Selective control of surface properties using hydrodynamic interactions†‡
Hassan
Masoud
and
Alexander
Alexeev
*
George W. Woodruff School of Mechanical Engineering, Atlanta, Georgia, USA. E-mail: alexander.alexeev@me.gatech.edu; Fax: +1 404 894 8496; Tel: +1 404 385 3659
First published on 5th October 2010
Abstract
Using computational modeling, we design nano-structured surfaces able to selectively regulate interactions between microchannel walls and flowing colloid–polymer suspensions. Depending on the geometry of nanoscopic posts lining internal channel surfaces, suspended nanoparticles and polymeric chains can be either hydrodynamically attracted to channel walls or repelled to the bulk fluid.
Of many challenges in designing functional systems operating at the micro/nano scale, one is to create materials that can “sense” changes in the local environment and respond to these changes without an external control.1 In practical microfluidic applications, there is a need in materials that can dynamically modify their effective surface properties in response to variations in the surroundings, such as changes in fluid velocity. Using computational modeling, we demonstrate that regularly-spaced nanoscopic posts protruding from internal surfaces of a microfluidic channel can be harnessed to regulate the surface ability to attract polymeric chains and nanoparticles dispersed in a solvent. Depending on the flow speed and direction, the topographically patterned surfaces can either hydrodynamically attract nanoscale objects suspended in the flowing fluid or prevent their depositions by repelling them away from solid walls. Furthermore, surfaces decorated with posts can discriminate nanoscopic entities with regard to their shape and, thus, can be utilized for separating colloid–polymer mixtures.
When a colloidal suspension flows in a microfluidic channel, the competition between diffusion and hydrodynamic effects sets the spatial distribution of the colloidal particles. Whereas the intensity of diffusion is governed by the physical properties of colloids and solvent, and therefore, cannot be readily changed for a given process, the magnitude of forces that suspension entities experience due to their hydrodynamic interactions with channel walls can be modified by altering the local surface geometry. Herein, we demonstrate how nanoscale wall topography can be used for hydrodynamically regulating the density distribution of a dilute colloidal suspension composed of a mixture of nanoparticles and polymeric chains. In our system, the internal surface of a microfluidic channel is lined with a regular array of rigid nanoscale posts that are tilted relative to the flow direction (Fig. 1). These sparse-distributed posts do not interact directly with suspended colloids, but rather induce regular fluid currents that affect the colloid distribution in the microchannel. The fluid currents transport nanoparticles and polymeric chains normal to the core flow direction and, depending on the post geometry, can either enhance or suppress the suspension migration to the patterned wall. In this way, the effective properties of nano-structured surfaces can be tailored using hydrodynamic forces.
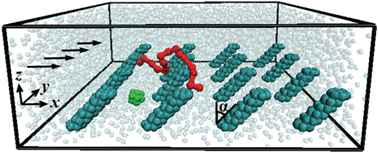 | ||
| Fig. 1 Snapshot illustrating suspension components in a microfluidic channel flow. The bottom channel wall is decorated with an array of tilted nano-posts. The arrows indicate the flow direction due to an imposed pressure gradient. For clarity, only one representative nanoparticle (green) and one polymer chain (red) are shown, whereas channel walls are not shown. | ||
We use dissipative particle dynamics2 (DPD) to examine the interactions between a nano-patterned surface and a dilute colloid–polymer suspension in a microfluidic channel formed by two parallel walls separated by a distance H (Fig. 1). DPD is a coarse-grained simulation technique that employs a momentum-conserving thermostat and soft repulsive interactions among beads representing clusters of molecules. This allows for simulations of physical phenomena occurring at relatively large time and spatial scales, while accurately capturing the relevant hydrodynamic effects. Indeed, DPD has been successfully used for simulating the dynamics of polymers and particles dispersed in Newtonian incompressible fluids.3
In DPD, the time evolution of the many-body system obeys the Newton's second law mdvi/dt = fi, where vi and fi are, respectively, the velocity and force on a bead i with mass m, and t is time. The equations of motion are integrated using the velocity-Verlet algorithm.2b The force on a bead is fi = ∑j(FCij + FDij + FRij), where the sum runs over all beads j within a cutoff radius rc around the bead i. The conservative force is given by FCij = aij(1 − ![[r with combining circumflex]](https://www.rsc.org/images/entities/i_char_0072_0302.gif) ij)
ij)![[r with combining circumflex]](https://www.rsc.org/images/entities/b_i_char_0072_0302.gif) ij, where aij is the repulsion between beads i and j,
ij, where aij is the repulsion between beads i and j, ![[r with combining circumflex]](https://www.rsc.org/images/entities/i_char_0072_0302.gif) ij = rij/rc and
ij = rij/rc and ![[r with combining circumflex]](https://www.rsc.org/images/entities/b_i_char_0072_0302.gif) ij = rij/rij with rij = |ri − rj|. The dissipative force is FDij = −γωD(rij)(
ij = rij/rij with rij = |ri − rj|. The dissipative force is FDij = −γωD(rij)(![[r with combining circumflex]](https://www.rsc.org/images/entities/b_i_char_0072_0302.gif) ijvij)
ijvij)![[r with combining circumflex]](https://www.rsc.org/images/entities/b_i_char_0072_0302.gif) ij and the random force is
ij and the random force is  , where vij = vi − vj. The coefficients γ and σ2 = 2kBTγ determine the strength of dissipative and random forces. Here, kB is the Boltzmann constant, and T is the temperature of the system. Moreover, weight functions ωD(rij) and ωR(rij) are coupled via ωD(rij) = [ωR(rij)]2, and ξij is a zero-mean Gaussian random variable of unit variance with ξij = ξji. The relations between weight functions and the strength of dissipative and random forces are set to ensure the thermodynamic equilibrium.4 The generalized form of the weight function is given by ωR(rij) = (1 −
, where vij = vi − vj. The coefficients γ and σ2 = 2kBTγ determine the strength of dissipative and random forces. Here, kB is the Boltzmann constant, and T is the temperature of the system. Moreover, weight functions ωD(rij) and ωR(rij) are coupled via ωD(rij) = [ωR(rij)]2, and ξij is a zero-mean Gaussian random variable of unit variance with ξij = ξji. The relations between weight functions and the strength of dissipative and random forces are set to ensure the thermodynamic equilibrium.4 The generalized form of the weight function is given by ωR(rij) = (1 − ![[r with combining circumflex]](https://www.rsc.org/images/entities/i_char_0072_0302.gif) ij)p with p = 1 for the standard DPD model.5 In our simulations, we set the time step Δt = 0.005, p = 0.25, m = 1, rc = 1, a = 25, kBT = 1 and the solvent number density n = 3. For all bead interactions except for solvent–solvent, we set γ = 4.5. For solvent–solvent interactions we set γss = 45 yielding the solvent kinematic viscosity ν equal to 3.4. Unless specified otherwise, all dimensional values are given in DPD units.
ij)p with p = 1 for the standard DPD model.5 In our simulations, we set the time step Δt = 0.005, p = 0.25, m = 1, rc = 1, a = 25, kBT = 1 and the solvent number density n = 3. For all bead interactions except for solvent–solvent, we set γ = 4.5. For solvent–solvent interactions we set γss = 45 yielding the solvent kinematic viscosity ν equal to 3.4. Unless specified otherwise, all dimensional values are given in DPD units.
The particle–polymer suspension is composed of polymeric chains and rigid particles immersed in a solvent. Each flexible polymeric chain consists of 16 DPD beads sequentially connected by finitely extensible nonlinear elastic (FENE) springs. The FENE potential is UFENE = −0.5ksr2max log[1 − |ri − rj|2/r2max], where ks = 10 is the stretching constant and rmax = 2 is the maximum spring extension. The polymer radius of gyration is Rg ≈ 1.33, as calculated from the equilibrium simulations. Rigid particles are constructed from 13 beads arranged in hexagonal close-packed spherical aggregates with the Stokes–Einstein radius RSE ≈ 0.7.6 The clusters of beads forming nanoparticles obey the rigid body dynamics.
The size of our computational domain is 20 × 20 × 10 in the x, y, and z directions, respectively. The top and bottom solid walls with thickness 1.5 are formed by freezing equilibrated beads beyond the channel boundaries and applying the bounce back rule at the solid–liquid interface.7 In the x and y directions, we impose a periodic boundary condition.
The bottom channel wall is decorated with an array of rigid posts arranged in a square pattern (Fig. 1). Posts are constructed using layers of 7 hexagonally close-packed beads. Each layer is rotated by 30° relative to its neighboring layers. The distance between adjacent layers is equal to the bead Stokes–Einstein diameter dSE ≈ 0.42.6 We perform the simulations for posts inclined α = ±45° (Fig. 1).
The low-Reynolds-number channel flow with Re = 0.5 is driven by a body force in the x direction that yields the particle Peclet number Pe = 10. Here, Pe> = 4VaveR2SE/HD with Vave being the averaged fluid velocity and D being the diffusion coefficient in equilibrium.
In the beginning of our simulations, we introduce a mixture of 20 polymeric chains and 20 nanoparticles that are randomly distributed in the solvent. In this dilute system, interactions between particles and chains practically do not affect the species distributions. Simulations are carried out for 107 time steps that are sufficient to collect the statistics regarding the spatial distributions inside the microchannel (ESI‡, Fig. S1). Below, we first describe the colloidal mixture in channels with non-adhesive walls with and without nanoscale posts, and then show how the combination of wall topography and adhesiveness can be harnessed for separating the mixture components.
In quiescent fluids, diffusive particles tend to distribute uniformly in the bulk fluid, whereas steric wall repulsion leads to the polymer depletion and particle ordering along fluid–solid interfaces (Fig. 2).8 When the suspension is subject to a low Re Poiseuille flow, hydrodynamic forces induce a cross-stream migration of the suspended components further decreasing the concentration near walls. This migration attributed to the non-linear interplay of hydrodynamic effects due to confining walls and shear rate gradients, changes the equilibrium distributions of suspension components (Fig. 2).9
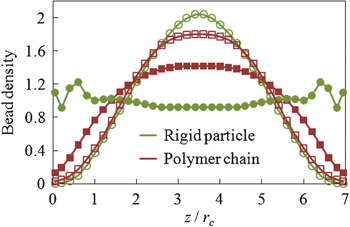 | ||
| Fig. 2 Normalized bead density profile of a dilute colloid–polymer suspension confined between two flat parallel walls in equilibrium (filled symbols) and in the presence of a unidirectional channel flow (empty symbols). | ||
To further gain control over the suspension density distribution, we introduce a regular array of posts that are attached to the channel bottom wall and tilted to an angle α = 45° in the direction of the fluid flow (Fig. 1). The gap between neighbor posts equals to 3.7 and is greater than the diameter of particles and chains to allow their free motion between posts. The post density is, however, sufficient to create near-wall fluid currents that direct particles and polymers towards the wall (ESI‡, Fig. S2). Indeed, Fig. 3a shows that the density distributions for both particles and chains exhibit peaks close to the patterned surface.10 Thus, the presence of posts makes the chemically non-adhesive surface hydrodynamically attractive to the suspended species (Fig. 3b).
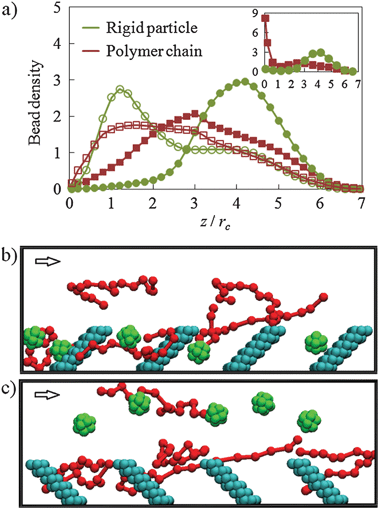 | ||
| Fig. 3 Dilute colloid–polymer suspension flowing in a microchannel lined with nanoscopic posts. Panel (a) shows normalized bead density profiles. The empty and filled symbols show the distributions in channels with posts tilted, respectively, along (α = 45°) and against (α = −45°) the fluid flow. The inset presents the density profile for a channel with a sticky wall and posts tilted α = −45°. Panels (b) and (c) show snapshots illustrating typical distributions of polymer chains (green) and nanoparticles (red) in channels with α = 45° and α = −45°, respectively. The arrows show the direction of fluid flow. For clarity, only five colloidal particles and five polymer chains are shown in each snapshot. | ||
At the limit of Re → 0, fluid flow is reversible.11 Thus, the change in post orientation relative to the flow direction should create circulatory flow structures able to repulse colloids away from the wall (ESI‡, Fig. S2). To test this, we simulated the flow of a particle–chain mixture in a channel with posts inclined α = −45°. As expected, we find that the particles are repulsed from the wall (Fig. 3c).10 This is also confirmed by the density distributions shown in Fig. 3a. Surprising, the hydrodynamics has a rather different effect on the distributions of particles and chains. Whereas particles move in the upper part of the channel, polymeric chains still remain relatively close to the bottom channel wall. We attribute this difference in cross-stream migration of particles and polymer chains to the chains' nonlinear behavior in fluid flow and possible entanglements with tilted posts.
This result indicates that patterned walls may be used for separating colloidal mixtures. To demonstrate such segregation of particles and chains, we introduce adhesion between the suspension and the bottom channel wall. To this end, we set a = 13 for the interactions of nanoparticles and chains with the structured wall. An inset in Fig. 3a shows the density distributions obtained in the simulation with an adhesive wall. Despite the fact that particles and chains are equally adhesive to the wall surface, only chains are trapped, whereas particles continue to move in the upper half of the channel. Thus, the structured wall was able to effectively separate the particle–polymer mixture.
Experimentally, our simulation results can be validated using dilute solutions of nanoparticles with the diameter about 100 nm and macromolecules with the radius of gyration about 200 nm immersed in water. In this case, the shear rate is about 1000 s−1 and post length is about 300 nm. We note that such nano-posts can be fabricated using photolithography or molding techniques.12
In summary, our simulations reveal that nanoscale patterning can be harnessed to effectively tailor the interactions between colloids and microchannel walls and separate particle–polymer mixtures. Decorated with a periodic array of nanoscopic posts, patterned walls induce regular fluid currents that hydrodynamically alter the distributions of suspended particles and polymers transported by a fluid through a microchannel. While the magnitude of hydrodynamic forces at the nanoscale is relatively weak, it is still sufficient to overcome the effect of diffusive motion, and thereby, can be used to regulate the colloid distributions. This opens an avenue for engineering smart materials with effective surface properties that vary depending on the environmental conditions.
Financial support from the ACS-Petroleum Research Fund is gratefully acknowledged.
Notes and references
- (a) T. P. Russell, Science, 2002, 297, 964–967 CrossRef CAS; (b) J. Atencia and D. J. Beebe, Nature, 2005, 437, 648–655 CrossRef CAS; (c) M. G. L. van den Heuvel, M. P. De Graaff and C. Dekker, Science, 2006, 312, 910–914 CrossRef CAS; (d) A. Alexeev and A. C. Balazs, Soft Matter, 2007, 3, 1500–1505 RSC; (e) O. B. Usta, M. Nayhouse, A. Alexeev and A. C. Balazs, J. Chem. Phys., 2008, 128, 235102 CrossRef; (f) J. Branscomb and A. Alexeev, Soft Matter, 2010, 6, 4066–4069 RSC.
- (a) P. J. Hoogerbrugge and J. M. V. A. Koelman, Europhys. Lett., 1992, 19, 155–160 CrossRef; (b) R. D. Groot and P. B. Warren, J. Chem. Phys., 1997, 107, 4423–4435 CrossRef CAS.
- (a) A. Alexeev, W. E. Uspal and A. C. Balazs, ACS Nano, 2008, 2, 1117–1122 CrossRef CAS; (b) J. A. Millan and M. Laradji, Macromolecules, 2009, 42, 803–810 CrossRef CAS.
- P. Espanol and P. Warren, Europhys. Lett., 1995, 30, 191–196 CrossRef CAS.
- X. J. Fan, N. Phan-Thien, S. Chen, X. H. Wu and T. Y. Ng, Phys. Fluids, 2006, 18, 063102 Search PubMed.
- W. X. Pan, D. A. Fedosov, G. E. Karniadakis and B. Caswell, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 78, 46706 CrossRef.
- D. A. Fedosov, I. V. Pivkin and G. E. Karniadakis, J. Comput. Phys., 2008, 227, 2540–2559 CrossRef.
- (a) D. A. Fedosov, G. E. Karniadakis and B. Caswell, J. Chem. Phys., 2008, 128, 144903 CrossRef; (b) Y. L. Chen, M. D. Graham, J. J. de Pablo, K. Jo and D. C. Schwartz, Macromolecules, 2005, 38, 6680–6687 CrossRef CAS; (c) L. Fang, H. Hu and R. G. Larson, J. Rheol. (Melville, NY, U. S.), 2005, 49, 127–138 Search PubMed.
- (a) M. Han, C. Kim, M. Kim and S. Lee, J. Rheol. (Melville, NY, U. S.), 1999, 43, 1157–1174 Search PubMed; (b) R. Khare, M. D. Graham and J. J. de Pablo, Phys. Rev. Lett., 2006, 96, 224505 CrossRef.
- We find a similar migration behavior in simulations in which only nanoparticles or chains were introduced.
- H. A. Stone, A. D. Stroock and A. Ajdari, Annu. Rev. Fluid Mech., 2004, 36, 381–411 CrossRef.
- M. K. Kwak, H. E. Jeong, T. I. Kim, H. Yoon and K. Y. Suh, Soft Matter, 2010, 6, 1849–1857 RSC.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Model validation and secondary flow structure. See DOI: 10.1039/c0cc02165b |
| This journal is © The Royal Society of Chemistry 2011 |
