Core–shell nanostructures for ultrasensitive detection of α-thrombin
Xia
Chen
,
Hongli
Liu
,
Xiaodong
Zhou
* and
Jiming
Hu
*
Key Laboratory of Analytical Chemistry for Biology and Medicine (Ministry of Education), College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072, China. E-mail: jmhu@whu.edu.cn; zhouxd@whu.edu.cn; Fax: +86-27-68752136; Tel: +86-27-68752439-8701
First published on 29th September 2010
Abstract
We have synthesized a stable, sensitive and specific surface-enhanced Raman tag, and demonstrated its application in human α-thrombin detection. The tag consists of aptamer-modified core–shell nanoparticles with hydrophobic Au@Ag as core and silica as shell encapsulating Raman active molecules. By taking advantage of the Raman signal enhancement effect by metallic nanostructures, high stability and robustness of glass-coated core–shell nanostructures and the recognition capabilities of aptamers, we designed a sandwich detection for protein identification with high selectivity and sensitivity. In this way, we realized the ultrasensitive detection of α-thrombin. GDNs (glass-coated, dye-tagged nanoparticles), which were conjugated with oligonucleotides or antibodies, were extremely soluble in water, and had mechanical and chemical stability, easily controllable-size distribution and friendly biocompatibility. Specifically, the glass coating renders the particles amenable to use in many solvents without altering the Raman spectral response and makes agglomeration a nonfactor. All these merits open the door of the real applications in diagnostics or medical investigations in complex biofluids, such as human plasma and serum. Using the aptamer-modified GDNs as Raman tags, we successfully performed the detection of α-thrombin in human plasma. Furthermore, the overall method have been proved effective and selective, and may be implemented for multiplex target analysis simultaneously.
Introduction
Detection of disease-related proteins has important applications in biomedical diagnosis. The lack of amplification methods for protein detection, compared to the polymerase chain reaction techniques for oligonucleotide targets,1 underscores the importance of developing new signal amplification strategies, which incorporate sensitive spectroscopic techniques and novel recognition systems. α-Thrombin, for example, a specific serine protease involved in the coagulation cascade,2 is crucial in physiological and pathological coagulation, and regulates many processes in inflammation and tissue repair at the vessel wall. So, establishing a high sensitive and selective method to detect α-thrombin is very important.Aptamers are single-stranded oligonucleotides selected from a huge combinatorial library by a process known as “systematic evolution of ligands by exponential enrichment” (SELEX). Numerous high-affinity and highly specific aptamers have been selected against a wide variety of target molecules including small inorganic or organic substances, peptides, proteins and viruses or cells.1,3,4 Thanks to their unique characteristics and chemical structure, aptamers offer themselves as ideal candidates for use in biosensors, including optical,5–10 electrochemical,11–14 and mass-sensitive aptasensors.15,16 Among these approaches, surface-enhanced Raman scattering (SERS) has several advantages that distinguish itself from others, such as enormous structure information contents about analyte, the narrow peak width avoiding overlapping in complex systems, without photobleaching and self-quenching, and excitable at any wavelength. However, until now, few researches have been done using SERS to develop aptasensors.17–22,24 Dong's group and C. Bazan's group20,24 had prepared nanoparticle-based Raman tags by the direct attachment of both Raman reporter and biomolecule to the metallic nanoparticles. Mulvaney's group22 and Chau's group23 prepared dye-embedded core–shell nanoparticle-based Raman tags. These tags, however, were either unstable in complex matrices or short of specific recognition capabilities which retarded the applications in the complex biofluilds. In this paper, we took α-thrombin as the sample protein, and developed a sensor system with high sensitivity, selectivity, and simplicity. Our method avoided the competition between the Raman dyes and the biomolecule, thus greatly extended the scope of the Raman active dyes. Our synthesized TBA-GDNs (α-thrombin binding aptamer modified GDNs) show the following benefits: (1) unique spectrum of each Raman reporter, so the background signal from the biological system can be avoided, supplying the possibility of usage as spectroscopic tags for multiplex analysis; (2) avoiding the problems of surface adsorption, substrate variations, and poor data reproducibility; (3) long-term stability in contrast with the naked metallic nanoparticles; (4) high sensitivity and selectivity; and (5) well-studied surface and amenable to a variety of functionalizations. We reported here a novel and simple method based on sol–gel technique to synthesize surface-enhanced Raman scattering tags.25,26 Based on the specific interaction between α-thrombin and α-thrombin binding aptamer (TBA), a great increase of probe intensity was observed as the concentration of α-thrombin went up. Control experiments were conducted by replacing α-thrombin with IgG, BSA or no protein, very weak or even no Raman signals were observed, indicating few binding of TBA-GDNs to the other proteins, and few non-specific adsorption of TBA-GDNs on the Au layer. It revealed the selectivity and specificity of the recognition system.
The goal of this work was to design an aptamer-based assay based on SERS detection for α-thrombin analysis in complex matrices, such as human plasma and serum. The results clearly proved our aptasensor's capability to recognize the target analyte in a protein-rich plasma. This provided a new and appealing tool for the important medical applications involved in the complex matrix. In general, the analysis of plasma proteins by aptamer-based sensing, as here reported for α-thrombin, could be further extended to different targets by changing the aptamers. Through this novel design, an SERS assay recognizing α-thrombin with high affinity, sensitivity, and specificity was developed, opening the possibility of an application to diagnosis or medical investigations.
Results and discussion
Herein, we have developed a sensitive, selective, and stable Raman tag, which takes advantage of strongly increased Raman signals generated by field enhancement and the stability, water-solubility of crosslinked shells under physiological conditions (e.g. high salt concentration). The main advantages of this assay lie in the following facts: narrow bands, robustness toward humidity, high sensitivity sufficient for trace level detection and absence of photobleaching over fluorescence-based counterparts; the reaction signal obtained from the labels themselves, avoiding the background noise originating from the biological system; extraordinary stability and easy functionalization of the silica shell. We have also observed that organic dyes with an isothiocyanate group or multiple sulfur atoms21,27,28 which can form strong chemical bond with Au@Ag nanoparticles are compatible with the silica encapsulation process and are an excellent group of Raman reporters due to their rich vibrational spectra and the possibility of combined surface enhancement. In this analytical technique, the concentration of analyte is proportional to the amount of Raman tags.Design, preparation, and characterization of GDNs
Fig. 1 shows the size and morphology of Raman tags. GDNs preparation involves first the synthesis of Au@Ag nanoparticles. Au colloidal particles have been applied extensively in gene analysis and antibody or antigen detection based on the advantages of easy preparation, high homogeneity, and biocompatibility. However, Au surface shows a lower enhancement in the visible light region in comparison with that of Ag. Therefore, we obtained core–shell particles by depositing Ag on preformed Au particles to achieve a higher signal than that from bare Au nanoparticles.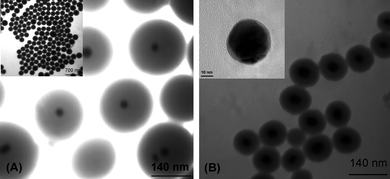 | ||
| Fig. 1 Transmission electron micrographs of nanoparticles: GDNs-based Raman tags. The diameter of Au@Ag core and the thickness of silica shell are (A) 30 ± 4, 80 ± 10 nm (B) 40 ± 5, 35 ± 5 nm, respectively. Inset of Fig. 1B is high resolution TEM image of Au@AgNPs (33 ± 3, 3 ± 2 nm). The latter one is taken as probe. | ||
Sol–gel process is used based on the ammonia-catalyzed hydrolysis of tetraethyl orthosilicate (TEOS), followed by nucleation and condensation onto the surface of Au@Ag nanoparticles. The thickness of amorphous silica shells can be well-controlled by regulating experimental conditions such as the concentration of precursor.
Fig. 2A shows the UV–vis spectra of the original Au@Ag nanoparticles and GDNs in aqueous solution. The 520 nm plasmon peak of Au@Ag nanoparticles shifts to 534 nm while the 402 nm plasmon peak disappears after glass coating. This phenomenon may be due to the slightly higher refractive index of silica relative to water, thus, the plasmon peak red shifts when the surfaces of Au@Ag nanoparticles are coated with silica shells.
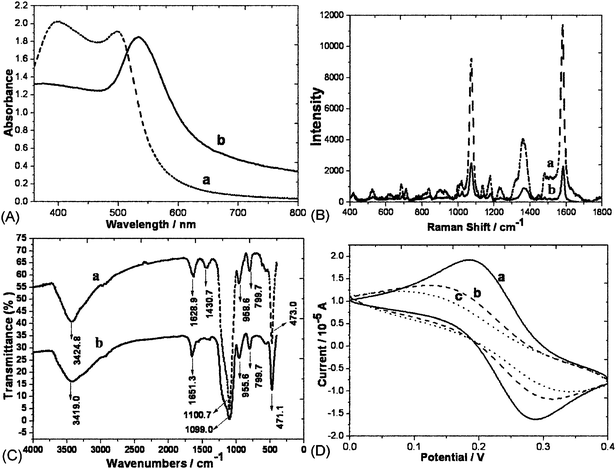 | ||
| Fig. 2 (A) UV-visible absorption spectra of Au@Ag core–shell nanoparticles before (a) and after (b) glass-coated. (B) SERS spectra of MBA on the Au@Ag surfaces before (a) and after (b) glass-coated. (C) The IR spectroscopy of GDNs before (a) and after (b) modified with aptamers. (D) Electrochemical cyclic voltammetry of (a) bare Au; (b) TBA modified Au; (c) 0.34 μM α-thrombin modified Au. | ||
The probe preparation involves firstly the covalent linking of Au@Ag nanoparticles with organic dyes. It is well-known that SERS is sensitive to the distance between Raman-active substrates and reporter molecules, only the first layer of molecules directly adsorbed on the substrate makes the main contribution. After screening several candidates, MBA is chosen as the choice for this work due to its commercial availability and spectral simplicity. The amount of MBA, however, must be controlled precisely to be low enough to prevent the aggregation of Au@Ag colloids and high enough to produce an intense SERS signal. The Raman spectrum of MBA clearly displays several strong peaks at 1081 and 1591 cm−1 which are assigned to the ν12 and ν8a aromatic ring vibrations, and other less intense modes, including δ (CH) (1130 − 1180 cm−1) and ν (COO-) (1380 − 1400 cm−1). As shown in Fig. 2B, there are no changes in all peak-locations after glass coating. In the following discussion, the strongest Raman band at 1591 cm−1 is chosen as a characteristic SERS peak for the analysis.
Modification of the nanoparticles
The biochemical surface modification of nanoparticles, which adds the desired functions to nanoparticles, is very important to the biochemical assay. Herein, the Au@Ag@SiO2 nanoparticle-based Raman tags are labeled with capture oligonucleotides. APTMS was introduced for the formation of the amino-modified GDNs, which were carboxylated by succinic anhydride treatment. Carboxyl-modified nanoparticles were dispersed in MES buffer containing EDC, NHS and amino-labeled oligonucleotides. We chose MES solution as buffer because it would not interfere or compete with the coupling reaction and the nanoparticles could disperse in it very well. Thereafter, the TBA-GDNs formed immediately after the reaction between amines on the oligonucleotides and the active ester intermediates which was first formed by the reaction between carboxyl groups and EDC. The coupling efficiency increased in the presence of NHS while nonspecific reaction decreased if glycine existed. Fig. 2C shows the IR spectroscopy of GDNs. The fact that the peaks 1628.9 cm−1 and 1430.7 cm−1 (δ-OH) disappeared while 1651.3 cm−1 (ν-CONH–) appeared could be the excellent proof of surface carboxylation. The resulting TBA-GDNs were dispersed in an 0.6 M NaCl PBS buffer containing 0.05% Triton X-100 and kept at 4 °C until use.Fabrication of sensing interface with a sandwich system (TBA/α-thrombin/TBA-GDNs)
Scheme 1 illustrates the overall strategy of employing SERS tags in a sandwich system. A sensing surface is constructed by attaching protein-specific aptamers onto a gold layer deposited over a silicon wafer, then further surface modification by 2-mercaptoethanol is required to minimize nonspecific adsorption. Thereafter, α-thrombin and TBA-GDNs are added and washed consecutively as described above. The SERS signature of the linker holding the GDNs together indicates the presence of the target protein. As shown in Fig. 3A, sensitive detection of α-thrombin is achieved by monitoring the strong SERS peaks of MBA, which greatly increases as the concentration of α-thrombin goes up. Our design reveals a highly sensitive method with a detection limit of α-thrombin as low as 10−13 mol L−1, which is comparable to or even better than fluorescence.29,30 | ||
| Scheme 1 Schematic illustration of the fabrication process for α-thrombin recognition. | ||
 | ||
| Fig. 3 (A) Average SERS spectra (6 × 6 points in an area of 36 μm × 36 μm) collected on samples treated with different concentrations of α-thrombin (a, 10−9 M; b, 10−10 M; c, 10−11 M; d, 10−12 M; e, 10−13 M; f, 10−14 M). The spectra have been arbitrarily shifted for better comparison. (B) SERS spectra obtained from the samples treated with (a) α-thrombin 10−9 M; (b) BSA 10−6 M; (c) IgG 10−6 M; (d) without capture aptamer TBA; (e) absence of protein. | ||
We have carried out control experiments to test the selectivity of this strategy. The aptamers on the SERS tags bind to the protein via a second recognition site, that two recognition events increase the selectivity of the method. Fig. 3B shows the results that strong SERS signals are observed only when using the target oligonucleotides while no obvious Raman signals are shown in the control experiments due to the failure linkage between Raman tags and α-thrombin. Selectivity is further confirmed by replacing the proteins with BSA and IgG, a high signal is obtained only when the specific protein exists, whereas the signal is much smaller or even negligible in the absence of the required aptamers or the blank solution. All the results demonstrate the high specificity of the assay. Thus, the designed recognition programme, in combination with TBA-GDNs, works well as a basis for protein detection.
 | ||
Fig. 4 Average SERS spectra collected on samples treated with diluted plasma after pretreatment (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). 100). | ||
Measurement of α-thrombin in complex biological matrices
In clinical tests, routine analysis of α-thrombin in plasma typically involves the enzyme-linked immunosorbent assay (ELISA), which is based on the sandwich principle and appropriate antibodies. Standardized procedures are expensive, and not all the proteins have a corresponding antibody/antigen, which thus highlights the need for a low cost and targeted detection methodology. Advances in DNA engineering and nanotechnology offer new possibilities for the rapid screening of α-thrombin. Herein, we combine the high sensitivity of a newly developed SERS nanoprobe with the high specificity of aptamer to achieve high sensitivity detection of α-thrombin, which is one order better than the clinical test (1.2 ng ml−1).31As demonstrated above, the presence of other proteins have no effect on the Raman response of the assay. The next series of experiments focus on evaluating the ability of our assay to detect α-thrombin in complex biological matrices such as plasma. Plasma is tested after pretreatment, consisting of fibrinogen quantitative precipitation which is necessary to avoid clotting. After detecting three plasma samples, we conclude that the concentration of α-thrombin transformed from prothrombin is around the 100 nM range, which is in good agreement with the literature.32 As can be seen in Fig. 4.
Conclusions
We have shown the design, synthesis, characterization, and function of SERS probe. GDNs-based Raman tags exhibited strong SERS intensity. The silica shells around the Au@Ag cores allowed Raman tags to exhibit extraordinary stability and easy functionalization which made the basis for the analysis of real samples. The overall method described in this work has been proved effective and selective, and this general procedure could be applied to other analytes in complex matrices. We also realized simple synthesis of the probe with various unique fingerprint reporters that could be combined with different aptamer/protein recognition pairs. Thus the extension to detection systems capable of multiplexing is therefore straightforward to conceptualize.Methods
Reagents
Human α-thrombin, silver nitrate, N-(3-dimethylaminopropy)-N-ethylcarbodiimidehydrochloride (EDC), succinic anhydride, 2-(N-morpholino)ethanesulfonic acid (MES), N-hydroxysuccinmide (NHS), and glycine were provided by Sigma. Tetraethoxysilane (TEOS), 3-aminopropyltrimethoxysilane (APTMS) were obtained from Wuhan silicon Corporation (China) and used as received. The oligonucleotides with the following sequences: 5′-NH2-(CH2)6GGTTGGTGTGGTTGG-3′, 5′-GGTTGGTGTGGTTGGCTATGG-SH-3′ were obtained from Sangon Biotechnology Inc. (Shanghai, China). Plasma samples were provided by volunteers in our group. HAuCl4·3H2O, trisodium citrate, 4-mercaptobenzoic acid (MBA) and other chemicals were all of analytical grade and used without any further purification unless otherwise specified. Ultrapure water with an electrical resistance larger than 18.2 MΩ was used throughout. The buffer for the experiments was 15 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM MgCl2. All the oligonucleotide solutions and the buffers in the experiments were prepared using sterile water.Instrumentations
SERS spectra were obtained using a confocal microprobe Raman spectroscopy (Jobin Yvon Horiba, France) with a 632.8 nm helium-neon laser. The objective used was an Olympus 50× long working distance lens with an air-cooled charge-coupled device detector. The slit width of the pinhole was set as 200 μm. The collection time of each SERS spectrum was 5 s over a spectral range from 400 to 1800 cm−1. TEM measurements were performed on a JEOL JEM-100CXII microscope. Infrared spectrum was achieved on a NICOLET 5700 FT-IR spectrometer. Electrochemical experiments were carried out in 5 mM Fe(CN)63− probe solution on a CHI 660A electrochemical workstation (CHI Instrument, Chenhua Co., Shanghai, China) with a conventional three-electrode test cell. Ag/AgCl (saturated KCl) electrode was used as the reference electrode, Au disk electrodes (d = 2 mm, Chenhua Co., Shanghai, China) served as the working electrodes, and Pt coil as the counter electrode.Preparation of Au@Ag@SiO2 nanoparticle-based SERS tags
Au@Ag nanoparticles were synthesized as was previously described.33 In brief, 49.5 mL of 0.01% (w/w) HAuCl4 was reduced by 0.5 mL of 1% sodium citrate solution at 100 °C under vigorous magnetic stirring for 15 min. Then, 4 mg AgNO3 was added to the boiling seed solution and, subsequently, 0.8 mL sodium citrate solution was added dropwise. The solution was left boiling for 1 h resulting in an orange-colored Au@Ag core–shell product. 2 mL Au@Ag colloids was added to 10 mL 2-propanol. Under continuous stirring, 20 μL of 10−3 M dye, 0.25 mL ammonia solution (30wt %) and various amounts of TEOS (Wuhan silicon Corporation) were consecutively added to the reaction mixture. The reaction was allowed to proceed for 1 h at room temperature. Due to the presence of negative charges on the surfaces of silica shells, the product could be well-dispersed in water without adding any surfactant. The core–shell particles were separated by centrifuging and dispersed into deionized water.The preparation of the Au@Ag@SiO2 Raman tags functionalized with oligonucleotides included the following processes. The Au@Ag@SiO2 Raman tags were first modified with amino groups by injecting 20 μL APTMS into the solution of 1 mL of Raman tags dispersed in 7 ml ethanol drop by drop at room temperature for 0.5 h under the conditions of magnetic stirring. Then, the particles were washed using ethanol and water successively by centrifugation and ultrasonication. The amino-modified particles were treated with 5 ml of 10% succinic anhydride in DMF solution and stirred for 3 h at room temperature. After centrifugation and washing with water, the resulting particles were dispersed in MES (pH 4.6) followed by the addition of the mixed solution containing 80 μL of EDC (0.1 M), 40 μL of NHS (0.05 M) and 200 μL of amine-modified oligonucleotides (6.6 μM) drop by drop for 4 h under magnetic stirring. After centrifugation, the particles were dispersed in an appropriate amount of glycine (20 mM) for 1 h. The resulting TBA-GDNs were dispersed in 1.8 mL of NaCl solution (0.6 M, 0.05% Triton X-100, PBS buffer) and stored at 4 °C until use. The final concentration of the nanoparticles is about 10−9 M.
SERS detection of α-thrombin
The Au substrate was cleaned with piranha solution for 10 min (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, concentrated H2SO4/30% H2O2), and rinsed with deionized water, sonicated in ethanol for 10 min. Subsequently, the thiolated TBA (6.6 μM, 15 mM Tris-HCl, pH 7.4) were deposited on the Au substrates and were allowed to sit overnight. The Au substrates were then immersed in a solution of 2-mercaptoethanol (Sigma, 1 mM) for one hour then thoroughly rinsed with water and dried under a gentle air stream. Various concentrations of α-thrombin were pipetted on each of the Au substrates that needed to be treated for another hour. The Au substrates were then washed with binding buffer and gently dried with air. The assembly processes were characterized by electrochemical measurements. Scheme 1 illustrates the overall strategy of using TBA-GDNs in a heterogeneous protein assay. To complete the sandwich system, TBA-GDNs were deposited on the Au substrates and washed in one hour.
1, concentrated H2SO4/30% H2O2), and rinsed with deionized water, sonicated in ethanol for 10 min. Subsequently, the thiolated TBA (6.6 μM, 15 mM Tris-HCl, pH 7.4) were deposited on the Au substrates and were allowed to sit overnight. The Au substrates were then immersed in a solution of 2-mercaptoethanol (Sigma, 1 mM) for one hour then thoroughly rinsed with water and dried under a gentle air stream. Various concentrations of α-thrombin were pipetted on each of the Au substrates that needed to be treated for another hour. The Au substrates were then washed with binding buffer and gently dried with air. The assembly processes were characterized by electrochemical measurements. Scheme 1 illustrates the overall strategy of using TBA-GDNs in a heterogeneous protein assay. To complete the sandwich system, TBA-GDNs were deposited on the Au substrates and washed in one hour.
Regeneration of Au substrates
The well-organized monolayers of thio-aptamers on Au substrates could be removed efficiently by NaBH4 solution (0.5 M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O/EtOH) developed by our group,34 which was simple and mild. Au substrates could be regenerated to fabricate new monolayers via this way without further treatments.
1 H2O/EtOH) developed by our group,34 which was simple and mild. Au substrates could be regenerated to fabricate new monolayers via this way without further treatments.
Pretreatment of plasma samples
To avoid sample clotting, fibrinogen was first precipitated from plasma samples. 1.25 mL of 2 M ammonium sulfate and 1 mL of 0.1 M sodium chloride were mixed with 0.25 mL of plasma, 3–4 min later, the mixture was centrifuged and the supernatant was kept back. The protein amount was evaluated by spectrophotometric measurements at 280 nm and a loss of protein content (40%) was detected after precipitation. α-Thrombin in plasma is present in the form of its precursor, prothrombin, which converted to α-thrombin via proteolytic processing. To generate the analyte, 0.03 M CaCl2 was added to the plasma (pH 7.1, absence of fibrinogen). The generation of α-thrombin was monitored via our assays.Acknowledgements
We gratefully acknowledge the financial support from National Natural Science Foundation of China (No. 20805034, 30772058, 20927003, 90913013, 20705025) and the National Key Laboratory of Chemo/Biosensing and Chemometrics of Hunan University. We also acknowledge Renjun Pei from Columbia University and Liang Feng for providing language help.References
- J. W. Liu, Z. H. Cao and Y. Lu, Chem. Rev., 2009, 109, 1948 CrossRef CAS
.
- J. Hu, P. C. Zheng, J. H. Jiang, G. L. Shen, R. Q. Yu and G. K. Liu, Anal. Chem., 2009, 81, 87 CrossRef CAS
.
- M. Famulok, J. S. Hartig and G. Mayer, Chem. Rev., 2007, 107, 3715 CrossRef CAS
.
- S. E. Osborne and A. D. Ellington, Chem. Rev., 1997, 97, 349 CrossRef CAS
.
- B. L. Li, H. Wei and S. J. Dong, Chem. Commun., 2007, 73 RSC
.
- J. Liu and Y. Lu, Nat. Protoc., 2006, 1, 246 Search PubMed
.
- R. Pei, J. Rothman, Y. L. Xie and M. N. Stojanovic, Nucleic Acids Res., 2009, 37, e59 CrossRef
.
- M. N. Stojanovic and D. W. Landry, J. Am. Chem. Soc., 2002, 124, 9678 CrossRef CAS
.
- M. N. Stojanovic, P. de Prada and D. W. Landry, J. Am. Chem. Soc., 2001, 123, 4928 CrossRef CAS
.
- H. Wei, B. L. Li, J. Li, E. K. Wang and S. J. Dong, Chem. Commun., 2007, 3735 RSC
.
- B. R. Baker, R. Y. Lai, M. S. Wood, E. H. Doctor, A. J. Heeger and K. W. Plaxco, J. Am. Chem. Soc., 2006, 128, 3138 CrossRef CAS
.
- R. Polsky, R. Gill, L. Kaganovsky and I. Willner, Anal. Chem., 2006, 78, 2268 CrossRef CAS
.
- Y. Xiao, B. D. Piorek, K. W. Plaxco and A. J. Heeger, J. Am. Chem. Soc., 2005, 127, 17990 CrossRef CAS
.
- A. A. L. Yi Xiao, Alan J. Heeger and Kevin W. Plaxco, Angew. Chem., Int. Ed., 2005, 44, 5456 CrossRef CAS
.
- A. Bini, M. Minunni, S. Tombelli, S. Centi and M. Mascini, Anal. Chem., 2007, 79, 3016 CrossRef CAS
.
- M. Liss, B. Petersen, H. Wolf and E. Prohaska, Anal. Chem., 2002, 74, 4488 CrossRef CAS
.
- J. W. Chen, J. H. Jiang, X. Gao, G. K. Liu, G. L. Shen and R. Q. Yu, Chem.–Eur. J., 2008, 14, 8374 CrossRef CAS
.
- J. W. Chen, X. P. Liu, K. J. Feng, Y. Liang, J. H. Jiang, G. L. Shen and R. Q. Yu, Biosens. Bioelectron., 2008, 24, 66 CrossRef CAS
.
- H. Cho, B. R. Baker, S. Wachsmann Hogiu, C. V. Pagba, T. A. Laurence, S. M. Lane, L. P. Lee and J. B.-H. Tok, Nano Lett., 2008, 8, 4386 CrossRef CAS
.
- L. Fabris, M. Dante, T. Q. Nguyen, J. B. H. Tok and G. C. Bazan, Adv. Funct. Mater., 2008, 18, 2518 CrossRef CAS
.
- Y. Liang, J. L. Gong, Y. Huang, Y. Zheng, J. H. Jiang, G. L. Shen and R. Q. Yu, Talanta, 2007, 72, 443 CrossRef CAS
.
- S. P. Mulvaney, M. D. Musick, C. D. Keating and M. J. Natan, Langmuir, 2003, 19, 4784 CrossRef
.
- P. J. Huang, L. L. Tay, J. Tanha, S. Ryan and L. K. Chau, Chem.–Eur. J., 2009, 15, 9330 CrossRef CAS
.
- Y. Wang, H. Wei, B. Li, W. Ren, S. Guo, S. Dong and E. Wang, Chem. Commun., 2007, 5220 RSC
.
- Y. Lu, Y. D. Yin, Z. Y. Li and Y. A. Xia, Nano Lett., 2002, 2, 785 CrossRef
.
- Y. D. Yin, Y. Lu, Y. G. Sun and Y. N. Xia, Nano Lett., 2002, 2, 427 CrossRef CAS
.
- J. L. Gong, J. H. Jiang, Y. Liang, G. L. Shen and R. Q. Yu, J. Colloid Interface Sci., 2006, 298, 752 CrossRef CAS
.
- J. L. Gong, Y. Liang, Y. Huang, J. W. Chen, J. H. Jiang, G. L. Shen and R. Q. Yu, Biosens. Bioelectron., 2007, 22, 1501 CrossRef CAS
.
- C. C. Huang, Z. H. Cao, H. T. Chang and W. H. Tan, Anal. Chem., 2004, 76, 6973 CrossRef CAS
.
- L. Q. Wang, L. Y. Li, Y. Xu, G. F. Cheng, P. A. He and Y. Z. Fang, Talanta, 2009, 79, 557 CrossRef CAS
.
- J. Bichler, M. Siebeck, R. Maschler, H. Pelzer and H. Fritz, Blood Coagulation Fibrinolysis, 1991, 2, 129 CrossRef CAS
.
- S. Centi, S. Tombelli, M. Minunni and M. Mascini, Anal. Chem., 2007, 79, 1466 CrossRef CAS
.
- W. Xie, L. Su, P. Donfack, A. G. Shen, X. D. Zhou, M. Sackmann, A. Materny and J. M. Hu, Chem. Commun., 2009, 5263 RSC
.
- M. Yuan, S. Zhan, X. Zhou, Y. Liu, L. Feng, Y. Lin, Z. Zhang and J. Hu, Langmuir, 2008, 24, 8707 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2010 |
