Self-assembly formation of multi-walled carbon nanotubes on gold surfaces
Jarrn-Horng
Lin
*a,
Ching-Shiun
Chen
b,
Mark H.
Rümmeli
*c and
Zhi-Yan
Zeng
a
aDepartment of Material Science, National University of Tainan, 33, Sec. 2, Shu-Lin St., Tainan, Taiwan 700, Republic of China. E-mail: janusjhlin@mail.nutn.edu.tw; Fax: +886 6 3017132; Tel: +886 6 2133111x647
bCenter for General Education, Chang Gung University, 259 Wen-Hwa 1st Road, Kwei-Shan, Tao-Yuan, Taiwan 333, Republic of China
cIFW Dresden, P.O. Box 270116, D-01171, Dresden, Germany
First published on 27th September 2010
Abstract
We report on the observation of self-assembled carbon nanostructures on a standard transmission electron microscopy (TEM) Au substrate formed via thermal chemical vapor deposition. Multi-walled carbon nanotubes (MWNTs) and other carbon nanostructures (CNs), such as carbon nanofibers and carbon nanoparticles (NPs), could be fabricated through structural transformation of metastable carbon layers on the Au surface during 800–850 °C with the thermal decomposition of ethylene. At these temperatures, we found that Au NPs will form immediately through the structural transformation of the Au grid surface in helium atmosphere. The Au NPs work as active centers to trigger the decomposition of ethylene into carbon atoms, which form metastable carbon layers or amorphous carbon nanobugs, and then form CNs via self-assembling. The growth of CNs was characterized by field-emission scanning electron microscopy (SEM), high-resolution TEM and RAMAN spectroscopy. The transformation of amorphous carbon nanobugs by electron beam irradiation is also recorded by in situ monitoring of TEM.
Introduction
Since the first report of carbon nanotubes (CNTs) in 1991, there have been numerous studies on the growth mechanism of CNTs. A widely accepted model, the vapor–liquid–solid (VLS) mechanism is often proposed to explain the growth of CNTs via catalytic chemical vapor deposition (CCVD).1 The explanation of VLS model for growing CNTs is generally relied on to explain the experiments of CCVD over iron-group metal catalysts, such as Fe, Co, Ni and their alloys. The high carbon solubility and thermal stability of these transition metal catalysts are regarded as the two main aspects of CNT synthesis by CCVD.Recent experimental studies show that the traditional low-activity metals, such as Cu,2–5 Ag,6 Pt,6 Pd6 and Au6–10 can also form CNTs in high yield. This breakthrough opens a new insight into the growth mechanisms of CNTs: the explanation of CNTs growth over metal catalysts with lower carbon solubility needs to be studied intensively. Moreover, the growth of CNTs can also be achieved through metal-free routes on different substrates, such as porous carbon,11 SiO2 nanoparticles (NPs),12,13 and nano diamond.14,15 A vapor–solid surface–solid (VSSS) model is proposed for the growth of single-walled CNTs (SWNTs).15 In the VSSS model, the size distribution and curvature of the non-metal NPs are the main deciding factors that regulate the growth of CNTs. In addition, a self-assembly model is also presented to explain the growth of multi-walled CNTs (MWNTs) on porous substrates.11 The pore structures, carbon diffusion and epitaxial growth are critical elements in this model. These approaches for metal-free CVD (MF-CVD) are at odds with the established models from CCVD synthesis over iron-group metals. Hence, there remains a need for an improved understanding of the mechanisms behind CNTs growth via metal-free or metal-assisted routes. This is particularly important for their application on the design of electronic devices, catalyst supports and polymer reinforcements. Furthermore, the combination of CNTs with copper-group metals and SiO2/Si substrate would be a revolutionary achievement for next-generation integration technology.
In addition, regarding the growth of CNTs guided by metal NPs via CCVD is reasonable. However, only a few interesting findings have been reported recently by Song et al.16 and Rümmeli et al.17 demonstrating that the growth of MWNTs could be formed by self-assembling even during the CCVD process. These findings open a new issue to discuss in the growth mechanism of CNTs over the well-known CCVD, which should be studied more at atomic-scale resolution to indicate the real functions of metal NPs.
Here, we present clear evidence showing the growth of MWNTs and other carbon nanostructures (CNs), such as carbon nanofibers (CNFs) and carbon nanoparticles (CNPs), from the self-assembly of metastable carbon layers on gold surfaces. The formation of MWNTs and other CNs are not guided by gold NPs, the movement of gold surface morphology at CNT growth temperatures creates highly-active geometric sites to self-assemble CNTs. This is the first time to directly observe the growth of CNTs on a metal surface via self-assembling without the aid of metal NPs. Our results provide an insight for better understanding of the real role of non-iron group metal catalysts in the growth mechanism of CNTs.
Experimental
A standard 200 mesh transmission electron microscopy gold grid was used as the support substrate. Prior to the synthesis of CNs, the grid surfaces were purified with 2 M nitric acid at room temperature for 4 h, and then thoroughly flushed with distilled water. To discuss the effect of surface morphology of the gold grid, typical samples were treated with aqua regia solution for 60 s to create surface roughness. Longer treatment times destroy the framework of the Au grid, which prevents TEM measurements. For the growth of CNs, the purified substrate was put into a tube furnace and heated to the required temperature (750–900 °C) in He flow (50 mL min−1). Once the synthesis temperature (750–900 °C) has been reached, the reaction was initiated by introducing a flowing C2H4/Ar mixture (flow rate = 50 mL min−1, C2H4/Ar mix = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) for typical time periods. Scanning electron microscopy (SEM, JEOL JSM-6700F) and transmission electron microscopy (TEM, JEOL AEM-3010 and JEM 2100) equipped with an energy dispersive spectrometer (EDS) were used to investigate the morphology and elemental analysis of the as-grown samples. Raman scattering spectroscopy (JOBIN-YVON T64000) was used with a laser excitation wavelength of 532 nm.
10) for typical time periods. Scanning electron microscopy (SEM, JEOL JSM-6700F) and transmission electron microscopy (TEM, JEOL AEM-3010 and JEM 2100) equipped with an energy dispersive spectrometer (EDS) were used to investigate the morphology and elemental analysis of the as-grown samples. Raman scattering spectroscopy (JOBIN-YVON T64000) was used with a laser excitation wavelength of 532 nm.
Results and discussion
We conducted the CNT growth process on a standard TEM gold grid at 750–900 °C without adding any metal catalysts, and used ethylene diluted with argon (C2H4/Ar = 1/10, flow rate 50 mL min−1) as the carbon source. The merits of using a standard TEM gold grid as the substrate to conduct CNT growth are to carry out the SEM, TEM, Raman measurements without any sampling preparations and to reduce any possible metal contamination from the sampling procedures.Fig. 1 shows representative SEM images of CNTs grown on standard and aqua regia treated TEM Au grids. Images a and b present the SEM micrographs of CNTs grown at the corner position of Au grid with different magnification. When an Au grid is pretreated in aqua regia for 60 s and then the CVD process applied, a noticeably higher CNT growth yield is obtained (images c and d). An Au grid can be regarded as bulk metal material, which, as yet, has not been reported to have any catalytic activity for growing CNTs. Considering previous studies and the VSSS model,15 we were surprised to find that CNTs could be fabricated on an Au thin film easily at elevated temperatures through thermal CVD.
 | ||
| Fig. 1 SEM images of (a) and (c) are representative micrographs of as-grown CNTs on TEM Au grid before and after treatment of aqua regia for 60 s. Images of (b) and (d) represent an enlarged area of (a) and (c), respectively. | ||
Dense and randomly formed clusters of CNTs are the main CNs displayed in Fig. 2a-b by SEM images in different close-view positions on the Au substrate. Often CNT clusters appeared at regions in which the surface was disturbed suggesting surface structural morphology may be important in the formation of the CNTs. Hollow and tangle-like structures of as-grown MWNTs are obtained as shown by TEM studies, e.g.Fig. 2c and 2d. The diameter distribution of the as-grown MWNTs ranged between 25–60 nm. Raman spectroscopy on the CNT samples (Fig. 2e) confirms sp2 carbon through the presence of the defect-induced D band (1330 cm−1) and tangential G band (1593 cm−1). Their relative ratio of IG/ID (= 0.875) is typical of low-crystallized MWNTs. Surprisingly, we did not observe any Au NPs inside or on the tip of the as-grown CNTs even when we used an angle-tilted sample holder for TEM measurements. This suggests that the growth model of CNTs on Au bulk surface is through a less well-known behavior.
 | ||
| Fig. 2 (a)-(b) SEM, (c) TEM and (d) HR-TEM images of random networks of CNTs grown on the edge or pore structures of Au substrate at 850 °C. (e) A typical Raman spectra of the as-grown CNTs. | ||
In the VSSS model, Takagi et al. pointed out that non-iron group metal NPs should dominate the growth of CNTs, the bottom- or tip-growth models will depend on the interaction of metal NPs and supported substrates.10 For the tip-growth model, the tip position of the as-grown CNTs should leave metal NPs with particle sizes usually consistent with the inner diameter of the as-grown CNTs. In our case, we hardly find any Au NPs on the top of the as-grown CNTs. Further examination of the as-grown MWNTs by HRTEM showed three kinds of structures were formed: MWNTs, CNFs and carbon NPs. Examples of these structures are given in Fig. 3a-c. Au NPs were also hardly found in the formation of the observed nanostructures. An important observation is that the MWNTs are always found on a short-range crystalline carbon layer that has formed on the Au substrate surface. Free stacking of the carbon layer is proposed to be produced from the decomposition of the carbon source via a self-assembling route. In Fig. 4a, a short lift-off MWNT was formed at 850 °C under the source of diluted ethylene for 5 min, its crystalline structure examined by HRTEM is consistent with that of the carbon layer shown in Fig. 4b. The graphic-like region of this layer presents a larger lattice distance (0.352 nm) compared with that of graphite (0.343 nm), which leads to further transformation into energy-stable structures. Recently, Homma et al. proposed that the deformation and lift-off of graphene-like structures on nano-diamond or SiO2 surfaces would dominate the CNT growth in metal-catalyst-free CVD.15,18
 | ||
Fig. 3 Typical TEM images of (a) carbon nanoparticles, (b) carbon nanofibers and (c) MWNTs grown on Au substrate by a mixture of C2H4/Ar (flow rate = 50 mL min−1, C2H4 /Ar mix = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at 850 °C for 30 min. 10) at 850 °C for 30 min. | ||
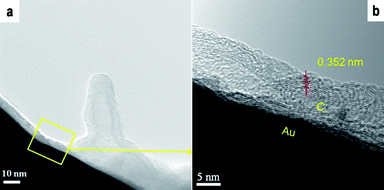 | ||
| Fig. 4 (a) Short-time formation of CNTs on Au grid surface and (b) short-range crystalline carbon layers covered on Au surface in a thermal CVD process at 850 °C for 5 min. | ||
For a longer reaction time at about 30 min, typical examples of the as-grown MWNTs are presented in Fig. 5a and 5b. The HRTEM studies reveal more detailed information on the structure of the as-grown MWNTs which have a unique inner morphology with both hollow and solid construction. The diameter of MWNTs ranged between 30 and 50 nm. It is apparent that their inner structures are quite different from the well-known structure of MWNTs. At the tip area of the as-grown MWNTs a solid-like structure with near 50 nm length was found, which was not observed in previous studies from CCVD synthesis. We are not aware of this structure being discussed in other studies with the tip-growth or bottom-growth models of CNTs via CCVD process. The tip-growth model has been ruled out by the results shown in Fig. 1 to Fig. 5. If the growth behavior of the as-grown MWNTs was catalytically guided by the Au NPs through the bottom-growth model on the surface of Au substrate, the formation of the carbon layer should cover Au NPs immediately and also break down their catalytic activity for growing CNTs. Here, we consider that the metastable carbon layer would play an important role for the growth of CNTs. The possible formation pathway of as-grown MWNTs in our observation is through self-assembly of the metastable carbon layer.
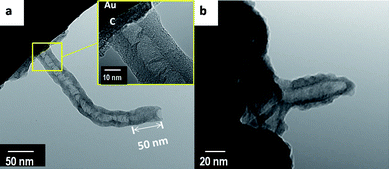 | ||
| Fig. 5 (a)-(b) TEM images of MWNTs were grown on a gold surface at 850 °C, the inset of (a) displayed a thick carbon layer deposited between MWNTs and the gold substrate. | ||
To verify the self-assembly model on Au grid surface, we systematically explored various synthesis parameters: (1) reaction time (5, 30, 120, 240 min) and (2) temperature (750, 800, 850 and 900 °C). If the growth of the CNTs were guided by Au NPs, longer reaction times with a typical temperature will lead to higher growth yields. Electron microscopy images are presented in Fig. 6 and 7. In Fig. 6 a–d, the SEM images depict the transformations of the surface morphology on the Au grid for temperatures ranging from 750 to 900 °C before introducing the carbon source ethylene. After the thermal CVD process shown in Fig. 6 e–h, a higher growth yield of CNTs is found in the temperature region of 800–850 °C. More interestingly, with a lower reaction temperature of 750 °C, only amorphous carbon layers are found on the Au substrate as shown in Fig. 6i. However, at the higher temperature of 900 °C, graphene layers are moving on the surface of the Au substrate and deform as they push against each other. This interesting finding is displayed in Fig. 6l. Only temperatures between 800 and 850 °C will contribute to the growth of CNTs as demonstrated in Fig. 6j and 6k.
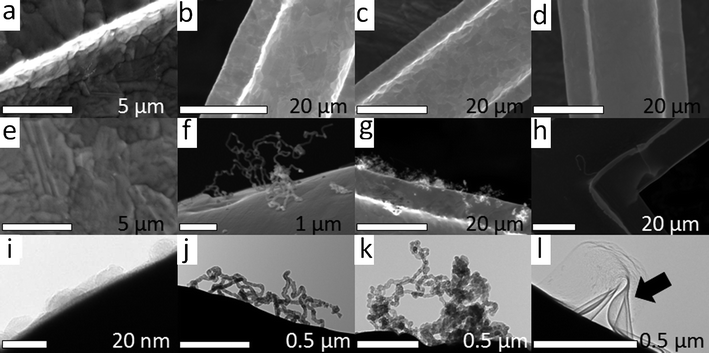 | ||
Fig. 6 (a)–(d) SEM images of Au substrate thermal-treated in helium at 750, 800, 850 and 900 °C for 30 min respectively, (e)–(h) SEM images of as-grown CNTs on Au substrates by a mixture of C2H4/Ar (flow rate = 50 mL min−1, C2H4 /Ar mix = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) for 30 min at 750, 800, 850 and 900 °C for 30 min respectively and (i)–(l) TEM images represent for SEM images of (e)–(h) respectively. The arrow in (l) displays the as-grown graphene structure. 10) for 30 min at 750, 800, 850 and 900 °C for 30 min respectively and (i)–(l) TEM images represent for SEM images of (e)–(h) respectively. The arrow in (l) displays the as-grown graphene structure. | ||
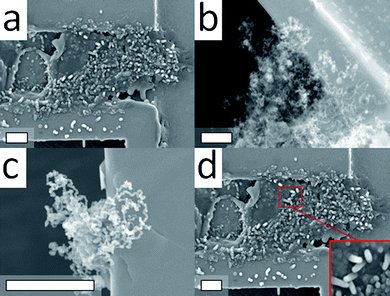 | ||
Fig. 7 (a)–(d) SEM images of as-grown CNTs on Au substrates by a mixture of C2H4/Ar (flow rate = 50 mL min−1, C2H4 /Ar mix = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at 850 °C for 5, 30, 120 and 240 min respectively. The inset in (d) shows that solid fiber-like structures form for the longer reaction time. The scale bar is 5 μm. 10) at 850 °C for 5, 30, 120 and 240 min respectively. The inset in (d) shows that solid fiber-like structures form for the longer reaction time. The scale bar is 5 μm. | ||
We also explored the role of reaction time on the CNT growth at 850 °C on an Au grid and examined the resultant results by SEM, e.g.Fig. 7 a–d. In the initial stage (< 5 min) of the CVD process, as displayed in Fig. 7a, we can clearly identify numerous nanobugs having formed on the surface of the Au substrate. Although longer reaction time will increase the growth rate of the CNTs, the thickness of the deposited carbon layer would also increase. Especially when the reaction time is at about 240 min, only CNFs are found on Au grid surface. The diameters of the as-grown CNFs are around 200–400 nm, which indicates the decomposition of the carbon source continues without any catalytic assistance from the Au NPs. The relationship between the thickness of the carbon layers and the reaction time is illustrated as a diagram shown in Fig. 8. This statistical survey of the thickness of the carbon layer with contact time between Au substrate and carbon source provides a clear evidence of the transformation of the metastable carbon layer. The data suggest that CNTs or CNFs would be formed by this pathway.
 | ||
Fig. 8 The diagram of the metastable carbon layer (MSCL) versus the reaction time of Au substrate by a mixture of CH4/Ar (flow rate = 50 mL min−1, C2H4 /Ar mix = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at 850 °C. The thickness of the MSCL was measured by the side-view of HRTEM analysis. 10) at 850 °C. The thickness of the MSCL was measured by the side-view of HRTEM analysis. | ||
Moreover, these data show several interesting results: (i) The growth yield of CNTs (determined through SEM and TGA, data not shown) improves noticeably with increasing reaction temperature from 800 to 850 °C. Obviously, at lower temperature (750 °C) or higher temperature (900 °C), the surface of gold hardly shows any CNTs present. At 750 °C, the gliding morphology on the Au surface is still inert to decomposing the carbon source, however the smooth surface of Au at 900 °C is attributed to surface melting (mp 1064 °C). (ii) In the initial growth stage (< 5 min) of CNTs on Au surface at 850 °C, it is easy to find numerous hemispherical nanobugs distributed on the Au surface. The diameter distribution of hemispherical nanobugs is in the range of 10 and 100 nm. However, longer growth times (>30 min.) led to their disappearance owing to being covered by carbon layers or as active sites to lift-off MWNTs.
Island-like carbon structures were proposed by several studies to deduce the growth model of CNTs by a non-metal assisted process.15,17 In our observations shown in Fig. 9a, we found that numerous hemispherical nanobugs (>5 nm) have formed. Closer views of the surface morphology of Au grids at 850 °C before and after introducing the carbon source ethylene were performed by HRTEM and displayed in Fig. 9a and 9b. The initial formation of hemisphere nanobugs is attributed to the transformation of the Au surface before the CNTs growth process at 850 °C. When the carbon source was subjected to Au substrate at the same temperature, most of the Au hemisphere nanobugs were immediately covered by carbon layers. We were surprised to find that the Au hemispherical nanobugs have superior activity for catalytic cracking of ethylene into carbon atoms, which spill over on the Au surface and formed a carbon layer or deforming into carbon nanobugs owing to the saturation of carbon solubility of Au NPs.
 | ||
| Fig. 9 (a) TEM image of Au nanobugs formed on Au grid surface by thermal treated in helium at 850 °C for 5 min. (b) TEM image of (a) after introducing diluted ethylene for 5 min. at the same temperature. (Yellow lines in Fig. 9b display the boundary of Au NPs and deposited carbon layers.) | ||
More interestingly, if the dissociation of ethylene is only determined by Au hemispherical nanobugs, then once covered with a thin carbon layer they should stop further carbon layers from forming. In Fig. 8, we demonstrate two different growth rates between reaction time and the thickness of a carbon layer, in the r1 stage, the deposited carbon forms quicker than that found in the r2 stage. This implies that the formation of carbon layers at short reaction times was controlled by Au NPs. Longer reaction times would cause poisoning of Au NPs due to deposited carbon. However, the cracking of ethylene continues with time even though at a lower rate. The thermal cracking of ethylene was found to be minor according to pure blank tests. This suggests the formed carbon layers are aiding the decomposition of the source. Several reports had proved that elemental carbon materials can have high catalytic activity in decomposition of carbon-bearing molecules.19–21 Our observations also indicate this.
A typical open-end MWNT was fabricated between the hemisphere nanobugs shown in Fig. 9b. Recently, Takeda et al. used in situ environmental TEM to reveal the mechanism of the close-ended form of the MWNTs over iron-based catalysts through CVD.22 In our observations, the open-end structure of the as-grown MWNTs is beyond the control of metal catalysts. The suggested the growth model is self-assembling from carbon layers, because the Au NPs were covered with a spillover carbon layer immediately. Furthermore, the diameter of CNTs should be regulated by the particle size of metal or non-metal NPs. In our findings shown in Fig. 10a and 10b, we nearly found Au NPs embedded in the center or outside of MWNTs, which further support our proposed self-assembling model.
 | ||
| Fig. 10 TEM images of (a) and (b) demonstrate the Au NPs are found outside or near the as-grown MWNTs. | ||
Thus, we propose the as-grown MWNTs form by the reconstruction of metastable carbon layer because its structure is quite different from that of metal-catalyzed MWNTs by the tip-growth or bottom-growth models. The above interesting findings are quite different from the usual VLS or VSSS growth models, which suggest the formation of NPs on the substrate would play a key role in the growth of such CNTs by metal-free chemical vapor deposition.
Although Cheng et al.12 and Takagi et al.15 reported simple methods to synthesize CNTs in the desired positions through MFCVD, the VSSS model seems reasonable for explaining the growth of SWNTs by MF-CVD, however direct evidence of the role of the non-metal NPs is still open to discussion. Moreover, a direct cloning of SWNTs from open-end SWNTs has been shown by Liu et al.,23 which means typically high active carbon-seed sites will have the ability to decompose carbon-bearing molecules and self-assembling of carbon atoms into CNTs.
In our observations, the gold NPs do not dominate the positions of the epitaxial growth of MWNTs, because MWCNTs are always found near the gold NPs not directly grown on them, even treated them at different temperatures. High-energy sites, such as edges, defects or Au nanobugs, on the Au substrate will be the active centers for the dissociation of hydrocarbons into carbon and hydrogen atoms. The diffusion of carbon atoms would collide together to form a metastable structure, such as the metastable carbon layers, and then deform and reconstruct into CNs through self-assembling along with the surface. In Fig. 11a, it is clearly demonstrated that carbon nanobugs are formed on the Au surface in the initial stage (less than 5 min) of the CNT growth process. Only carbon, gold and copper (coming from the TEM sample holder) elements were traced by EDS analysis shown in Fig. 11b. The newborn carbon nanobugs were unstable and would be transformable by a continuous electron beam radiation in a TEM chamber, the continuously in situ TEM images were monitored in Fig. 11c–f. For longer CNTs growth time (above 10 min), MWNTs form from the carbon nanobugs under a continuous supply of carbon atoms by the dissociation of ethylene. A typical TEM image is displayed in Fig. 11g. The original structure of the MWNTs shown in Fig. 11g shows fully solid-like CNFs, which after exposure to the electron beam (<1 s) in a TEM chamber become porous and solid-like structures near the tip. Although we have no direct measurements by in situ environmental TEM to monitor the CNTs growth process step by step accompanying the decomposition of a carbon source, it does not contradict our results in ex-situ TEM. Furthermore, these metastable CNTs have not been reported in any previous CCVD studies. The possible formation route is self-assembled through MF-CVD on high active sites of the metastable carbon layer. Even though a detailed study of the process is still required, our observations provide new insights for growing CNTs by MF-CVD.
 | ||
| Fig. 11 (a) SEM image of carbon nanobugs formed on a gold surface. (b) Typical EDS spectra for the carbon nanobugs grown on Au surface. (c)–(f) The transformation images of a typical carbon nanobugs on the Au surface exposed in electron beams under the TEM measurement. (g) A newborn MWNT exposed in electron beams and formed porous structures on the Au surface. The scale bar is 200 nm. | ||
Conclusions
We present a self-assembly model for growing CNTs through a CCVD process on an Au bulk surface. Self-assembly of MWNTs was performed by the reconstruction of metastable carbon layers. This approach can provide valuable implications for understanding the growth mechanism of CNTs via a metal-free catalytic process.Acknowledgements
Financial support from the National Science Council of Republic of China (98-2113-M-024-001-) is gratefully acknowledged.References
- P. J. F. Harris, Carbon, 2007, 45, 229–239 CrossRef CAS.
- W. Zhou, Z. Han, J. Wang, Y. Zhang, Z. Jin, X. Sun, Y. Zhang, C. Yan and Y. Li, Nano Lett., 2006, 6, 2897–2990.
- J. H. Lin, C. S. Chen, H. L. Ma, C. Y. Hsu and H. W. Chen, Carbon, 2007, 45, 223–225 CrossRef CAS.
- F. S. Ou, M. M. Shaijumon, L. Ci and P. M. Ajayan, Carbon, 2007, 45, 1713–1716 CrossRef CAS.
- S. Bals, J. Batenburg, J. Verbeeck, J. Sijbers and G. V. Tendeloo, Nano Lett., 2007, 7, 3669–3674 CrossRef CAS.
- J. N. Wang, L. F. Su and Z. P. Wu, Cryst. Growth Des., 2008, 8, 1741–1747 CrossRef CAS.
- D. Takagi, Y. Homma, H. Hibino, S. Suzuki and Y. Kobayashi, Nano Lett., 2006, 6, 2642–2645 CrossRef CAS.
- S. Bhaviripudi, E. Mile, S. A. Steiner, A. T. Zare, M. S. Dresselhaus and A. M. Belcher, J. Am. Chem. Soc., 2007, 129, 1516–1517 CrossRef CAS.
- C. Luo, L. Liu, K. Jiang, L. Zhang, Q. Li and S. Fan, Carbon, 2008, 46, 440–444 CrossRef CAS.
- D. Takagi, Y. Kobayashi, H. Hibino, S. Suzuki and Y. Homma, Nano Lett., 2008, 8, 832–835 CrossRef CAS.
- J. H. Lin, C. S. Chen, H. L. Ma, C. Y. Hsu and H. W. Chen, Carbon, 2008, 46, 1619–1623 CrossRef CAS.
- B. Liu, W. Ren, L. Gao, S. Li, S. Pei, C. Liu, C. Jiang and H. M. Cheng, J. Am. Chem. Soc., 2009, 131, 2082–2083 CrossRef CAS.
- S. Huang, Q. Cai, J. Chen, Y. Qian and L. Zhang, J. Am. Chem. Soc., 2009, 131, 2094–2095 CrossRef CAS.
- F. Rao, T. Li and Y. Wang, Carbon, 2009, 47, 3580–3584 CrossRef CAS.
- D. Takagi, Y. Kobayashi, H. Hibino and Y. Homma, J. Am. Chem. Soc., 2009, 131, 6922–6923 CrossRef CAS.
- J. Song, G. Du, C. Song, J. Zhao, S. Feng, J. Zheng and Z. Zhu, J. Mater. Chem., 2009, 19, 7725–7729 RSC.
- M. H. Rummeli, F. Schäffel, A. Bachmatiuk, D. Adebimpe, G. Trotter, F. Börrnert, A. Scott, E. Coric, M. Sparing, B. Rellinghaus, P. G. McCormick, G. Cuniberti, Martin Knupfer, L. Schultz and B. Büchner, ACS Nano, 2010, 4, 1146–1152 CrossRef CAS.
- H. Liu, D. Takagi, S. Chiashi and Y. Homma, Carbon, 2010, 48, 114–122 CrossRef CAS.
- N. Muradov, Catal. Commun., 2001, 2, 89–94 CrossRef CAS.
- N. Muradov, F. Smith and G. Bokerman, J. Phys. Chem. C, 2009, 113, 9737–9747 CrossRef CAS.
- J. L. Pinilla, I. Suelves, M. J. Lazaro and R. Moliner, J. Power Sources, 2009, 192, 100–106 CrossRef CAS.
- H. Yoshida, S. Takeda, T. Uchiyama, H. Kohno and Y. Homma, Nano Lett., 2008, 8, 2082–2086 CrossRef CAS.
- Y. Yao, C. Feng, J. Zhang and Z. Liu, Nano Lett., 2009, 9, 1673–1677 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
