Mimicking multipass transmembrane proteins: synthesis, assembly and folding of alternating amphiphilic multiblock molecules in liposomal membranes†‡
Takahiro
Muraoka
a,
Tatsuya
Shima
a,
Tsutomu
Hamada
b,
Masamune
Morita
b,
Masahiro
Takagi
b and
Kazushi
Kinbara
*a
aInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1, Katahira, Aoba-ku, Sendai 980-8577, Japan. E-mail: kinbara@tagen.tohoku.ac.jp; Fax: +81 22-217-5612; Tel: +81 22-217-5612
bSchool of Materials Science, Japan Advanced Institute of Science and Technology, 1-1, Asahidai, Nomi, Ishikawa 923-1292, Japan
First published on 17th September 2010
Abstract
Alternating amphiphilic multiblock molecules 1–4, involving fluorescent hydrophobic units, were designed as mimics for multipass transmembrane proteins. Fluorescence spectroscopy of 1–4 in liposomal membranes suggested the face-to-face stacking of the hydrophobic units to give folded structures as well as intermolecular assemblies.
Synthetic molecules mimicking the structure and function of proteins have been attracting keen interest for development of molecular devices and medicines.1Membrane proteins are important targets, where several synthetic ion channels,2 including tubes,3 ribbons,4 and barrels as mimics of β-barrel structures5 have been developed so far. On the other hand, multipass transmembrane (MTM) proteins remain mostly unexplored targets, although they provide one of the major structural motifs of the membrane proteins.6MTM proteins usually consist of α-helices connected by hydrophilic residues, where their ternary structures are stabilized by the helix–helix interaction.7 In this communication, as simple structural mimics of MTM proteins, we report alternating amphiphilic multiblock molecules 1–4, consisting of linearly connected hydrophilic and hydrophobic moieties (Scheme 1). We found that the hydrophobic units of 1–4, 1,4-bis(4-phenylethynyl)benzene (BPEB) units, tend to form face-to-face stacking in the membrane, to possibly give folded structures8 like MTM proteins (Fig. 1).
 | ||
| Scheme 1 Molecular structures of 1–4. | ||
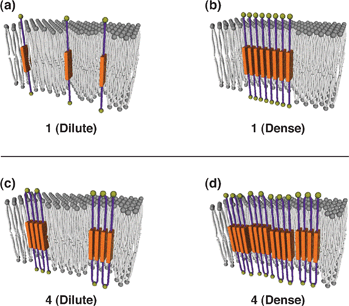 | ||
| Fig. 1 Plausible models for the assemblies of 1 and 4 in a liposomal membrane. (a) 1 and (c) 4 in dilute conditions. (b) 1 and (d) 4 in dense conditions. | ||
The BPEB unit is known to fluoresce at 390 nm upon excitation around 320 nm.9 Since the face-to-face stacking of BPEB units results in a bathochromic shift of the emission band (λem = 440 nm), the assembling events of this unit are able to be monitored by means of fluorescence spectroscopy. BPEB units of 2–4 were connected by hydrophilic units composed of a benzoate group bearing two tetraethylene glycol chains. 1–4 were prepared by repeating Williamson synthesis between phenolic OH and TEG tosylate (see Supplementary Information‡). Finally, hydroxyl groups were introduced to both termini of 1–4. 1–4 tend to be soluble in relatively polar organic solvents such as THF, DMF and DMSO, while hardly soluble in water and non-polar solvents such as hexane and CH2Cl2.
First, we investigated the aggregation behavior of BPEB units of 1–4 in solution. The fluorescence spectra of 1–4 in THF (2.0 × 10−5 M) showed two emission peaks (λex = 313 nm) in all cases, where no obvious difference was observed in the spectral profiles among them (λem; 1: 367, 384 nm, 2: 366, 384 nm, 3: 367, 385 nm, 4: 367, 384 nm) (Fig. 2, blue lines). These emission profiles indicate that the face-to-face stacking of BPEB units hardly occurs in THF.9 In sharp contrast, in a THF–water (1/9) mixture, 1–4 (2.0 × 10−5 M) showed red-shifted emission peaks in each case (λex = 313 nm, λem = 1: 426, 451 nm, 2: 427, 450 nm, 3: 450 nm, 4: 428, 452 nm) (Fig. 2, red lines). Moreover, absorption spectra of 1–4 showed a hypsochromic shift in a THF–water (1/9) mixture compared with those in THF (see Supplementary Information Fig. S1‡). These spectral changes strongly indicate that BPEB units form cofacial planar H-aggregates in aqueous conditions. Dynamic light-scattering (DLS) analyses of 1–4 (2.0 × 10−5 M) in a THF–water (1/9) mixture revealed the presence of particles with average hydrodynamic diameters of ca. 100 nm in each case (see Supplementary Information Fig. S2‡).
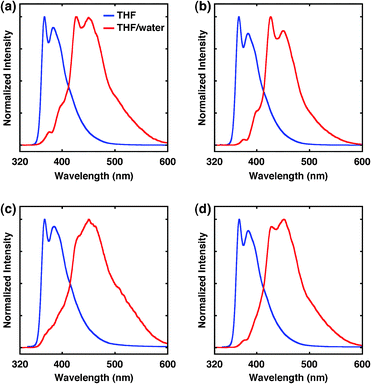 | ||
| Fig. 2 Fluorescence spectra of (a) 1, (b) 2, (c) 3 and (d) 4 in THF (blue) and THF–water = 1/9 (red) with excitation at 313 nm. | ||
The aggregation behavior of 1–4 in the liposomal membranes was then investigated using giant unilamellar liposomes of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), which can be directly observed by optical microscopy.10 Actually, phase-contrast microscopy (200 μM DOPC containing 1–4, [1–4]/[DOPC] = 0.10) clearly visualized μm-sized unilamellar liposomes in water (Fig. 3a–d). Of importance, these liposomes were also visualized by fluorescence microscopy monitoring at >420 nm (λex = 330–385 nm) (Fig. 3e–h), which demonstrates successful incorporation of 1–4 into the liposomal membranes under these conditions.
![Optical micrographs of DOPC giant unilamellar liposomes containing (a), (e) 1, (b), (f) 2, (c), (g) 3 and (d), (h) 4 ([1–4]/[DOPC] = 0.10). (a)–(d) Phase-contrast and (e)–(h) fluorescence micrographs (λex; 330–385 nm, λobsd; >420 nm). Scale bars: 10 μm.](/image/article/2011/CC/c0cc02420a/c0cc02420a-f3.gif) | ||
| Fig. 3 Optical micrographs of DOPC giant unilamellar liposomes containing (a), (e) 1, (b), (f) 2, (c), (g) 3 and (d), (h) 4 ([1–4]/[DOPC] = 0.10). (a)–(d) Phase-contrast and (e)–(h) fluorescence micrographs (λex; 330–385 nm, λobsd; >420 nm). Scale bars: 10 μm. | ||
Fluorescence spectroscopy of DOPC liposomes prepared with 1 in water (200 μM DOPC containing 1, [1]/[DOPC] = 0.00050) showed emission peaks at 366 and 384 nm (λex = 315 nm, Fig. 4a). The spectral profile is quite similar to that of 1 in THF (Fig. 2a, blue line). Namely, at this concentration, 1 does not form the face-to-face assemblies in the membrane (Fig. 1a). However, of interest, when the amount of 1 was increased, broad emission appeared at >400 nm with a decrease in the intensity of the original emission. At molar ratio [1]/[DOPC] = 0.10, the spectrum showed the maximum emission at 426 nm. Hence, the BPEB unit of 1 is capable of assembling in the membrane to form face-to-face stacking at relatively high concentrations (Fig. 1b). In sharp contrast to 1, 4 in the membrane showed emission bands at 428 and 448 nm (λex = 313 nm) even at very low concentration ([4]/[DOPC] = 0.00050, Fig. 4d). Furthermore, 200-fold increase in the amount of 4 ([4]/[DOPC] = 0.10) resulted in only a slight change in the spectral profile; the fluorescence exhibited little dependency on the concentration of 4. Thus, BPEB units of 4 are likely to form intramolecular face-to-face stacking, allowing 4 to adopt a folded structure like MTM proteins (Fig. 1c). However, slightly intensified emission at λem > 480 nm (Fig. 4d) also suggests a possible contribution of intermolecular stacking at higher concentrations ([4]/[DOPC] > 0.0050) (Fig. 1d). Interestingly, not only 4 but also 2 bearing two BPEB units showed emission at 426 nm (λex = 313 nm) even at a highly diluted condition ([2]/[DOPC] = 0.00050), while 3 showed red-shifted emission (425 nm) only at high concentrations ([3]/[DOPC] = 0.050–0.10). This apparent even–odd effect may suggest formation of dimeric assemblies of BPEB units, which play some roles in folding and assembling of 1–4 in the membrane. It is worthy of note that in spite of intermolecular assembling of 1–4 in the membrane, no μm-scale domain structure like lipid rafts11 was observed by fluorescence microscopy of the liposomes including 1–4.§
![Fluorescence spectra of 200 μM DOPC giant unilamellar liposomes in water containing (a) 1, (b) 2, (c) 3 and (d) 4 with excitation at 315 nm. [1–4]/[DOPC] = 0.00050, 0.0050, 0.050 and 0.10.](/image/article/2011/CC/c0cc02420a/c0cc02420a-f4.gif) | ||
| Fig. 4 Fluorescence spectra of 200 μM DOPC giant unilamellar liposomes in water containing (a) 1, (b) 2, (c) 3 and (d) 4 with excitation at 315 nm. [1–4]/[DOPC] = 0.00050, 0.0050, 0.050 and 0.10. | ||
In conclusion, the BPEB unit was found to have strong capabilities to be involved in a liposomal membrane and form face-to-face stacking. These features could be applied for controlled folding of molecules as was found for alternating amphiphilic multiblock molecules 2 and 4, which can be regarded as structural mimics of two- and four-transmembrane proteins, respectively. MTM proteins exhibit diverse functions, such as cell-adhesion of four-transmembrane proteins, claudins,12 ion permeation of tetrameric two-transmembrane proteins, potassium channels,13 and signal transduction of seven-transmembrane proteins, G proteins.14 The functions of 1–4 are now under investigation.
We thank Prof. M. Shimomura and Dr T. Higuchi for assistance in DLS measurements. We also thank Ms Yuko Kishimoto for support in preparation and microscopy of liposomes. This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices (Institute of Multidisciplinary Research for Advanced Materials, Tohoku University)”, and partially supported by the Ministry of Education, Science, Sports and Culture, Japan, Grant-in-Aid for Young Scientists S and Asahi Glass Foundation to K. K.
Notes and references
- H.-A. Klok, Angew. Chem., Int. Ed., 2002, 41, 1509 CrossRef CAS; S. M. Butterfield and J. Rebek, Jr., J. Am. Chem. Soc., 2006, 128, 15366 CrossRef CAS; O. Khakshoor, B. Demeler and J. S. Nowick, J. Am. Chem. Soc., 2007, 129, 5558 CrossRef CAS; J. A. Robinson, Chimia, 2007, 61, 84 CrossRef CAS.
- A. L. Sisson, M. R. Shar, S. Bhosale and S. Matile, Chem. Soc. Rev., 2006, 35, 1269 RSC; A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev., 2007, 36, 348 RSC; G. W. Gokel and N. Barkey, New J. Chem., 2009, 33, 947 RSC.
- I. Tabushi, Y. Kuroda and K. Yokota, Tetrahedron Lett., 1982, 23, 4601 CrossRef CAS; J.-H. Fuhrhop, U. Liman and U. Koesling, J. Am. Chem. Soc., 1988, 110, 6840 CrossRef CAS; M. R. Ghadiri, J. R. Granja and L. K. Buehler, Nature, 1994, 369, 301 CrossRef CAS; T. M. Fyles and C. C. Tong, New J. Chem., 2007, 31, 655 RSC; M. Jung, H. Kim, K. Baek and K. Kim, Angew. Chem., Int. Ed., 2008, 47, 5755 CrossRef CAS.
- M. A. Schmitt, B. Weisblum and S. H. Gellman, J. Am. Chem. Soc., 2004, 126, 6848 CrossRef CAS.
- W.-Y. Yang, J.-H. Ahn, Y.-S. Yoo, N.-K. Oh and M. Lee, Nat. Mater., 2005, 4, 399 CrossRef CAS; C. P. Wilson and S. J. Webb, Chem. Commun., 2008, 4007 RSC; N. Sakai, J. Mareda and S. Matile, Acc. Chem. Res., 2008, 41, 1354 CrossRef CAS; A. Satake, M. Yamamura, M. Oda and Y. Kobuke, J. Am. Chem. Soc., 2008, 130, 6314 CrossRef CAS; R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nat. Chem., 2010, 2, 533 Search PubMed.
- P. J. F. Henderson, Curr. Opin. Cell Biol., 1993, 5, 708 CrossRef CAS; E. Wallin and G. von Heijne, Protein Sci., 1998, 7, 1029 CAS; D. Oesterhelt, Curr. Opin. Struct. Biol., 1998, 8, 489 CrossRef CAS; S. Subramaniam, Curr. Opin. Struct. Biol., 1999, 9, 462 CrossRef CAS.
- J. H. Perlman, A.-O. Colson, W. Wang, K. Bence, R. Osman and M. C. Gershengorn, J. Biol. Chem., 1997, 272, 11937 CrossRef CAS; G. V. Nikiforovich, M. Zhang, Q. Yang, G. Jagadeesh, H.-C. Chen, L. Hunyady, G. R. Marshall and K. J. Catt, Chem. Biol. Drug Des., 2006, 68, 239 CrossRef CAS; Molecular Biology of the Cell, ed. B. Alberts, A. Johnson, J. Lewis, M. Raff and K. Roberts, Garland Science, New York, USA, 2007, 5th edn, p. 632 Search PubMed; W.-K. Lee, J. J. Han, B.-S. Jin, D. W. Boo and Y. G. Yu, Biochem. Biophys. Res. Commun., 2009, 390, 815 Search PubMed; J.-Y. Shim, Biophys. J., 2009, 96, 3251 CrossRef CAS.
- Y. Zhao and J. S. Moore, Foldamers: Structure, Properties, and Applications, ed. S. Hecht and I. Huc, Wiley-VCH, Weinheim, Germany, 2007, pp. 75–108 Search PubMed; J. J. van Gorp, J. A. J. M. Vekemans and E. W. Meijer, Chem. Commun., 2004, 60 Search PubMed.
- M. Levitus, K. Schmieder, H. Ricks, K. D. Shimizu, U. H. F. Bunz and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2001, 123, 4259 CrossRef CAS.
- T. Hamada, Y. Miura, K. Ishii, S. Araki, K. Yoshikawa, M. Vestergaard and M. Takagi, J. Phys. Chem. B, 2007, 111, 10853 CrossRef CAS; K. Ishii, T. Hamada, M. Hatakeyama, R. Sugimoto, T. Nagasaki and M. Takagi, ChemBioChem, 2009, 10, 251 CrossRef CAS; M. Morita, M. Vestergaard, T. Hamada and M. Takagi, Biophys. Chem., 2010, 147, 81 CrossRef CAS.
- J. F. Hancock, Nat. Rev. Mol. Cell Biol., 2006, 7, 456 CrossRef CAS; T. Hamada, M. Morita, Y. Kishimoto, Y. Komatsu, M. Vestergaard and M. Takagi, J. Phys. Chem. Lett., 2010, 1, 170 Search PubMed.
- L. Elkouby-Naor and T. Ben-Yosef, Int. Rev. Cell Mol. Biol., 2010, 279, 1 Search PubMed.
- D. A. Doyle, J. M. Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait and R. MacKinnon, Science, 1998, 280, 69 CrossRef CAS.
- K. L. Pierce, R. T. Premont and R. J. Lefkowitz, Nat. Rev. Mol. Cell Biol., 2002, 3, 639 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Synthesis, UV-Vis absorption spectra and profiles of dynamic light scattering analyses of 1–4, and preparation of giant liposomes. See DOI: 10.1039/c0cc02420a |
| § Circular dichroism spectra of 2 and 4 gave no significant signal even at high concentration ([2, 4]/[DOPC] = 0.10, [DOPC] = 200 μM) in a long light path length quartz cuvette (100 mm). |
| This journal is © The Royal Society of Chemistry 2011 |
