Facile synthesis of linear-dendritic cholesteryl-poly(ε-caprolactone)-b-(L-lysine)G2 by thiol-ene and azide-alkyne “click” reactions†
Irakli
Javakhishvili
a,
Wolfgang H.
Binder
b,
Susanne
Tanner
b and
Søren
Hvilsted
*a
aTechnical University of Denmark, Department of Chemical and Biochemical Engineering, Danish Polymer Centre, Building 423, DK-2800, Kgs. Lyngby, Denmark. E-mail: sh@kt.dtu.dk; Tel: +45 4525 2965
bMartin-Luther University Halle-Wittenberg, Faculty of Natural Sciences II/Institute of Chemistry, Lehrstuhl Makromolekulare Chemie, von Danckelmannplatz 4, D-06120 Halle (Saale), Germany. Tel: +49 (0)345 55 25930
First published on 14th January 2010
Abstract
The construction of a linear-dendritic block copolymer consisting of terminal cholesteryl moiety, poly(ε-caprolactone), and a second generation L-lysine dendron has been accomplished by the combination of copper(I) catalyzed azide-alkyne and UV-triggered thiol-ene “click” reactions. Ring-opening polymerization of ε-caprolactone initiated by 5-hexyn-1-ol and Mitsunobu coupling with 4-pentenoic acid provide hetero-telechelic poly(ε-caprolactone) bearing terminal alkyne and alkenegroups. It is then employed in the sequential “click” reactions with the azide-functionalized dendritic wedge and thiocholesterol. Near to quantitative functionalization of the intermediate and final products has been attained as confirmed by NMR spectroscopy and MALDI-TOF spectrometry.
Introduction
Amphiphilic linear-dendritic block copolymers have gained significant importance due to their peculiar properties that drive self-assembly into various shapes. Different combinations of the constituting blocks have been prepared, and their capacity of forming complex supramolecular architectures has been investigated.1Hedrick and co-workers have designed linear-dendritic amphiphiles that resemble naturally occuring phospholipids.2 The hydrophilic dendrons based on 2,2-bis(hydroxymethyl)propionic acid (bis-MPA) (A) were combined with the linear segment of the hydrophobic poly(ε-caprolactone) (PCL) (B) viaring-opening polymerization (ROP) of ε-caprolactone (ε-CL) initiated by the dendrons possessing multiple functionalities. The symmetric linear-dendritic triblock copolymer (ABA) was also prepared via the Mitsunobu coupling of the linear-dendritic block copolymer (AB) with the acid-functionalized dendrons.2 Fréchet and co-workers3 obtained linear-dendritic block copolymers by divergently growing bis-MPA based dendrons from poly(ethylene glycol) (PEG) chain-end.
A step-wise synthetic protocol that affords well-defined structures with a controlled number of peripheral functional groups available for subsequent functionalization can render self-assembled aggregates stimuli responsive, and prompts the application of such nanocarriers as drug delivery devices.4 Thus, poly(ethylene oxide) (PEO)-dendritic polylysine and PEO-dendritic polyester have been prepared, and then peripheral functional groups have been modified by acid-labile hydrophobic moieties to obtain pH responsive micelles.4
Tomalia and co-workers5 have prepared core-shell nanostructures from precise monodisperse, linear-dendritic polymers. First a thiol functionalized di-dendron is conjugated with single strand DNA. Together with the other linear-dendritic copolymer incorporating appropriate complementary DNA as the linear segment, these structures may be used as supramacromolecular components to build core-shell nanostructures possessing base-paired DNA cores surrounded by four dendrons.5
Cao and co-workers developed the route towards poly(L-lactide)-b-dendritic poly(L-lysine) based on the metal free ROP of the L-lactide initiated by the hydroxyl end-capped Boc-protected poly(L-lysine) dendrons.6 The authors have pointed out the role of poly(L-lysine) as a functional vector for DNA transfection.6
Zubarev and Stupp7 demonstrated a synthetic pathway leading to hybrid structures: dendron rodcoils. These macromolecules comprise dendritic, rod-like, and coil-like molecular segments, and have been obtained via convergent or divergent strategies.7 Amphiphilic “tree-shaped” comb-dendritic block copolymer has been prepared with hydrophobic, rigid comb block, poly(γ-n-dodecyl-L-glutamate), that results in the formation of extremely stable micelles, and highly functional dendritic exterior – the polyester dendron modified with PEG – that can be used to attach biocompatible ligands for efficient targeting.8Self-assembly of this linear-dendritic hybrid block copolymer in solution provides a potential route to core-shell micellar nanostructures with various surface functionalities. The semirigid rod nature of the comb block determines the unique shape of the macromolecular structure and assists self-assembly.8 Stupp and co-workers have also reported about the synthesis of the amphiphilic rodcoil dendrons where the dendritic wedge was connected with the rigid moiety via the polyester spacer.9 Preparation of these intricate structures involved ROP of L-lactide initiated by cholesterol, and substitution of the hydroxyl terminus of this cholesteryl-oligo(L-lactic acid) with the L-lysine dendron. While liquid chrystalline character of the cholesteryl moiety could drive self-assembly of the rodcoil dendron, the L-lysine wedge could provide sterics to direct the nanostructure formation. Furthermore, both cholesterol and L-lysine could interact with the cell membrane, and thus promote and facilitate cell adhesion.9
However, this synthetic procedure, though subtle, is quite tedious and demanding. With the advance of the copper(I) catalyzed azide-alkynecycloaddition10 (CuAAC) and UV initiated thiol-ene11 “click” reactions, and their successful implementation in the field of the macromolecular chemistry,12 we believe that the synthetic approach towards the rodcoil dendrons can be accelerated, and made more robust and versatile: all three constituent blocks can be coupled to avoid the default presence of either a cholesterol or L-lysine wedge as is the case in the strategies discussed above.6,9CuAAC “click” reaction has previously been employed in the preparation of unsymmetrical dendrimers,13 dendronized linear polymers14 as well as linear-dendritic block copolymers,15 and thus has proved to be a highly efficient and convenient approach.
Herein, we present the synthetic layout for the preparation of the rodcoil dendron based on cholesterol, PCL, and dendritic L-lysine. Since Hawker and co-workers pointed out the efficiency and compatibility of these orthogonal “click” reactions,16 we believe this is one of the first successful attempts to employ this strategy in building such multifunctional macromolecular architecture alongside Haddleton and co-workers, where CuAAC reaction has been coupled with thio-Michael addition,17 and Anseth and co-workers, where copper-free “click” chemistry has been conjugated with thiol-ene photocoupling.18
Experimental
Materials and methods
ε-CL (Fluka, ≥99%) was dried over CaH2, and distilled under reduced pressure. Tetrahydrofuran (THF) (Sigma-Aldrich, 99.9%), N,N-dimethylformamide (DMF) (Fluka, 99.8%), toluene (Sigma-Aldrich, 99.9%), and triethylamine (TEA) (Sigma-Aldrich, 99%) were dried over CaH2, and distilled under nitrogen flow. Tin octoate (Sn(Oct)2) (Sigma, ∼95%) was distilled under reduced pressure. Methanol (Sigma-Aldrich, 99.9%), heptane (Sigma-Aldrich, 99%), dichloromethane (DCM) (Sigma-Aldrich, 99.8%), dioxane (Riedel-de Haën, 99%), ethyl acetate (Sigma-Aldrich, 99.7%), 5-hexyn-1-ol (Aldrich, 96%), 4-pentenoic acid (Aldrich, 97%), L-lysine (Fluka, purum; crystallized; ≥98%), di-tert-butyl dicarbonate (Aldrich, 97%), N,N′-dicyclohexylcarbodiimide (DCC) (Aldrich, 99%), 3-bromo-1,2-propanediol (Fluka, ≥96%), diethyl azodicarboxylate (DEAD) (Aldrich), triphenylphosphine (TPP) (Fluka, ∼97%), sodium azide (Aldrich, 99%), CuI (Aldrich, 98%), thiocholesterol (Aldrich), trifluoroacetic acid (TFA) (Sigma-Aldrich, ≥98%), 2,2-dimethoxy-2-phenylacetophenone (DMPA) (Aldrich, 99%), 4-di(methylamino)pyridine (4-DMAP) (Fluka, >98%) were used as received.Characterization by NMR spectroscopy was conducted on Bruker 300 MHz spectrometer using CDCl3 or DMSO-d6 as solvents (both from Aldrich). The coupling constants are given in Hz. Molecular weights and polydispersity indices were estimated by size exclusion chromatography (SEC) on Viscotek 200 instrument using two PLgel mixed-D columns (Polymer Laboratories (PL)), assembled in series, and a refractive index detector. SEC samples were run in THF at room temperature (1 mL min−1). Molecular weights were calculated using polystyrene (PS) standards from PL. Mass spectra were acquired by matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry using a Bruker autoflex III smartbeam mass spectrometer, equipped with the laser that produces pulses at 337 nm. Samples using 2,5-dihydroxyacetophenon as matrix and NaTFA as additive were prepared by dissolving the polymer in THF at a concentration of 20 mg mL−1. A 10 μL aliquot of this solution was added to 100 μL of a 20 mg mL−1 matrix solution, and 1 μL NaTFA as a THF solution (20 mg mL−1) was added as cationzation agent.
All reactions were carried out under the nitrogen atmosphere unless otherwise stated.
Synthesis
1: Boc protection of L-lysine was carried out according to the procedure reported elsewhere.192: 1 (5.00 g, 14.45 mmol), 3-bromo-1,2-propanediol (1.02 g, 6.58 mmol), and 4-DMAP (0.17 g, 1.39 mmol) were placed in a 250 mL two-neck flask, and dissolved in DCM (60 mL). The reaction mixture was stirred and cooled in an ice/water bath, and the solution of DCC (3.00 g, 14.54 mmol) in DCM was injected. Afterward, the ice/water bath was removed, and the reaction was carried out at room temperature for 24 h. After completion of the reaction, the mixture was filtered to remove dicyclohexylurea, and extracted with dilute NaHCO3 (2 × 50 mL), dilute NaHSO4 (1 × 50 mL), distilled water (1 × 50 mL), and brine (1 × 50 mL). The solution was dried over anhydrous MgSO4, and concentrated under reduced pressure. Yield: 4.64 g (87%).
δ H (300 MHz; CDCl3) 5.10–5.45 (3H, br, CHNHBoc and OCH2CHO), 4.55–4.85 (2H, br, CH2NHBoc), 4.37–4.53 (m, OCH2CHO), 4.14–4.37 (m, OCH2CHO and CHNHBoc) (4.14–4.53 corresponds to 4H), 3.38–3.60 (2H, m, CH2Br), 2.95–3.25 (4H, m, CH2NHBoc), 1.58–2.00 (m, CH2CH2CHNHBoc), 1.25–1.58 (m, BocNHCH2CH2CH2CH2CHNHBoc and C(CH3)3) (1.25–2.00 corresponds to 48H).
3: 2 (4.58 g, 5.64 mmol) and sodium azide (0.73 g, 11.23 mmol) were placed in a round-bottom flask, and dissolved in DMF (50 mL). The reaction mixture was stirred at 40 °C overnight. Afterward, it was diluted with distilled water (70 mL), and extracted with ethyl acetate (7 × 50 mL). The organic layers were combined, and washed with distilled water (6 × 100 mL), brine (1 × 75 mL), and distilled water (1 × 100 mL). The solution was then dried over anhydrous MgSO4, and concentrated on rotary-evaporator. Yield: 3.96 g (91%).
δ H (300 MHz; CDCl3) 5.10–5.40 (3H, br, CHNHBoc and OCH2CHO), 4.60–4.83 (2H, br, CH2NHBoc), 4.31–4.45 (1H, m, OCH2CHO), 4.15–4.31 (3H, m, OCH2CHO and CHNHBoc), 3.40–3.60 (2H, m, CH2N3), 2.95–3.20 (4H, m, CH2NHBoc), 1.60–1.97 (m, CH2CH2CHNHBoc), 1.28–1.60 (m, BocNHCH2CH2CH2CH2CHNHBoc and C(CH3)3) (1.28–1.97 corresponds to 48H). The resonances at 4.11 (q), 2.03 (s), 1.25 (t) correspond to the residual ethyl acetate.
4: Ring opening polymerization of ε-CL was conducted as reported earlier.20 Degree of polymerization estimated by 1H NMR is 20, Mn = 2380 Da. SEC: Mn = 4200 Da, Mw = 4700 Da, Mw/Mn = 1.11.
δ H (300 MHz; CDCl3) 4.05 (40H, t, J1,3 6.7, (CH2)4CH2OC(O) and C(O)OCH2(CH2)3C≡CH), 3.64 (2H, t, J1,3 6.5, (CH2)4CH2OH), 2.30 (t, J1,3 7.5, OC(O)CH2(CH2)4), 2.23 (td, J1,4 2.6, J1,3 7.1, CH2C≡CH) (2.18–2.40 corresponds to 42 H), 1.96 (1H, t, J1,4 2.6, CH2C≡CH), 1.50–1.81 (84H, m, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2C≡CH), 1.25–1.50 (40H, m, OC(O)CH2CH2CH2CH2CH2).
5: The Mitsunobu coupling was performed under the same conditions as in ref. 20. SEC: Mn = 4100 Da, Mw = 4500 Da, Mw/Mn = 1.11. δH (300 MHz; CDCl3) 5.80 (1H, m, CH2CH = CH2), 5.00 (2H, m, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.05 (40H, t, J1,3 6.7, (CH2)4CH2OC(O) and C(O)OCH2(CH2)3C≡CH), 2.36 (m, OC(O)CH2CH2CH
CH2), 4.05 (40H, t, J1,3 6.7, (CH2)4CH2OC(O) and C(O)OCH2(CH2)3C≡CH), 2.36 (m, OC(O)CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 2.28 (t, J1,3 7.5, OC(O)CH2(CH2)4), 2.21 (td, J1,4 2.6, J1,3 7.1, CH2C≡CH) (2.16–2.42 corresponds to 46H), 1.94 (1H, t, J1,4 2.6, CH2C≡CH), 1.48–1.80 (84H, m, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2C≡CH), 1.20–1.48 (40H, m, OC(O)CH2CH2CH2CH2CH2).
CH2), 2.28 (t, J1,3 7.5, OC(O)CH2(CH2)4), 2.21 (td, J1,4 2.6, J1,3 7.1, CH2C≡CH) (2.16–2.42 corresponds to 46H), 1.94 (1H, t, J1,4 2.6, CH2C≡CH), 1.48–1.80 (84H, m, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2C≡CH), 1.20–1.48 (40H, m, OC(O)CH2CH2CH2CH2CH2).
6: A 50 mL two-neck flask was charged with 5 (1.4 g, 0.57 mmol), 3 (0.93 g, 1.2 mmol), CuI (0.11 g, 0.58 mmol), and THF (19 mL). The reaction mixture was stirred at room temperate until the polymer dissolved completely. Then TEA (0.32 mL, 2.3 mmol) was introduced. The reaction was conducted at 35 °C for 24 h. Afterward, the mixture was diluted with THF, and treated with basic Al2O3. The solution was then filtered, concentrated under reduced pressure, and precipitated into the large excess of cold MeOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. The block copolymer was isolated viafiltration, and dried in the vacuum oven at room temperature until constant weight. SEC: Mn = 5100 Da, Mw = 5600 Da, Mw/Mn = 1.10. δH (300 MHz; CDCl3) 7.5 (1H, br, Ar), 5.80 (1H, m, CH2CH = CH2), 5.10–5.60 (br, CHNHBoc and OCH2CHO), 5.00 (m, CH2CH
1) mixture. The block copolymer was isolated viafiltration, and dried in the vacuum oven at room temperature until constant weight. SEC: Mn = 5100 Da, Mw = 5600 Da, Mw/Mn = 1.10. δH (300 MHz; CDCl3) 7.5 (1H, br, Ar), 5.80 (1H, m, CH2CH = CH2), 5.10–5.60 (br, CHNHBoc and OCH2CHO), 5.00 (m, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.85–4.93 (br m, CH2NHBoc, OCH2CHO, CHNHBoc, CH2Ar, (CH2)4CH2OC(O) and C(O)OCH2(CH2)3Ar) (3.85–5.60 corresponds to 53H), 2.93–3.22 (4H, br, CH2NHBoc), 2.58–2.85 (2H, br, C(O)O(CH2)3CH2Ar), 2.03–2.55 (44H, br m, OC(O)CH2CH2CH
CH2), 3.85–4.93 (br m, CH2NHBoc, OCH2CHO, CHNHBoc, CH2Ar, (CH2)4CH2OC(O) and C(O)OCH2(CH2)3Ar) (3.85–5.60 corresponds to 53H), 2.93–3.22 (4H, br, CH2NHBoc), 2.58–2.85 (2H, br, C(O)O(CH2)3CH2Ar), 2.03–2.55 (44H, br m, OC(O)CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and OC(O)CH2(CH2)4), 1.50–1.97 (m, CH2CH2CHNHBoc, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2Ar), 1.05–1.60 (m, BocNHCH2CH2CH2CH2CHNHBoc, C(CH3)3 and OC(O)CH2CH2CH2CH2CH2) (1.05–1.97 corresponds to 172H).
CH2 and OC(O)CH2(CH2)4), 1.50–1.97 (m, CH2CH2CHNHBoc, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2Ar), 1.05–1.60 (m, BocNHCH2CH2CH2CH2CHNHBoc, C(CH3)3 and OC(O)CH2CH2CH2CH2CH2) (1.05–1.97 corresponds to 172H).
7: 6 (150 mg, 0.046 mmol), thiocholesterol (187 mg, 0.46 mmol), DMPA (42 mg, 0.16 mmol), and toluene (1.2 mL) were placed in a 15 mL round-bottom open vial equipped with a magnetic stirring bar. Having dissolved all the solid components, the vial was placed under the UV lamp (365 nm), and irradiated for 1.5 h while stirring under air. The reaction was monitored by SEC and 1H NMR. After completion of the reaction, the mixture was diluted with DCM, and precipitated into the large excess of cold heptane. The polymer was isolated on the filter paper, washed with heptane, and dried in the vacuum oven at room temperature. SEC: Mn = 5900 Da, Mw = 6800 Da, Mw/Mn = 1.15.
δ
H
(300 MHz; CDCl3) 7.5 (1H, br, Ar), 5.05–5.70 (4H, br, thiocholesterol CH = C, CHNHBoc and OCH2CHO), 3.86–4.96 (48H, br m, CH2NHBoc, OCH2CHO, CHNHBoc, CH2Ar, (CH2)4CH2OC(O) and C(O)OCH2(CH2)3Ar), 2.97–3.22 (4H, br, CH2NHBoc), 2.44–2.83 (5H, m, OC(O)(CH2)3CH2SCH, SCHCH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, C(O)O(CH2)3CH2Ar), 2.30 (44H, t, J1,3 7.5, OC(O)CH2(CH2)3SCH, OC(O)CH2(CH2)4, and SCHCH2C
CH, C(O)O(CH2)3CH2Ar), 2.30 (44H, t, J1,3 7.5, OC(O)CH2(CH2)3SCH, OC(O)CH2(CH2)4, and SCHCH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH (this multiplet is dominated by the large triplet from the PCL backbone)), 1.55–1.97 (m, CH2CH2CHNHBoc, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2Ar), 1.25–1.60 (m, BocNHCH2CH2CH2CH2CHNHBoc, C(CH3)3 and OC(O)CH2CH2CH2CH2CH2), 0.99 (s, CH3CC
CH (this multiplet is dominated by the large triplet from the PCL backbone)), 1.55–1.97 (m, CH2CH2CHNHBoc, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2Ar), 1.25–1.60 (m, BocNHCH2CH2CH2CH2CHNHBoc, C(CH3)3 and OC(O)CH2CH2CH2CH2CH2), 0.99 (s, CH3CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 0.91 (s, 3H, CH3CH(CH)CH2), 0.87 (s, CH3CH(CH3)CH2), 0.85 (s, CH3CH(CH3)CH2), 0.67 (s, CH3C(CH)CH). Other signals originating from the cholesteryl moiety overlap with the peaks corresponding to the block copolymer, and, therefore, cannot be unambiguously assigned.
CH), 0.91 (s, 3H, CH3CH(CH)CH2), 0.87 (s, CH3CH(CH3)CH2), 0.85 (s, CH3CH(CH3)CH2), 0.67 (s, CH3C(CH)CH). Other signals originating from the cholesteryl moiety overlap with the peaks corresponding to the block copolymer, and, therefore, cannot be unambiguously assigned.
8: A 25 mL two-neck flask was charged with 7 (123 mg, 0.034 mmol) and DCM (3.6 mL). The flask was immersed in an ice/water bath, and TFA (0.75 mL) was injected. The reaction mixture was stirred for 30 min in the ice/water bath, and for 90 min at room temperature. Then it was concentrated under reduced pressure, and the product was dried in the vacuum oven at room temperature.
δ
H
(300 MHz; DMSO-d6) 8.30–8.80 (6H, br, CHNH3OOCCF3), 7.65–8.05 (br, CH2NH3OOCCF3), 7.94 (s, Ar) (7.65–8.05 corresponds to 7H), 5.20–5.70 (2H, br m, thiocholesterol CH = C and OCH2CHO), 4.55–4.90 (2H, br, CH2Ar), 3.80–4.55 (44H, m, OCH2CHO and CHNH3OOCCF3, (CH2)4CH2OC(O) and C(O)OCH2(CH2)3Ar), 2.42–2.94 (br, CH2NH3OOCCF3, OC(O)(CH2)3CH2SCH, SCHCH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, C(O)O(CH2)3CH2Ar, overlaps with DMSO-d6), 2.27 (44H, t, J1,3 7.1, OC(O)CH2(CH2)3SCH, OC(O)CH2(CH2)4, and SCHCH2C
CH, C(O)O(CH2)3CH2Ar, overlaps with DMSO-d6), 2.27 (44H, t, J1,3 7.1, OC(O)CH2(CH2)3SCH, OC(O)CH2(CH2)4, and SCHCH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 1.42–2.00 (m, CH2CH2CHNH3OOCCF3, F3CCOONH3CH2CH2CH2CH2, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2Ar), 1.18–1.42 (m, OC(O)CH2CH2CH2CH2CH2), 0.86–0.94 (m, CH3CC
CH), 1.42–2.00 (m, CH2CH2CHNH3OOCCF3, F3CCOONH3CH2CH2CH2CH2, OC(O)CH2CH2CH2CH2CH2 and C(O)OCH2CH2CH2CH2Ar), 1.18–1.42 (m, OC(O)CH2CH2CH2CH2CH2), 0.86–0.94 (m, CH3CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH and CH3CH(CH)CH2), 0.85 (s, CH3CH(CH3)CH2), 0.83 (s, CH3CH(CH3)CH2), 0.64 (s, CH3C(CH)CH).
CH and CH3CH(CH)CH2), 0.85 (s, CH3CH(CH3)CH2), 0.83 (s, CH3CH(CH3)CH2), 0.64 (s, CH3C(CH)CH).
Results and discussions
ROP of ε-CL initiated by 5-hexyn-1-ol and catalyzed by tin(II) octoate at elevated temperature in bulk has been reported by Schubert and co-workers.21 The polydispersity index (PDI) of 1.19 was achieved. We have shown that better control over the reaction can be gained by conducting it at moderate temperature with high catalyst concentration.20 Thus, PCL with the PDI = 1.11 and high end-group fidelity has been obtained. The degree of polymerization estimated by 1H NMR was 20. Esterification of the hydroxyl chain end with 4-pentenoic acid according to Mitsunobu protocol20 afforded hetero-telechelic PCL bearing alkyne and alkene functional groups. The α-alkenyl-ω-alkynyl-PCL had narrow molecular weight distribution (PDI = 1.11), and near to quantitative functionality as confirmed by 1H NMR (Fig. S2†) and MALDI-TOF analysis (Fig. S6†).The synthesis of the second generation dendron of L-lysine, (L-lysine)G2 (3), with the azide functional group in the focal point was carried out according to Scheme 1.
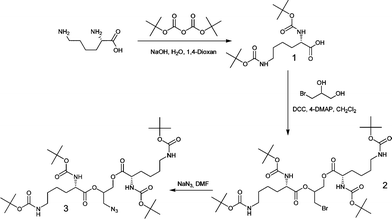 | ||
| Scheme 1 Preparation of bis-(di-Boc-L-lysine)-3-azido-1,2-propandiol | ||
This approach allows immediate incorporation of the primary bromo function in the core. Therefore, it is not necessary to introduce bromoalkyl moiety by additional esterification reaction as would be the case in the standard procedure of building dendrons.13 Subsequent nucleophilic substitution furnishes azide functional dendritic wedge of the second generation with high yield and purity.
Thus, 3-bromo-1,2-propandiol was reacted with Nα,Nε-di-Boc-L-lysine (1.1 eq) in the presence of DCC and 4-DMAP. The purification step did not require column chromatography, and sufficed with extractions. Afterward, 2 was converted to the corresponding azide by treating it with sodium azide (2 eq) at 40 °C overnight. The heteronuclear single quantum coherence (HSQC) NMR experiment (Fig. S1†) confirmed full conversion under these reaction conditions. The structure was further verified by MALDI-TOF spectrometry (Fig. S4 and S5†).
Azide-alkyne and UV initiated thiol-ene “click” reactions were conducted as depicted in Scheme 2.
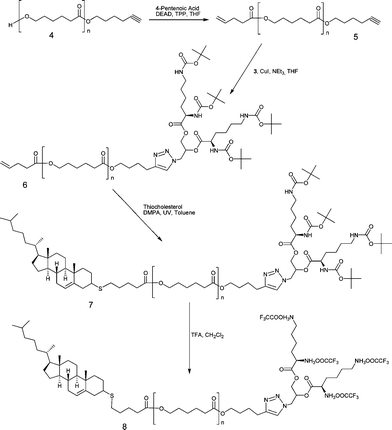 | ||
| Scheme 2 CuAAC and thiol-ene “click” reactions followed by the removal of Bocprotecting groups | ||
CuAAC reaction between α-alkenyl-ω-alkynyl-PCL5 and bis-(di-Boc-L-lysine)-3-azido-1,2-propandiol3 (2 eq) was catalyzed by CuI (1 eq in comparison to the alkyne). The “click” components were reacted in THF in the presence of NEt3 at 35 °C for 24 h.22 The excess of 3 was removed by precipitation in MeOH–H2O mixture. The product was analyzed by SEC, NMR, and MALDI-TOF.
SEC revealed monomodal, symmetrical trace, which was shifted to the higher molecular weight (Fig. 1), and low PDI of 1.10. This implies that no chain scission took place nor did the polymer chains remain unreacted. Near to quantitative functionalization was attained as judged from NMR experiments. Both 1H and HSQCNMR data indicated almost full conversion as the resonances corresponding to the alkyne functional group could no longer be detected. Furthermore, the peaks ascribed to the dendritic wedge and triazol ring (h) emerged (Fig. 2). The signals assigned to the alkene moiety were observed at 5.00 ppm and 5.80 ppm (p and o). The integrals of the resonances from the alkene end-group (5.80 ppm, o) and CH2NHBoc (2.93–3.22 ppm, c) were in good agreement with the theoretical values.
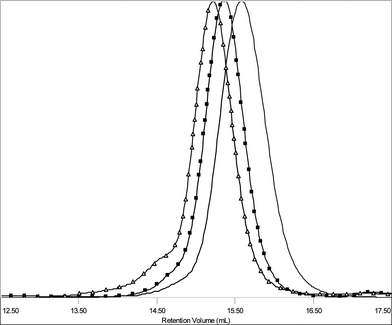 | ||
| Fig. 1 Normalized SEC traces of α-alkenyl-ω-alkynyl-PCL (5) (Mn = 4100 Da, Mw/Mn = 1.11), alkenyl-PCL-b-(di-Boc-L-lysine)G2 (6) (Mn = 5100 Da, Mw/Mn = 1.10) ■, cholesteryl-PCL-b-(di-Boc-L-lysine)G2 (7) (Mn = 5900 Da, Mw/Mn = 1.15) △. | ||
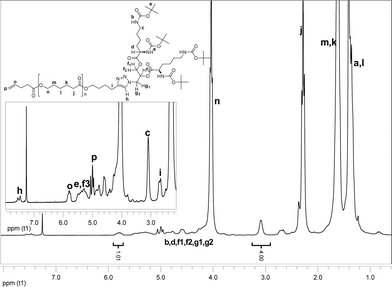 | ||
| Fig. 2 1H NMR spectrum of alkenyl-PCL-b-(di-Boc-L-lysine)G2 (6). The spectrum was recorded in CDCl3. | ||
The MALDI-TOF analysis produced a spectrum with the signal spacing of approximately 114 Da (Fig. 3), corresponding to the molecular weight of one repeating unit in the PCL chain. Two series were observed: one fitting the experimental molecular weight of the alkenyl-PCL-b-(di-Boc-L-lysine)G2 (6) as the M-Na+-series, while the other minor series being assignable to the M-Li+-series with the loss of two of the Bocgroups during the ionization process.
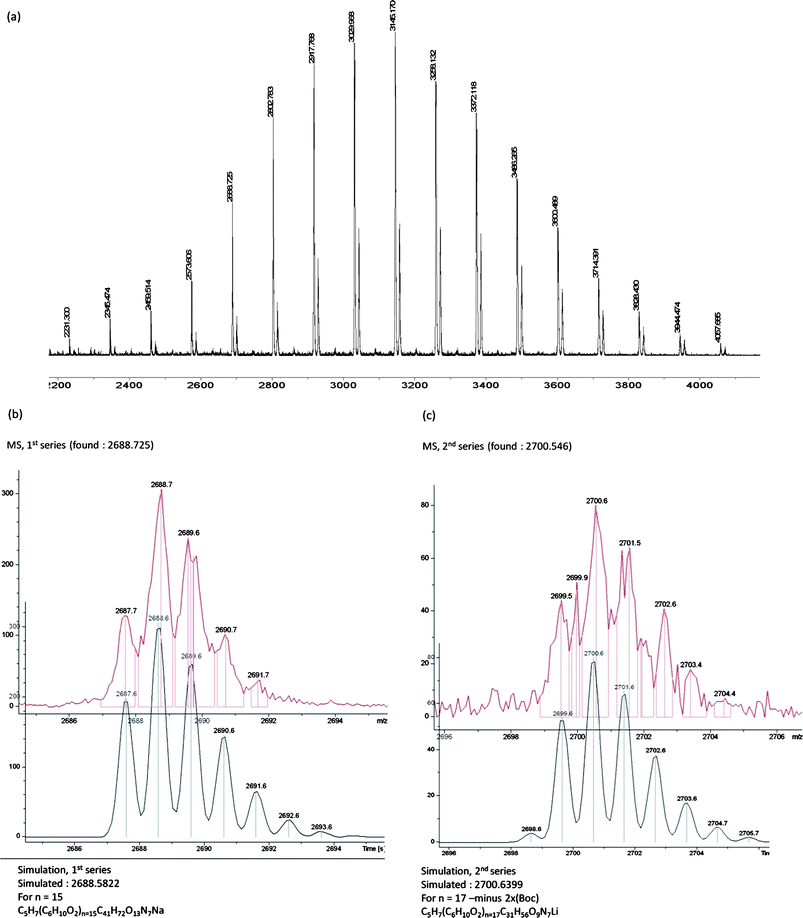 | ||
| Fig. 3 MALDI-TOF spectrum of alkenyl-PCL-b-(di-Boc-L-lysine)G2 (6): (a) full spectrum, (b) expansion (top) and simulation (bottom) of 1st-series, (c) expansion (top) and simulation (bottom) of 2nd series. | ||
The cholesteryl moiety was introduced via the thiol-ene “click” reaction between the alkenyl-PCL-b-(di-Boc-L-lysine)G2 and thiocholesterol (Scheme 2). The solution of 6, thiocholesterol, and DMPA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 molar ratio) in toluene was stirred and irridiated at 365 nm in the presence of oxygen. Large excess of the thiol and the photoinitiator were taken to overcome the obstacles posed by rigid structure and hindered mobility of thiocholesterol,23 and to achieve near to quantitative functionalization as concluded after investigations by NMR and MALDI-TOF. In the 1H NMR spectrum (Fig. 4) the signals from the alkene chain end disappeared completely, and the resonances originating from the cholesteryl moiety (q, r, and s) could be observed. The MALDI-TOF analysis revealed the species of the molecular weight that correspond to 7 (Fig. 5). The signal spacing of 114 Da indicative of the repeating unit of the PCL was again observed. In total, three series were observed as M–Na+-adducts with the main series being the M–Na+-series of 7, while the other two series indicated again the loss of either one or two Boc moieties (Fig. S7†).
3.5 molar ratio) in toluene was stirred and irridiated at 365 nm in the presence of oxygen. Large excess of the thiol and the photoinitiator were taken to overcome the obstacles posed by rigid structure and hindered mobility of thiocholesterol,23 and to achieve near to quantitative functionalization as concluded after investigations by NMR and MALDI-TOF. In the 1H NMR spectrum (Fig. 4) the signals from the alkene chain end disappeared completely, and the resonances originating from the cholesteryl moiety (q, r, and s) could be observed. The MALDI-TOF analysis revealed the species of the molecular weight that correspond to 7 (Fig. 5). The signal spacing of 114 Da indicative of the repeating unit of the PCL was again observed. In total, three series were observed as M–Na+-adducts with the main series being the M–Na+-series of 7, while the other two series indicated again the loss of either one or two Boc moieties (Fig. S7†).
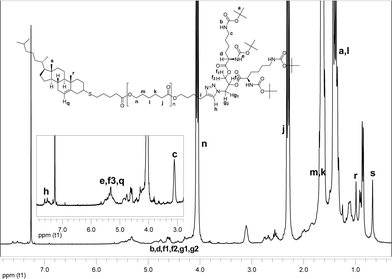 | ||
| Fig. 4 1H NMR spectrum of Cholesteryl-PCL-b-(di-Boc-L-lysine)G2 (7). The spectrum was recorded in CDCl3. | ||
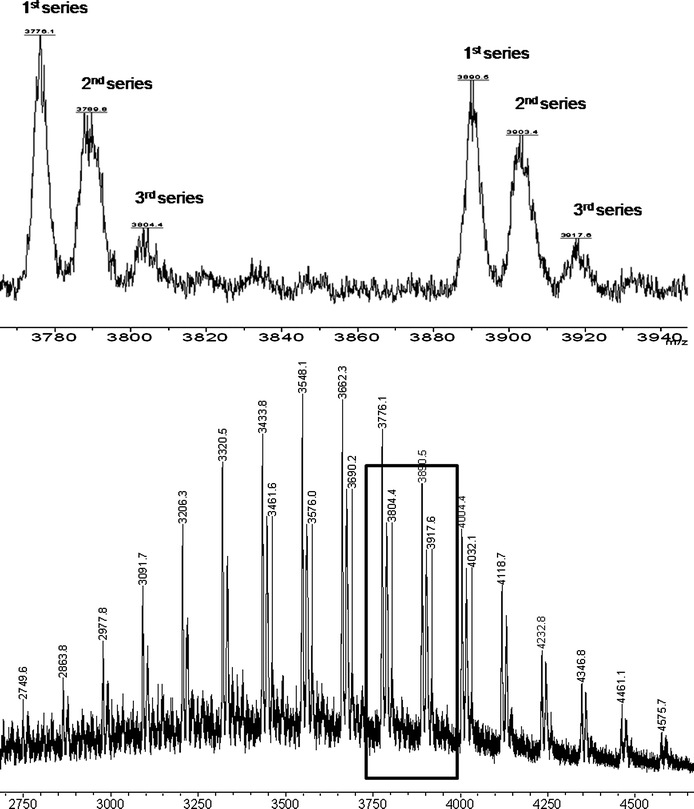 | ||
| Fig. 5 MALDI-TOF spectrum of Cholesteryl-PCL-b-(di-Boc-L-lysine)G2 (7), bottom: full spectrum; top: expansion of the indicated region. | ||
However, the SEC trace had a small shoulder (Fig. 1), though PDI was still fairly low (1.15). The appearance of the shoulder could tentatively be ascribed to the intramolecular side reactions of radical nature that could take place during the thiol-ene “click” reaction.
The final step involved deblocking of the amine functionalities. 7 was treated with TFA in CH2Cl2. The solvent and the excess of the acid were removed in vacuo, and the product was analyzed by NMR and MALDI-TOF. In 1H NMR spectrum (Fig. S3†) the signal corresponding to tert-butyl group was not observed, while the broad peaks related to the free amine groups were detected at 8.30–8.80 ppm and 7.65–8.05 ppm. The triazoleproton resonated at 7.94 ppm. Such a dramatic shift could be explained by the cardinal changes in solubility of the macromolecule (the spectrum was recorded in DMSO-d6). MALDI-TOF mass spectrometry showed several series, proving the final structure with sequential loss of the trifluoroacetate moieties during the ionization process (Fig. 6, S8–S12†).
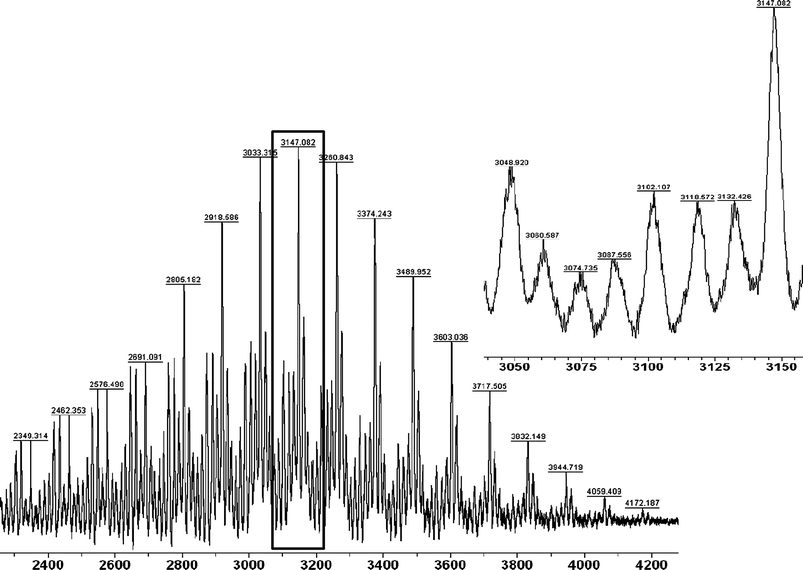 | ||
| Fig. 6 MALDI-TOF spectrum of Cholesteryl-PCL-b-(L-lysine)G2 (8). | ||
Thus, a series of peaks with loss of trifluoroacetate moieties was detected by simulation of the isotope pattern of the respective peaks and subsequent comparison with the measured (relatively broad) mass peaks. In addition, fragments with truncated structures stemming from the relatively hard ionization conditions and the reflectron-modus were also detected. However, in accordance with the NMR data, the formation of the structure of 8 was unambiguously proved by MALDI-TOF mass spectrometry as well.
Conclusions
In conclusion, we have demonstrated the effectiveness and flexibility of the orthogonal azide-alkyne and thiol-ene “click” reactions in preparation of the linear-dendritic cholesteryl-PCL-b-(L-lysine)G2. The “click” reactions considerably shorten the synthetic cascade for building such macromolecules compared to the conventional esterification reactions. Moreover, the versatility of the heterofunctional PCL in combination with these orthogonal “click” reactions provides greater degree of freedom for incorporation of different structural elements, and thus opens new avenues to much larger library of complex amphiphilic architectures.Notes and references
- I. Gitsov, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5295 CrossRef CAS.
- A. Würsch, M. Möller, T. Glauser, L. S. Lim, S. B. Voytek, J. L. Hedrick, C. W. Frank and J. G. Hilborn, Macromolecules, 2001, 34, 6601 CrossRef.
- H. Ihre, O. L. Padilla De Jesús and J. M. J. Fréchet, J. Am. Chem. Soc., 2001, 123, 5908 CrossRef CAS.
- E. R. Gillies, T. B. Jonsson and J. M. J. Fréchet, J. Am. Chem. Soc., 2004, 126, 11936 CrossRef CAS.
- C. R. DeMattei, B. Huang and D. A. Tomalia, Nano Lett., 2004, 4, 771 CrossRef CAS.
- Y. Li, Q. Li, F. Li, H. Zhang, L. Jia, J. Yu, Q. Fang and A. Cao, Biomacromolecules, 2006, 7, 224 CrossRef CAS.
- E. R. Zubarev and S. I. Stupp, J. Am. Chem. Soc., 2002, 124, 5762 CrossRef CAS.
- L. Tian and P. T. Hammond, Chem. Mater., 2006, 18, 3976 CrossRef CAS.
- H.-A. Klok, J. J. Hwang, J. D. Hartgerink and S. I. Stupp, Macromolecules, 2002, 35, 6101 CrossRef CAS.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (b) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS.
- K. L. Killops, L. M. Campos and C. J. Hawker, J. Am. Chem. Soc., 2008, 130, 5062 CrossRef CAS.
- (a) W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2008, 29, 952 CrossRef CAS; (b) R. K. Iha, K. L. Wooley, A. M. Nyström, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620 CrossRef CAS.
- P. Wu, M. Malkoch, J. N. Hunt, R. Vestberg, E. Kaltgrad, M. G. Finn, V. V. Fokin, K. B. Sharpless and C. J. Hawker, Chem. Commun., 2005, 5775 RSC.
- B. Helms, J. L. Mynar, C. J. Hawker and J. M. J. Fréchet, J. Am. Chem. Soc., 2004, 126, 15020 CrossRef CAS.
- (a) C. Hua, S.-M. Peng and C.-M. Dong, Macromolecules, 2008, 41, 6686 CrossRef CAS; (b) J. del Barrio, L. Oriol, R. Alcalá and C. Sánchez, Macromolecules, 2009, 42, 5752 CrossRef CAS.
- L. M. Campos, K. L. Killops, R. Sakai, J. M. J. Paulusse, D. Damiron, E. Drockenmuller, B. W. Messmore and C. J. Hawker, Macromolecules, 2008, 41, 7063 CrossRef CAS.
- L. Nurmi, J. Lindqvist, R. Randev, J. Syrett and D. M. Haddleton, Chem. Commun., 2009, 2727 RSC.
- C. A. DeForest, B. D. Polizzotti and K. S. Anseth, Nat. Mater., 2009, 8, 659 CrossRef CAS.
- P. Agrawal, U. Gupta and N. K. Jain, Biomaterials, 2007, 28, 3349 CrossRef CAS.
- I. Javakhishvili and S. Hvilsted, Biomacromolecules, 2009, 10, 74 CrossRef CAS.
- R. Hoogenboom, B. C. Moore and U. S. Schubert, Chem. Commun., 2006, 4010 RSC.
- R. Riva, S. Schmeits, F. Stoffelbach, C. Jérôme, R. Jérôme and Ph. Lecomte, Chem. Commun., 2005, 5334 RSC.
- N. ten Brummelhuis, C. Diehl and H. Schlaad, Macromolecules, 2008, 41, 9946 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and MALDI-TOF spectra. See DOI: 10.1039/b9py00303g |
| This journal is © The Royal Society of Chemistry 2010 |
