Implications of citrate concentration during the seeded growth synthesis of gold nanoparticles†‡
Anna A.
Volkert
,
Varuni
Subramaniam
and
Amanda J.
Haes
*
University of Iowa, Department of Chemistry, 204 IATL, Iowa City, Iowa 52242, USA. E-mail: amanda-haes@uiowa.edu; Fax: +1 319-335-1270; Tel: +1 319-384-3695
First published on 7th October 2010
Abstract
Increasing the citrate concentration during the seeded growth synthesis of gold nanoparticles yields materials with decreased aspect ratios and increased defect densities. The stability of these nanoparticles is attributed to variations in their overall Gibb's free energy.
In recent years, the tunable chemical and physical properties of nanoparticles are more easily exploited because of improvements in direct1 and seeded2growth synthetic techniques which yield discrete nanomaterial sizes with various shapes (i.e. spheres,1 rods,3 and nanoflowers).4 In all cases, the stability of a solution-phase nanostructure depends on its overall Gibb's free energy, which is dictated by inherent structural properties (i.e. crystallinity, shape, size) and external environment (i.e. ionic strength, pH).
During nanoparticle growth, atom lattice reorganization results in reduced numbers of internal and surface defects thereby minimizing the overall energy and surface tension of the system.5 To further decrease nanoparticle free energy, capping agents such as citrate or CTAB can bind preferentially to the relatively higher energy crystal planes and if this occurs during growth, asymmetric nanoparticle morphologies will develop.2 Moreover, the ionic speciation of the nanoparticle solution will influence the double layer thickness on the nanoparticle which improves its stability by reducing surface energy but can interrupt and reduce the uniform flux of growth species to these same surfaces.
Once synthesized, nanoparticle stability will dictate their ultimate usefulness for applications, shelf-life, as well as their chemical and physical properties. As a result, various methods are used to monitor the stability of nanostructures. Flocculation parameter studies,6 for instance, yield semi-quantified information by monitoring a size-dependent chemical or physical property of a nanomaterial as a function of time and/or solution pH.6,7
Herein, we report a novel strategy to vary gold nanoparticle structure and resulting stability in solution by varying citrate concentration during seeded growth syntheses. Citrate stabilized gold (Au@citrate) nanoparticles with a relative standard deviation (RSD) less than 10% were considered monodisperse.8 As shown in Fig. 1, these nanomaterials are synthesized in three steps. First, gold nanoparticle seeds are synthesized using standard citrate reduction techniques.1 The as-synthesized seeds (diameter, d = 10.8 ± 0.8 nm, number of samples analyzed, N = 225) are diluted with nanopure water (18.2 MΩ cm−1) and grown via the addition of gold salt and hydroxylamine hydrochloride (reducing agent).1 The first generation seeds are monodisperse (d = 18.0 ± 1.8 nm, N = 203). Next, six second generation gold nanoparticle samples are synthesized from the first generation nanoparticles. Citrate, a stabilizing agent, is added to 25 mL of ∼0.2 nM first generation nanoparticles in incremental concentrations (0.10, 0.30, 0.50, 0.70, 0.90, or 1.10 mM) using either a 0.10 or 2.0 M sodium citrate tribasic dihydrate stock solution. Next, 200.0 mM hydroxylamine hydrochloride (141 μL) is added to the nanoparticle solution and allowed to equilibrate. After 15 minutes, the pH of the nanoparticle solution is measured (Table S1, ESI‡) and 250 μL HAuCl4 (1% w/v) is added and stirred for at least four hours in the dark at room temperature to ensure equilibrated nanoparticle growth. Using the measured pH values, the ionic strengths of each sample was calculated (Table S1) as described in the ESI.‡
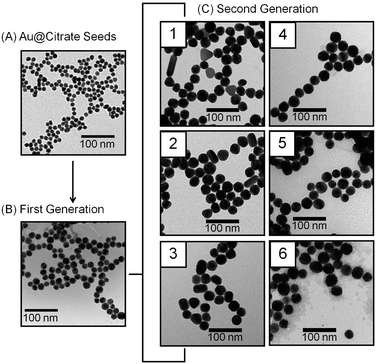 | ||
| Fig. 1 Representative TEM images. (A) Au@citrate (d = 10.8 ± 0.8 nm), (B) first generation (d = 18.0 ± 1.8 nm), and (C) second generation nanoparticles prepared in (1) 0.10, (2) 0.30, (3) 0.50, (4) 0.70, (5) 0.90, and (6) 1.10 mM citrate concentrations, respectively. | ||
Representative transmission electron microscopy (TEM) images and analysis of seed, first, and second generation gold nanoparticles are found in Fig. 1, Table 1 and Table S2 (ESI‡). Qualitative and quantitative inspections of these TEM data compare the shape and size homogeneity among the second generation nanomaterials. In all cases; the minimum, mean, and maximum nanoparticle dimensions as well as the mean aspect ratios are evaluated (including standard deviations and percent relative standard deviations (% RSD)).
| Sample | [Citrate]/mM | Mean diameter/nm | Mean aspect ratio | Aspect ratio > 2 | N |
|---|---|---|---|---|---|
| a The control sample was grown in the presence of 0.10 mM citrate and pH 4.97. | |||||
| 1 | 0.10 |
31.0 ± 2.7
(9%) |
1.30 ± 0.43
(33%) |
8% | 138 |
| 2 | 0.30 |
31.4 ± 2.6
(8%) |
1.27 ± 0.39
(31%) |
7% | 146 |
| 3 | 0.50 |
30.2 ± 2.6
(9%) |
1.22 ± 0.20
(16%) |
2% | 131 |
| 4 | 0.70 |
30.3 ± 3.0
(10%) |
1.16 ± 0.07
(6%) |
0% | 129 |
| 5 | 0.90 |
30.0 ± 3.6
(12%) |
1.11 ± 0.04
(4%) |
0% | 145 |
| 6 | 1.10 |
30.4 ± 4.1
(13%) |
1.13 ± 0.05
(6%) |
0% | 114 |
| Controla | 0.10 |
32.2 ± 3.4
(11%) |
1.27 ± 0.37
(29%) |
6% | 119 |
Several important trends are noted. First, the average nanoparticle size for all six samples is ∼30 nm. Second, gold nanoparticles grown in the presence of 0.10, 0.30, and 0.50 mM citrate contain a small population of rod-like structures with aspect ratios greater than 2 (8%, 7%, and 2%, respectively) whereas no rod-like structures are observed for materials grown in 0.70, 0.90, and 1.10 mM citrate (Fig. S1, ESI‡). Above 1.10 mM citrate concentrations, spherical but heterogeneous nanoparticles (% RSD > 30%) form (data not shown). Third, the average aspect ratio for the six second generation nanoparticle samples are 1.30 (±33%), 1.27 (±31%), 1.22 (±16%), 1.16 (±6%), 1.11 (±4%), and 1.13 (±6%) for 0.10 to 1.10 mM citrate concentrations, respectively.
These data suggest that citrate (the stabilizing agent) and/or solution pH influence(s) the homogeneous growth of gold nanoparticles. Previous studies revealed that as the AuCl4− to hydroxylamine ratio decreased2 or solution pH increased (3.5–7.5),9nanoparticle homogeneity improved. To understand the role of citrate and/or pH in these homogeneous growth syntheses, a control study was performed where nanoparticles were grown in the presence of 0.10 mM citrate (most heterogeneous sample) at pH = 4.97 (pH of most homogeneous sample). The pH was adjusted using 1.00 M NH4OH. Interestingly, this control sample yielded similar structures to the nanoparticles grown in 0.10 mM citrate (Fig. S2, ESI‡). Analysis of these data are summarized in Table 1 and reveal that ∼6% of rod-like structures with aspect ratios greater than 2 and a mean aspect ratio of 1.27 (±29%) form, thereby indicating that citrate concentration is more important than pH for homogeneous nanostructure growth in these conditions.
Clearly, citrate stabilizes nanoparticles during growth by impacting double layer properties and/or the inherent rate of gold reduction by hydroxylamine on the nanoparticle surface. Regardless of concentration, citrate preferentially adsorbs onto the gold nanoparticle surface vs. other ions in solution (i.e.AuCl4−, Cl−, etc.).2 At low (<0.70 mM) citrate concentrations, the formation of nanoparticles with aspect ratios greater than 2 indicate faceted growth. At higher citrate concentrations, no nanoparticles with aspect ratios greater than 2 are observed, and the mean nanoparticle diameters are statistically similar.
To further investigate subsequent impacts of citrate present during growth, the optical properties of the nanoparticles are monitored using extinction spectroscopy (HR4000, Ocean Optics). Extinction spectra of gold nanoparticles are a measure of the localized surface plasmon resonance (LSPR) which will vary predictably based on the composition, shape, size, and homogeneity as well as the local dielectric environment surrounding the nanostructures.10 Only nanoparticle samples grown where no rod-like nanostructures formed are evaluated ([citrate] = 0.70–1.10 mM). These samples are centrifuged three times (2773 × g for 20–25 minutes), washed in water (pH adjusted to 11 with NaOH) to remove excess citrate from the nanoparticle solution, and suspended in pH 12 buffer (sodium phosphate, conductivity 3.0 mS cm−1) to ensure a consistent dielectric environment.
The three second generation gold nanoparticle samples all exhibit similar extinction maxima, λmax, which are centered at ∼520 nm (Fig. S3, ESI‡). Closer evaluation of these data, however, reveals slight differences in the LSPR peak widths (full width at half maxima, Γ). As citrate concentration increases from 0.70, 0.90, to 1.10 mM, the Γ systematically increases from 102 nm, 106 nm, to 114 nm, respectively. Because the average extinction maxima among the samples are statistically similar, this slight dampening or broadening of the LSPR band is likely attributed to increased electron scattering in the nanostructures—a phenomenon which arises with increased surface and internal defects.11
To understand why LSPR dampening is occurring, the Debye length present during nanoparticle growth is calculated using the ionic strengths found in Table S1, ESI‡ and the Debye–Hückel parameter.12 For nanoparticles grown in 0.70, 0.90, and 1.10 mM citrate concentrations, Debye lengths are 5.0, 4.6, and 4.2 nm, respectively. As the citrate concentration increases, the Debye length decreases by compressing the double layer and increasing the association of citrate with the gold surface. As the degree of interaction between a capping agent (citrate) and the metal surface increases, metal reduction will be limited in the growth plane.13 We hypothesize that citrate at relatively higher concentrations likely disrupts the flux of AuCl4− to the nanoparticles during growth which results in more internal and/or surface defects, and as a result, induces plasmon dampening.
If citrate impacts both nanoparticle structure (morphology) and defect density, then the stability of nanoparticles grown in these studies could vary. As a result, nanoparticle stability is semi-quantified using flocculation parameter estimations. To collect these data, nanoparticles are centrifuged (2773 × g for 20–25 min) and resuspended in 3.0 mS cm−1sodium acetate and phosphate buffer (pH 4 and 12, respectively) three times. The citrate concentration in all the samples is maintained at ∼0.02 mM after centrifugation. TEM is used to evaluate structural differences for the nanostructures before and after centrifugation. With the exception of nanoparticles grown in the presence of 0.10 mM citrate, statistically similar nanoparticle structures are observed. As a result, the nanoparticle sample grown in the presence of 0.10 mM citrate was not evaluated for stability (Fig. S4, ESI‡).
To monitor flocculation, extinction spectra were collected every 5 seconds. Spectra collected in pH 4 buffer were baseline adjusted and normalized to the spectra collected in the pH 12 buffer to account for slight variations in spectrometer function and nanoparticle concentrations. Fig. 2A reveals the time dependent LSPR spectra of gold nanoparticles grown in the presence of 0.70 mM citrate. As flocculation increases, the extinction intensity at 520 nm decreases while a new lower energy band centered at 650 nm intensifies. After 100 seconds, the extinction intensities which correspond to both wavelengths decrease indicating irreversible nanoparticle aggregation/precipitation. Similar behavior was observed for all nanoparticle samples. Next, the integrated area between ∼600–800 nm was calculated using Grams AI to quantify the degree of nanoparticle flocculation as a function of time (Fig. S5, ESI‡). Nanoparticle flocculation increases with time (at pH 4) and at different rates for the various nanoparticle samples. In order to compare the various nanoparticle samples, the first derivative of the flocculation curves were plotted vs.citrate concentration (Fig. 2B).
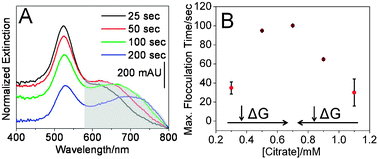 | ||
| Fig. 2 Optical characterization of Au nanoparticles. (A) Time-dependent extinction spectra for nanoparticles (0.70 mM citrate) in buffer (pH 4). The shaded area was integrated to calculate flocculation. (B) Maximized flocculation time as a function of citrate growth concentrations. Error bars represent averaged flocculation parameters over a 60 second window centered at the maximized flocculation time. | ||
Two important trends are observed in these data. First, nanoparticle samples which contain rod-like nanoparticles (aspect ratios > 2) flocculated within ∼90 seconds. We attribute this result to the relatively high Gibb's free energy (i.e. surface tension) of rod-like structures vs. spheres.14 Second, for the three nanoparticle samples which did not contain rod-like structures (i.e. aspect ratios > 2) but exhibited similar mean diameters and extinction maxima wavelengths; the time required to achieve maximized flocculation increased with decreased citrate concentration present during growth. As indicated previously by the LSPR dampening studies, increased internal and/or external atomic defect densities are likely forming with increased citrate concentrations. As the number of defects in the nanoparticles increases, the surface energies of the resulting nanostructures also increase.15 Because the nanoparticle sample grown in 0.70 mM citrate exhibited the narrowest LSPR band; defect density, and as a result, Gibb's free energy is minimized yielding the most stable sample.
In summary, gold nanoparticles were grown in the presence of varying citrate concentrations. Citrate stabilized these nanostructures during growth and impacted the overall solution pH and ionic strength. We observed that increased citrate concentrations initially reduced the population of rod-like structures (aspect ratios > 2). In comparison to previous studies, citrate concentration rather than pH was found to dominate the formation of these rod-like structures. Between citrate concentrations of 0.70–1.10 mM, no rod-like structures formed; however, the LSPR band of these samples dampened with increased citrate concentration. This spectroscopic characterization suggested that the number of internal and/or external atomic defects was increasing. This hypothesis was further supported via time-dependent flocculation studies which indicated that the materials with the most dampened LSPR properties were the most unstable nanoparticles. The citrate-dominated double layer likely impedes nanoparticle growth at the relatively higher citrate concentrations. It is known that nanoparticle aging reduces surface energy and defect density through atom reorganization;16,17 therefore, future studies which evaluate trends in LSPR dampening and flocculation as a function of aging and solution temperature will further validate these findings.
Notes and references
- K. R. Brown, D. G. Walter and M. J. Natan, Chem. Mater., 2000, 12, 306–313 CrossRef CAS.
- L. Cao, T. Zhu and Z. Liu, J. Colloid Interface Sci., 2006, 293, 69–76 CrossRef CAS.
- N. N. Long, L. V. Vu, C. D. Kiem, S. C. Doanh, C. T. Nguyet, P. T. Hang, N. D. Thien and L. M. Quynh, J. Phys.: Conf. Ser., 2009, 187, 012026 CrossRef.
- X. Zou, E. Ying and S. Dong, Nanotechnology, 2006, 17, 4758 CrossRef CAS.
- G. Cao, Nanostructures and Nanomaterials: Synthesis, Properties and Applications, Imperial College Press, 2004 Search PubMed.
- C. S. Weisbecker, M. V. Merritt and G. M. Whitesides, Langmuir, 1996, 12, 3763–3772 CrossRef CAS.
- K. S. Mayya, V. Patil and M. Sastry, Langmuir, 1997, 13, 3944–3947 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Langmuir, 2001, 17, 6782–6786 CrossRef CAS.
- X. Ji, X. Song, J. Li, Y. Bai, W. Yang and X. Peng, J. Am. Chem. Soc., 2007, 129, 13939–13948 CrossRef CAS.
- C. L. Haynes, A. J. Haes, A. D. McFarland and R. P. Van Duyne, in Nanoparticles with Tunable Localized Surface Plasmon Resonances, ed. J. R. Lakowicz, Plenum Press, New York, 2003 Search PubMed.
- U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Springer, 1995 Search PubMed.
- R. J. Hunter, Introduction to Modern Colloid Science, Oxford University Press, 1993 Search PubMed.
- A. Quintanilla, V. C. L. Butselaar-Orthlieb, C. Kwakernaak, W. G. Sloof, M. T. Kreutzer and F. Kapteijn, J. Catal., 2010, 271, 104–114 CrossRef CAS.
- M. N. Magomedov, Phys. Solid State, 2004, 46, 954–968 CrossRef CAS.
- W. D. Callister Jr., Materials Science and Engineering and Introduction, Wiley, 2007 Search PubMed.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- L. Bisson, C. Boissiere, C. Sanchez, C. Tomazeau and D. Uzio, Mater. Res. Soc. Symp. Proc., 2007, 1017.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Description of ionic strength calculations and TEM and optical analysis. See DOI: 10.1039/c0cc02075c |
| This journal is © The Royal Society of Chemistry 2011 |
