Exponential growth of functional poly(glutamic acid)dendrimers with variable stereochemistry†
Sebastian
Hartwig
,
Mary M.
Nguyen
and
Stefan
Hecht
*
Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, 12489 Berlin, Germany. E-mail: sh@chemie.hu-berlin.de; Fax: (+49) 30 2093-6940; Tel: (+49) 30 2093-7365
First published on 25th November 2009
Abstract
Polyglutamate dendrimers up to the fourth generation have been prepared via an accelerated iterative divergent/convergent binomial synthesis, which allows incorporation of either all-(L) or (D-alt-L) stereochemistry in the peptide backbone and enables versatile postfunctionalization of the dendritic core and periphery.
Dendrimers are discrete macromolecules with a well defined and perfectly branched structure, presenting attractive functional scaffolds for biomedical and pharmaceutical applications.1–3 In view of their high potential biocompatibility and biodegradability, particularly peptidedendrimers composed of branched AB2-type‡amino acids, i.e.lysine4 on the one hand or glutamic5–7 and aspartic acid8 on the other hand, are particularly interesting targets. Two of the main advantages of dendrimers are the possibility of precise attachment of many functional groups in close proximity (multivalency) and their adjustable size, both of which can be tuned by generation number. In the case of higher generation dendrimers these features enable not only the attachment of a large number of peripheral functionalities interacting with the environment but also other beneficial effects, such as enhanced circulation times in vivo.9 However, the eminent bottleneck in the use of dendrimers for any type of application requiring significant amounts of material is their demanding synthesis, limiting their size and availability.§ Nowadays, dendrimers are prepared via either divergent10 or convergent11 syntheses, including double-stage convergent,12 accelerated,13 or exponential growth14 approaches. Here, we report on the unprecedented accelerated synthesis of polyglutamatedendrimers up to the fourth generation via an iterative divergent/convergent exponential growth approach.14,15 Our synthesis provides exquisite control of backbone stereochemistry,16i.e. either all-(L) or (D-alt-L), to tune structure and biodegradability,17 and allows for regioselective introduction of functional groups.
The incorporation of an alternating (D,L)-stereochemistry into a macromolecule can be conveniently accomplished by using a dimeric building block as repeat unit. To achieve rapid dendrimer growth, the exponential synthesis strategy is very attractive.14 As the dipeptide is extended at the side chain functionalities and not along the backbone, our synthesis requires orthogonal temporary protecting groups at the N-terminus and the carboxylate side chains while maintaining a permanent protecting group at the C-terminus. For this purpose, we chose the acid labile Boc-group as the N-terminal protecting group, while the carboxylic acid side chains were protected with benzyl esters, readily cleavable by hydrogenolysis. The C-terminus was masked as methyl ester instead, allowing for cleavage via saponification at a later stage of the synthesis.
The dendrimer synthesis is based on the G1 dipeptide building blocks 1a and 1b, readily prepared from commercially available Boc-L-Glu(Z) via a 3-step route involving esterification of the C-terminus, Boc-cleavage, and subsequent coupling with either Boc-L-Glu(Z) or Boc-D-Glu(Z).† This synthesis route provided access to both monomers in almost quantitative yields and in excellent purity, which were then subjected to our exponential growth procedure to give both series of the corresponding G2 and G4 dendrons4a/b and 7a/b (Scheme 1).
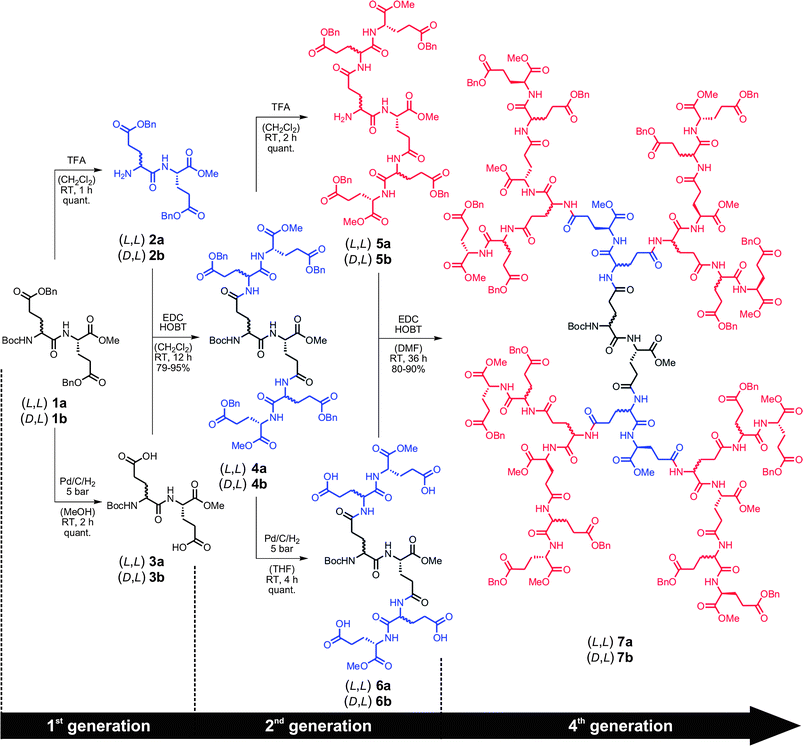 | ||
| Scheme 1 Synthesis of the (L,L)- and (D,L)-G4 dendrons7a and 7b starting from the (L,L)- and (D,L)-G1 dipeptide monomers 1a and 1b, respectively, via an accelerated exponential growth approach. | ||
The dendron growth sequence starts with removal of the orthogonal Boc and Zprotecting groups to yield the N- and Cγ-deprotected dipeptides2a/b and 3a/b, respectively. All deprotection reactions proceeded smoothly and gave quantitative yields of the respective activated fragments, which were used without further purification. The subsequent coupling afforded both G2 dendrons4a and 4b in high yields. Purification of 4a and 4b was readily achieved by column chromatography and repetitive precipitation in diethyl ether. Again, deprotection of the N-terminal Boc and Cγ-Z groups gave the activated dendrons5a/b and 6a/b, respectively, in quantitative yields. Without further purification, the orthogonal fragments were coupled to yield the desired G4 dendrons7a and 7b in excellent yields. Please note that purification of the G4 dendrons could easily be achieved by precipitation in diethyl ether and extensive washing of the solid residue with diethyl ether and MeOH, yielding the desired products as white powders of high purity.
The GPC-traces of the peptidedendrons (Fig. 1, bottom) nicely illustrate the rapid growth of the hydrodynamic volume as a function of generation. Interestingly, the corresponding molecular masses determined by GPC, calibrated with polystyrene standards, correlate reasonably well with the real molecular masses of the molecules. As expected for discrete dendritic macromolecules, polydispersities determined by GPC were close to unity (PDI ≤ 1.04). Assuming well solvated peptide repeat units in DMF, the increase in hydrodynamic volume can qualitatively be visualized by molecular models of each generation (Fig. 1, top). The absence of structural defects, potentially arising from incomplete deprotection or coupling steps, was verified by both mass spectrometry and 1H NMR spectroscopy.†
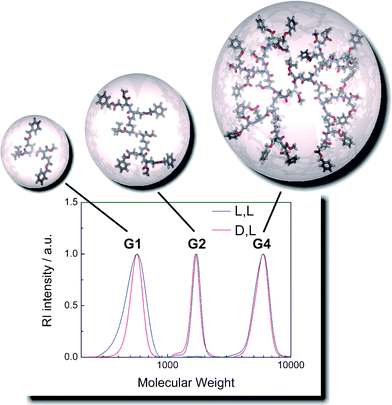 | ||
| Fig. 1 Overlay of the GPC-traces of the (D)-alt-(L) G1, G2, and G4 dendrons1b, 4b, and 7b (red traces) including schematic representations of the three-dimensional shape of each generation. For comparison, the all-(L) series (1a, 4a, and 7a) is shown in blue (GPC in DMF at 70 °C, calibrated with narrow polystyrene standards). | ||
Unfortunately, further growth of the G8 dendrons by another divergent/convergent cycle failed. Initial Boc-deprotection proceeded smoothly yielding the G4 dendron with a single free amine functionality at the focal point and also Z-deprotection gave the G4 dendron with 16 terminal free carboxylic acid functionalities. However, subsequent coupling of both fragments did not lead to formation of the desired G8 dendron and only starting material was isolated. Most likely, the focal amine functionality of the G4 dendron was sterically too shielded and hence not accessible for the activated terminal acid groups. Such inhibition of further dendritic growth in the iterative divergent/convergent binomial synthesis has been observed by Moore and coworkers in the case of meta-phenylene ethynylene dendrons.14
The peptidedendrons serve as versatile scaffolds, allowing for solubilization of various functional moieties in water. These functional entities could be attached either covalently to the focal amine group (or non-covalently in the dendritic interior) while terminal deprotection of the dendrons would furnish dendritic polyelectrolytes with many solubilizing carboxylate groups. Therefore, for example water-insoluble dyes could be encapsulated within a given dendritic shell18 and thus be transferred into a physiological environment for various biomedical and biosensing purposes. In this context, (metallo)porphyrins constitute a very attractive class of dyes with unique photophysical and photochemical features, in particular with regard to their singlet oxygen generation ability6 as an important prerequisite for photodynamic therapy.19 In order to covalently attach a tetraphenylporphyrin (TPP) core to the dendritic interior, i.e. the focal amine functionality, 5-(4′-carboxyphenyl)-10,15,20-triphenylporphyrin20 was prepared and successfully coupled to the D,L-G4 dendrimer7b. The fully protected G4 dendron carrying a porphyrin focal point 8 is insoluble in water and remains in the organic layer, whereas after saponification 9 is readily water-soluble (Fig. 2).
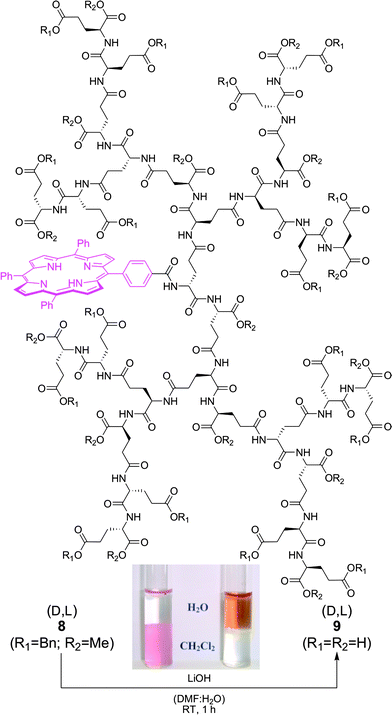 | ||
| Fig. 2 The G4 dendron, carrying a porphyrin focal point, is insoluble in water in its completely protected form 8 (left) while saponification provides the fully deprotected derivative 9 with good water solubility (right). | ||
In summary, polyglutamatedendrons up to the fourth generation have been synthesized using an exponential growth approach and were readily functionalized both at their focal point as well as periphery. Due to the precise control over dimension, core and exterior functionality21 as well as backbone stereochemistry, these dendrons and derived dendrimers constitute a versatile platform for the design of functional macromolecules. Ongoing work in our laboratories is concerned with exploiting this dendrimer family as structure-directing building blocks in polyelectrolyte complexes and as scaffold in bio-applications.
Generous support by the German Research Foundation (DFG via SFB 765) and the Fonds der Chemischen Industrie for providing a doctoral Kekulé-Fellowship to Sebastian Hartwig are gratefully acknowledged. Mary Nguyen was supported by the DAAD (RISE internship).
Notes and references
- R. Duncan and L. Izzo, Adv. Drug Delivery Rev., 2005, 57, 2215 CrossRef CAS.
- R. Haag and F. Kratz, Angew. Chem., Int. Ed., 2006, 45, 1198 CrossRef CAS.
- M. W. Grinstaff, Chem.–Eur. J., 2002, 8, 2838 CrossRef CAS.
-
R. G. Denkewalter, J. F. Kolc and W. J. Lukasavage, U.S. Patent, 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410
410![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688, 1983, p. 9.
688, 1983, p. 9. - F. E. Appoh, D. S. Thomas and H. B. Kraatz, Macromolecules, 2005, 38, 7562 CrossRef CAS.
- S. A. Vinogradov, L. W. Lo and D. F. Wilson, Chem.–Eur. J., 1999, 5, 1338 CrossRef CAS.
- L. J. Twyman, A. E. Beezer, R. Esfand, B. T. Mathews and J. C. Mitchell, J. Chem. Res. (S), 1998, 758 RSC.
- G. Palui, F.-X. Simon, M. Schmutz, P. J. Mesini and A. Banerjee, Tetrahedron, 2008, 64, 175 CrossRef CAS.
- H. Maeda, Adv. Drug Delivery Rev., 1991, 6, 181 CrossRef CAS.
- D. A. Tomalia, H. Baker, J. Dewald, M. Hall, C. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Polym. J. (Tokyo), 1985, 17, 117 Search PubMed.
- S. M. Grayson and J. M. J. Fréchet, Chem. Rev., 2001, 101, 3819 CrossRef CAS.
- K. L. Wooley, C. J. Hawker and J. M. J. Fréchet, J. Am. Chem. Soc., 1991, 113, 4252 CrossRef CAS.
- A. W. Freeman and J. M. J. Fréchet, Org. Lett., 1999, 1, 685 CrossRef CAS.
- T. Kawaguchi, K. L. Walker, C. L. Wilkins and J. S. Moore, J. Am. Chem. Soc., 1995, 117, 2159 CrossRef CAS.
- T. Ozaki and A. Shoji, Makromol. Chem. Rapid Commun., 1982, 3, 157 CrossRef CAS.
- S. E. Gibson and J. T. Rendell, Chem. Commun., 2008, 922 RSC , and references therein.
- M. Werle and A. Bernkop-Schnürch, Amino Acids, 2006, 30, 351 CrossRef CAS.
- S. Hecht and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2001, 40, 74 CrossRef CAS.
- R. Bonnett, Chem. Soc. Rev., 1995, 24, 19–33 RSC.
- S. Matile, N. Berova, K. Nakanishi, J. Fleischhauer and R. W. Woody, J. Am. Chem. Soc., 1996, 118, 5198–5206 CrossRef CAS.
- Please note the potential for interior functionalization by saponification of the interior methyl esters groups. For a review on interior dendrimer functionalization, see: S. Hecht, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 1047 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis details and characterization data. See DOI: 10.1039/b9py00217k |
| ‡ In polymer chemistry, A and B groups are commonly used to depict chemical functionalities of orthogonal, i.e.nucleophilic and electrophilic , reactivity. |
| § Only few dendrimer types, such as PPI or PAMAMdendrimers, are commercially available thus far. |
| This journal is © The Royal Society of Chemistry 2010 |
