3 nm, respectively; (b), (c) and (d) show the size evolution of Pd NCs from (a) with different volume of growth solution from 50 μl, 100 μl to 200 μl, respectively. Similarly, (f), (g) and (h) and (j), (k) and (l) evolve from (e) and (i), respectively by varying the volume of growth solution to control the size of Pd NCs. Reaction time was 1 h for all reactions. | ||
In summary, we have reported the rational synthesis of monodispersed sub-10 nm of Pd NCs with fine-tunable size with a multifunctional peptide Q7, in aqueous solution at room temperature. The specific peptide molecule, Q7, bound to the Pd NC surface and acted as a stabilizer to mediate Pd crystal nucleation and growth. At the nucleation stage, Q7 bound to and helped stabilize the different-sized small Pd NC nuclei achieved (i.e. 2.2 nm, 2.7 nm and 3.0 nm) using different concentrations of the external reducing agent, NaBH4. At the growth stage, W in Q7 played the dual role of binding to and reducing the precursor onto the existing nuclei, which led to the further controllable growth of the Pd NCs. By using the variable sizes of nuclei as seeds, and by introducing different amounts of precursors Pd NCs with tunable sizes from 2.6 to 6.6 nm were achieved with good size distribution.
Acknowledgements
We acknowledge support from the ONR under award N00014-08-1-0985, and ARO Proposal No. 54709-MS-PCS. We also thank Electron Imaging Center of Nanomachines for the TEM support and Prof. Y. Tang for the LC-MS support.References
- (a) L. Schlapbach and A. Zuttel, Nature, 2001, 414, 353 CrossRef; (b) Y. Nishihata, J. Mizuki, T. Akao, H. Tanaka, M. Uenishi, M. Kimura, T. Okamoto and N. Hamada, Nature, 2002, 418, 164 CrossRef CAS; (c) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (d) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS; (e) D. B. Pacardo, M. Sethi, S. E. Jones, R. R. Naik and M. R. Knecht, ACS Nano, 2009, 3, 1288 CrossRef CAS; (f) C. Jiang, S. Ranjit, Z. Duan, Y. L. Zhong, K. P. Loh, C. Zhang and X. Liu, Nanoscale, 2009, 1, 391 RSC.
- (a) J. Turkevich and G. Kim, Science, 1970, 169, 873 CrossRef CAS; (b) R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2004, 108, 8572 CrossRef CAS; (c) Y. Piao, Y. Jang, M. Shokouhimehr, I. S. Lee and T. Hyeon, Small, 2007, 3, 255 CrossRef CAS.
- (a) X. Wang, J. Zhuang, Q. Peng and Y. Li, Nature, 2005, 437, 121 CrossRef CAS; (b) S.-W. Kim, J. Park, Y. Jang, Y. Chung, S. Hwang, T. Hyeon and Y. W. Kim, Nano Lett., 2003, 3, 1289 CrossRef CAS; (c) M. T. Reetz and M. Maase, Adv. Mater., 1999, 11, 773 CrossRef CAS; (d) L. A. Gugliotti, D. L. Feldheim and B. E. Eaton, Science, 2004, 304, 850 CrossRef CAS; (e) J. Liu, F. He, T. M. Gunn, D. Zhao and C. B. Roberts, Langmuir, 2009, 25, 7116 CrossRef CAS; (f) M. Ganesan, R. G. Freemantle and S. O. Obare, Chem. Mater., 2007, 19, 3464 CrossRef CAS; (g) Y. Xiong, H. Cai, B. J. Wiley, J. Wang, M. J. Kim and Y. Xia, J. Am. Chem. Soc., 2007, 129, 3665 CrossRef CAS.
- (a) S. O'Brien, L. Brus and C. B. Murray, J. Am. Chem. Soc., 2001, 123, 12085 CrossRef CAS; (b) Y. Sun and Y. Xia, Science, 2002, 298, 2176 CrossRef CAS; (c) B. Lim, M. Jiang, J. Tao, P. H. C. Camargo, Y. Zhu and Y. Xia, Adv. Funct. Mater., 2009, 19, 189 CrossRef CAS; (d) Y. Li, G. P. Whyburn and Y. Huang, J. Am. Chem. Soc., 2009, 131, 15998 CrossRef CAS; (e) Z. R. Dai, S. Sun and Z. L. Wang, Nano Lett., 2001, 1, 443 CrossRef CAS.
- (a) C.-C. You, M. De, G. Han and V. M. Rotello, J. Am. Chem. Soc., 2005, 127, 12873 CrossRef CAS; (b) X. Kou, S. Zhang, Z. Yang, C.-K. Tsung, G. D. Stucky, L. Sun, J. Wang and C. Yan, J. Am. Chem. Soc., 2007, 129, 6402 CrossRef CAS; (c) J. H. Choi, K. H. Chen, J.-H. Han, A. M. Chaffee and M. S. Strano, Small, 2009, 5, 672–675 CrossRef CAS; (d) J. Slocik, M. Stone and R. Naik, Small, 2005, 1, 1048 CrossRef CAS; (e) R. M. Kramer, C. Li, D. C. Carter, M. O. Stone and R. R. Naik, J. Am. Chem. Soc., 2004, 126, 13282 CrossRef CAS; (f) L. Berti and G. A. Burley, Nat. Nanotechnol., 2008, 3, 81 CrossRef CAS.
- (a) S. R. Whaley, D. S. English, E. L. Hu, P. F. Barbara and A. M. Belcher, Nature, 2000, 405, 665 CrossRef CAS; (b) S. Brown, Nat. Biotechnol., 1997, 15, 269 CAS.
- (a) I. A. Banerjee, L. Yu and H. Matsui, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14678 CrossRef CAS; (b) R. R. Naik, S. J. Stringer, G. Agarwal, S. E. Jones and M. O. Stone, Nat. Mater., 2002, 1, 169 CrossRef CAS; (c) J. Samson, A. Varotto, P. C. Nahirney, A. Toschi, I. Piscopo and C. M. Drain, ACS Nano, 2009, 3, 339 CrossRef CAS.
- (a) Y. Li, E. Boone and M. A. El-Sayed, Langmuir, 2002, 18, 4921 CrossRef CAS; (b) A. Nemamcha, J.-L. Rehspringer and D. Khatmi, J. Phys. Chem. B, 2006, 110, 383 CrossRef CAS.
- (a) S. E. Habas, H. Lee, V. Radmilovic, G. A. Somorjai and P. Yang, Nat. Mater., 2007, 6, 692 CrossRef CAS; (b) X. Kou, W. Ni, C.-K. Tsung, K. Chan, H.-Q. Lin, G. Stucky and J. Wang, Small, 2007, 3, 2103 CrossRef CAS.
- M. Sarikaya, C. Tamerler, A. K. Y. Jen, K. Schulten and F. Baneyx, Nat. Mater., 2003, 2, 577 CrossRef CAS.
- S. Si and T. Mandal, Chem.–Eur. J., 2007, 13, 3160 CrossRef CAS.
- K. T. Nam, Y. J. Lee, E. M. Krauland, S. T. Kottmann and A. M. Belcher, ACS Nano, 2008, 2, 1480 CrossRef CAS.
- (a) V. K. LaMer and R. H. Dinegar, J. Am. Chem. Soc., 1950, 72, 4847 CrossRef CAS; (b) C. B. Murray, C. R. Kagan and M. G. Bawendi, Annu. Rev. Mater. Sci., 2000, 30, 545 CrossRef CAS.
- A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310 CrossRef CAS.
- K. R. Brown, D. G. Walter and M. J. Natan, Chem. Mater., 2000, 12, 306 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. Experimental details for peptide selection, peptide synthesis and Pd NCs synthesis; Q7 peptide sequence molecular structure and characterization; TEM images of Pd NCs. See DOI: 10.1039/c0nr00194e/ |
| This journal is © The Royal Society of Chemistry 2010 |
