Dual nanocomposite multihollow polymer microspheres prepared by suspension polymerization based on a multiple pickering emulsion †
Quanxing
Gao
,
Chaoyang
Wang
*,
Hongxia
Liu
,
Yunhua
Chen
and
Zhen
Tong
*
Research Institute of Materials Science, South China University of Technology, Guangzhou 510640, China. E-mail: zhywang@scut.edu.cn; mcztong@scut.edu.cn; Fax: (+) 86-20-87112886; Tel: (+) 86-20-87112886
First published on 16th December 2009
Abstract
A solid-stabilized multiple w/o/w or o/w/o emulsion was prepared in a two-step process. Various nanocomposite polymer microspheres with multihollow armored closed pores were fabricated easily by suspension polymerization of the multiple Pickering stabilized emulsions .
Self-assembly of solid particles at the liquid–liquid interface to stabilize so-called Pickering emulsions has been well documented and offers a straightforward pathway for the production of organized nanostructures.1,2 Pickering emulsion droplets have been used as templates to prepare highly controlled elastic membranes and other supracolloidal structures.3–7 In addition, some attempts have been made to improve the stability of multiple or double emulsions by introducing solid particles. Both particles and surfactants have been employed usually in combination to form multiple emulsions .1,8
Recently, Pickering emulsion droplets have also been used as versatile polymerization vessels for fabricating hybrid polymer particles and capsules with supracolloidal structures.9–13 The solid particles first self-assemble at the liquid–liquid interface and act as effective stabilizers during the polymerization process without the need for any conventional stabilizers. After the polymerization, the particles armour the surface of the resultant polymer beads. Such solid-stabilized heterogeneous polymerizations are more attractive in preparation of hybrid beads than the conventional emulsion and suspension polymerizations. Polymerizations based on Pickering emulsion include Pickering miniemulsion polymerization, Pickering suspension polymerization, Pickering dispersion polymerization and Pickering emulsion interface-initiated atom transfer radical polymerization (PEII-ATRP).9–13 Porous or multihollow polymer matrices and nanocomopsites are attracting increasing amounts of attention due to their wide applications, which are often prepared by polymerization of the multiple emulsions stabilized by the surfactants.14 In this work, we report our studies on suspension polymerization based on multiple emulsion droplets using different nanoparticles as stabilizers. We use water-in-oil-in-water (w/o/w) and oil-in-water-in-oil (o/w/o) multiple emulsions as templates to facilely fabricate dual nanocomposite multihollow hydrophobic polymer microspheres and hydrophilic polymer microgels, respectively.
Multiple emulsions can form in oil/water mixtures containing two types of particles differing only in their hydrophobicity. Hydrophilic Fe2O3nanoparticles of about 5 nm can stabilize o/w emulsions while hydrophobic fumed silica with primary diameter of 5–30 nm are used to obtain w/o emulsions . Multiple w/o/w Pickering emulsions with styrene as the oil phase are prepared in a similar two-step process to that reported by Binks' group.1 The first step involves the formation of a primary and simple w/o emulsion by homogenizing water (ϕw = 0.2) into a dispersion of hydrophobic silicananoparticles in styrene with 2,2′-azobisisobutyronitrile (AIBN) via ultrasonification. In the second step, the w/o emulsion just made is re-emulsified into an aqueous dispersion of hydrophilic Fe2O3nanoparticles by shaking by hand. The volume fraction of w/o emulsion in the final multiple emulsion is 0.17. Subsequently, the produced w/o/w emulsion is polymerized for 8 h at 60 °C and dual nanocomposite polystyrene (PS) microspheres with aqueous multi-cores or dual nanocomposite multihollow PS microparticles are obtained as shown in Fig. 1.
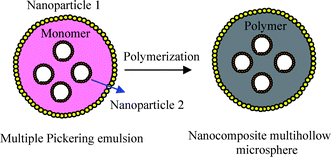 | ||
| Fig. 1 A schematic representation of suspension polymerization based on a multiple Pickering emulsion for preparing nanocomposite multihollow polymer microspheres. | ||
Light microscopy images of the primary water-in-styrene (w/o) emulsion (a) and the relative multiple water-in-styrene-in-water (w/o/w) Pickering emulsion (b) are illustrated in Fig. 2. It is obvious that many water drops exists inside styrene globules in Fig. 2b and the structure of multiple emulsion is formed. The first simple emulsion is not destroyed in the re-emulsification process and keeps its original structure in the second multiple emulsion . The size and size distribution of the simple emulsion and the multiple emulsion are estimated on counting 200 beads with the optical microscope (Carl Zeiss, German). Water droplets in the primary w/o emulsion are approximately 1.5 μm in diameter. Styrene globules in multiple w/o/w emulsion are from several micrometres to tens micrometres with an average diameter of 50 μm. The average size of water droplets inside styrene globules is the same as that in the primary w/o emulsion . Both the simple emulsion and the multiple emulsions are stable to coalescence for more than 6 months in the time range of experimental observation. The major instability in surfactant multiple emulsions results from the diffuse of surfactant through from inner to outer interfaces or vice versa. In Pickering emulsion , the solid particles which irreversibly locate at the liquid–liquid interface act as the mechanical barriers against coalescence, thereby the migration of particles between the interfaces is expected to be minimal after emulsion formation and the long-termed stable emulsion is obtained.1
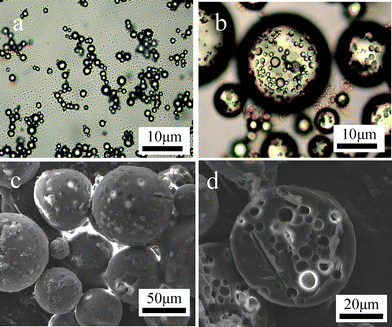 | ||
| Fig. 2 Light microscopy images of (a) primary w/o emulsion stabilized by silicananoparticles and (b) multiple w/o/w emulsion stabilized by Fe2O3nanoparticles from re-emulsification of primary w/o emulsion ; SEM images of (c) nanocomposite multihollow PS microspheres and (d) one of microspheres being cut into halves. | ||
Good stability of the multiple Pickering emulsion is necessary for the subsequent suspension polymerization. Polymerization can be carried easily out with no need for stirring after the robust multiple Pickering emulsion is generated. Silica and Fe2O3nanoparticles act as the effective stabilizer during polymerization and building blocks for creating organic-inorganic hybrid composites after polymerization. The resulting nanocomposite PS microspheres with dual hybrid by silica and Fe2O3 are brown and weakly magnetic due to the outer shell of Fe2O3nanoparticles. The morphology of microspheres is observed using scanning electron microscopy (SEM, Philips XL 30) and SEM photos are shown in Fig. 2c and d. Nanocomposite PS microspheres still keep spherical and the size of PS microspheres with an average diameter of 50 μm is in rough agreement with that of monomer globules before polymerization, which demonstrate good stabilization during polymerization. Fig. 2d is an electron micrograph for a sectioned bead. It can be clearly seen that the inner structure of nanocomposite PS microspheres is porous and each pore is not interconnected. Therefore, pristine nanocomposite PS microspheres should have multi-pores. The independent pores inside the microspheres also suggests that the high stabilization of the multiple emulsion in the polymerization process. The size and amount of pores in microspheres can be tuned by the original structure of the multiple emulsion . Moreover, according to the energy spectrum analysis (EDX) of an intact bead and a sectioned one (see ESI for details† ), it is found that inorganic nanoparticles mainly aggregate at the inner and outer interfaces of multihollow bead.
The molecular weight (Mn) of the PS in the nanocomposite is about 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with a polydispersity of 2.95. The FTIR spectrum of nanocomposite microspheres is recorded on a Bruker Vector33 FTIR spectrometer (see ESI for details† ). The bands at 698 and 755 cm−1 can be attributed to flexural vibrations (δC–H) of the benzene ring and those at 1449, 1491, and 1601 cm−1 can be attributed to benzene ring vibrations (νC–C) of PS. The peak at 540 cm−1 which is the result of the Fe–O vibration indicates the presence of Fe2O3 in the polymer matrix. Asymmetric and symmetric stretching of the Si–O bonds (1154 and 1026 cm−1, respectively), symmetric stretching of the Si–OH (silanols) around 950 cm−1, and the band at 1068 cm−1 corresponds to the vibration of Si–O–Si in network of the silica are also found in the spectrum. From inductively coupled plasma atomic emission spectroscopy (ICP-AES), Si and Fe contents in the beads is 0.23% and 0.48%, respectively.
000 with a polydispersity of 2.95. The FTIR spectrum of nanocomposite microspheres is recorded on a Bruker Vector33 FTIR spectrometer (see ESI for details† ). The bands at 698 and 755 cm−1 can be attributed to flexural vibrations (δC–H) of the benzene ring and those at 1449, 1491, and 1601 cm−1 can be attributed to benzene ring vibrations (νC–C) of PS. The peak at 540 cm−1 which is the result of the Fe–O vibration indicates the presence of Fe2O3 in the polymer matrix. Asymmetric and symmetric stretching of the Si–O bonds (1154 and 1026 cm−1, respectively), symmetric stretching of the Si–OH (silanols) around 950 cm−1, and the band at 1068 cm−1 corresponds to the vibration of Si–O–Si in network of the silica are also found in the spectrum. From inductively coupled plasma atomic emission spectroscopy (ICP-AES), Si and Fe contents in the beads is 0.23% and 0.48%, respectively.
Besides, the stable toluene-in-water-in-toluene (o/w/o) Pickering emulsion was prepared by a similar two-step process. Water globules in an o/w/o multiple emulsion contain acrylamide (Am), N,N′-methylenebisacrylamide (BIS), 2,2-azobis(2-methylpropionamidine)dihydrochloride ( V−50, a photo-initiator) and NaCl. The Laponite RD clay with a diameter of 25–30 nm and thickness of 1 nm is used as the stabilizer for the primary o/w emulsion and hydrophobic silica as the stabilizer for the secondary w/o emulsions . From Fig. 3a, we can clearly distinguish the water globules, each of them containing many internal oil droplets. The dual hybrid multihollow microgels are prepared viasuspension polymerization of Am dissolved in the water globules, which is carried out in an ice-water bath under 30 mW cm−2 UV radiation for 5 min. Hybrid PAm microspheres are labelled with fluorescein isothiocyanate (FITC) for confocal laser scanning microscopy (CLSM) observation. Fluorescence image of microgels redispersed in water is presented in Fig. 3b. The hybrid microgels have a good dispersibility in water. The PAm microgels with multiple compartment structure are clearly observed in the Fig. 3b. Moreover, rhodamine B-stained clay is used to produce multihollow hybrid PAm microgels for CLSM testing (see ESI for details† ). It is surprising that the is clay located on both inner and outer interfaces of the gel beads, not only on the inner interface. The possible reason is the diffuse of clay through from the inner to outer interface due to the existing of NaCl. We also investigate suspension polymerization of other hydrophilic monomer such as N-isopropylacrylamide (NIPAm) based on multiple Pickering emulsion and the multihollow and dual hybrid PNIPAm microgels are obtained.
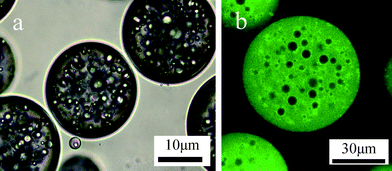 | ||
| Fig. 3 (a) A light microscopy image of multiple o/w/o emulsion stabilized by clay and silicananoparticles and (b) a fluorescence image of nanocomposite multihollow PAm microgels redispersed in water. | ||
In summary, dual nanocomposite multihollow hydrophobic or hydrophilic polymer microspheres could be facilely prepared viasuspension polymerization based on multiple Pickering emulsion . Embedding inorganic nanoparticles will endow polymeric matrices with versatile functionality, such as optical, magnetic and electric properties. Aqueous or oil multi-cores will enable the polymer microspheres to encapsulate an abundance of substances, such as drug, protein, enzyme and other biomolecules. This kind of hybrid multihollow polymer microspheres is expected to have wide potential applications in materials science and biotechnology .
Acknowledgements
This work was supported by the National Natural Science Foundation of China (20874030 and 50973034), the Scientific and Technologic Program of Guangzhou Municipality (2007J1-C0351) and NCET-07-0306.Notes and references
- (a) B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21 CrossRef CAS; (b) R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 100–102, 503 CrossRef CAS.
- (a) B. P. Binks, R. Murakami, S. P. Armes, S. Fujii and A. Schmid, Langmuir, 2007, 23, 8691 CrossRef CAS; (b) B. P. Binks, R. Murakami, S. P. Armes and S. Fujii, Langmuir, 2006, 22, 2050 CrossRef CAS; (c) D. L. Cheung and S. A. F. Bon, Phys. Rev. Lett., 2009, 102, 066103 CrossRef.
- (a) O. D. Velev, K. Furusawa and K. Nagayama, Langmuir, 1996, 12, 2374 CrossRef CAS; (b) H. Y. Koo, S. T. Chang, W. S. Choi, J. H. Park, D. Y. Kim and O. D. Velev, Chem. Mater., 2006, 18, 3308 CrossRef CAS; (c) V. Rastogi, S. Melle, O. G. Calderon, A. A. Garcia, M. Marquez and O. D. Velev, Adv. Mater., 2008, 20, 4263 CrossRef CAS.
- (a) Y. Lin, H. Skaff, A. Boker, A. D. Dinsmore, T. Emrick and T. P. Russell, Science, 2003, 299, 226 CrossRef CAS; (b) E. Glogowski, R. Tangirala, J. B. He, T. P. Russell and T. Emrick, Nano Lett., 2007, 7, 389 CrossRef CAS; (c) J. T. Russell, Y. Lin, A. Boker, L. Su, P. Carl, H. Zettl, J. B. He, K. Sill, R. Tangirala, T. Emrick, K. Littrell, P. Thiyagarajan, D. Cookson, A. Fery, Q. Wang and T. P. Russell, Angew. Chem., Int. Ed., 2005, 44, 2420 CrossRef CAS; (d) H. Skaff, Y. Lin, R. Tangirala, K. Breitenkamp, A. Boker, T. P. Russell and T. Emrick, Adv. Mater., 2005, 17, 2082 CrossRef CAS; (e) Y. Lin, A. Boker, H. Skaff, D. Cookson, A. D. Dinsmore, T. Emrick and T. P. Russell, Langmuir, 2005, 21, 191 CrossRef CAS; (f) Y. Lin, H. Skaff, A. Boker, A. D. Dinsmore, T. Emrick and T. P. Russell, J. Am. Chem. Soc., 2003, 125, 12690 CrossRef CAS; (g) T. Li, Z. W. Niu, T. Emrick, T. R. Russell and Q. Wang, Small, 2008, 4, 1624 CrossRef CAS.
- (a) A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch and D. A. Weitz, Science, 2002, 298, 1006 CrossRef CAS; (b) J. W. Kim, A. Fernandez-Nieves, N. Dan, A. S. Utada, M. Marquez and D. A. Weitz, Nano Lett., 2007, 7, 2876 CrossRef CAS; (c) D. B. Lawrence, T. Cai, Z. Hu, M. Marquez and A. D. Dinsmore, Langmuir, 2007, 23, 395 CrossRef CAS; (d) M. F. Hsu, M. G. Nikolaides, A. D. Dinsmore, A. R. Bausch, V. D. Gordon, X. Chen, J. W. Hutchinson and D. A. Weitz, Langmuir, 2005, 21, 2963 CrossRef CAS.
- (a) T. Ngai, H. Auweter and S. H. Behrens, Macromolecules, 2006, 39, 8171 CrossRef CAS; (b) L. M. Croll, H. D. H. Stover and A. P. Hitchcock, Macromolecules, 2005, 38, 2903 CrossRef CAS; (c) P. F. Noble, O. J. Cayre, R. G. Alargova, O. D. Velev and V. N. Paunov, J. Am. Chem. Soc., 2004, 126, 8092 CrossRef CAS; (d) O. J. Cayre, P. F. Noble and V. N. Paunov, J. Mater. Chem., 2004, 14, 3351 RSC; (e) H. W. Duan, D. Y. Wang, N. S. Sobal, M. Giersig, D. G. Kurth and H. Möhwald, Nano Lett., 2005, 5, 949 CrossRef CAS.
- (a) C. Y. Wang, H. X. Liu, Q. X. Gao, X. X. Liu and Z. Tong, ChemPhysChem, 2007, 8, 1157 CrossRef CAS; (b) H. X. Liu, C. Y. Wang, Q. X. Gao, X. X. Liu and Z. Tong, Int. J. Pharm., 2008, 351, 104 CrossRef CAS; (c) H. X. Liu, C. Y. Wang, Q. X. Gao, J. X. Chen, B. Y. Ren, X. X. Liu and Z. Tong, Int. J. Pharm., 2009, 376, 92 CrossRef CAS.
- (a) K. P. Oza and S. G. J. Frank, J. Dispersion Sci. Technol., 1989, 10, 163 CrossRef CAS; (b) T. Sekine, K. Yoshida, F. Matsuzaki, T. Yanaki and M. Yamaguchi, J. Surfactants Deterg., 1999, 2, 309 Search PubMed; (c) B. R. Midmore and T. M. Herrington, Prog. Colloid Polym. Sci., 1999, 112, 115 CAS; (d) S. Arditty, V. Schmitt, J. Giermanska-Kahn and F. Leal-Calderon, J. Colloid Interface Sci., 2004, 275, 659 CrossRef CAS; (e) N. Garti, A. Aserin, I. Tiunova and H. Binyamin, J. Am. Oil Chem. Soc., 1999, 76, 383 CrossRef CAS.
- (a) S. Cauvin, P. J. Colver and S. A. F. Bon, Macromolecules, 2005, 38, 7887 CrossRef; (b) T. Chen, P. J. Colver and S. A. F. Bon, Adv. Mater., 2007, 19, 2286 CrossRef CAS; (c) S. A. F. Bon and P. J. Colver, Langmuir, 2007, 23, 8316 CrossRef CAS; (d) S. A. F. Bon, S. Cauvin and P. J. Colver, Soft Matter, 2007, 3, 194 RSC; (e) S. A. F. Bon and T. Chen, Langmuir, 2007, 23, 9527 CrossRef CAS; (f) P. J. Colver, C. A. L. Colard and S. A. F. Bon, J. Am. Chem. Soc., 2008, 130, 16850 CrossRef CAS.
- (a) A. Schmid, J. Tonnar and S. P. Armes, Adv. Mater., 2008, 20, 3331 CrossRef CAS; (b) D. Dupin, A. Schmid, J. A. Balmer and S. P. Armes, Langmuir, 2007, 23, 11812 CrossRef CAS; (c) A. Schmid, S. Fujii, S. P. Armes, C. A. P. Leite, F. Galembeck, H. Minami, N. Saito and M. Okubo, Chem. Mater., 2007, 19, 2435 CrossRef CAS; (d) S. Sacanna, W. K. Kegel and A. P. Philipse, Phys. Rev. Lett., 2007, 98, 158301 CrossRef CAS; (e) S. Sacanna and A. P. Philipse, Adv. Mater., 2007, 19, 3824 CrossRef CAS.
- (a) T. Hasell, J. X. Yang, W. X. Wang, J. Li, P. D. Brown, M. Poliakoff, E. Lester and S. M. Howdle, J. Mater. Chem., 2007, 17, 4382 RSC; (b) J. Yang, T. Hasell, W. X. Wang, J. Li, P. D. Brown, M. Poliakoff, E. Lester and S. M. Howdle, J. Mater. Chem., 2008, 18, 998 RSC; (c) J. Zhang, K. Q. Chen and H. Y. Zhao, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2632 CrossRef CAS; (d) K. Q. Chen, Y. F. Yang, Q. N. Sa, L. Q. Shi and H. Y. Zhao, Polymer, 2008, 49, 2650 CrossRef CAS; (e) Y. N. Wu, J. Zhang and H. Y. Zhao, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1535 CrossRef CAS.
- (a) D. J. Voorn, W. Ming and A. M. van Herk, Macromolecules, 2006, 39, 2137 CrossRef CAS; (b) J. Jeng, T. Y. Chen, C. F. Lee, N. Y. Liang and W. Y. Chiu, Polymer, 2008, 49, 3265 CrossRef CAS; (c) X. D. He, X. W. Ge, H. R. Liu, M. Z. Wang and Z. C. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 933 CrossRef CAS; (d) X. D. He, X. W. Ge, H. R. Liu, M. Z. Wang and Z. C. Zhang, Chem. Mater., 2005, 17, 5891 CrossRef CAS; (e) B. Liu, W. Wei, X. Z. Qu and Z.Z. Yang, Angew. Chem., Int. Ed., 2008, 47, 3973 CrossRef CAS; (f) A. Walther, M. Hoffmann and A. H. E. Mueller, Angew. Chem., Int. Ed., 2008, 47, 711 CrossRef CAS.
- (a) Y. H. Chen, C. Y. Wang, J. X. Chen, X. X. Liu and Z. Tong, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1354 CrossRef CAS; (b) H. X. Liu, C. Y. Wang, Q. X. Gao, J. X. Chen, X. X. Liu and Z. Tong, Mater. Lett., 2009, 63, 884 CrossRef CAS; (c) Q. X. Gao, C. Y. Wang, H. X. Liu, C. H. Wang, X. X. Liu and Z. Tong, Polymer, 2009, 50, 2587 CrossRef CAS.
- (a) J. J. Crevecoeur, L. Nelissen and P. J. Lemstra, Polymer, 1999, 40, 3685 CrossRef CAS; (b) J. J. Crevecoeur, L. Nelissen and P. J. Lemstra, Polymer, 1999, 40, 3691 CrossRef CAS; (c) A. Desforges, M. Arpontet, H. Deleuze and O. Mondain-Monval, React. Funct. Polym., 2002, 53, 183 CrossRef CAS; (d) D. Stefanec and P. Krajnc, React. Funct. Polym., 2005, 65, 37 CrossRef CAS; (e) D. Stefanec and P. Krajnc, Polym. Int., 2007, 56, 1313 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the experimental procedures and characterization data. See DOI: 10.1039/b9py00255c |
| This journal is © The Royal Society of Chemistry 2010 |
