Evaluation of arsenic biotransformation by Iberian green frog during metamorphosis†
Malgorzata A.
Bryszewska
a,
Estrella
Sanz
b,
Jon
Sanz-Landaluze
b,
Riansares
Muñoz-Olivas
b,
Manuel E.
Ortiz-Santaliestra
c and
Carmen
Cámara
*b
aTechnical University of Lodz, Faculty of Biotechnology and Food Sciences, Institute of General Food Chemistry, ul. Stefanowskieg 4/10, 90-924, Lodz, Poland. E-mail: malgorzata.bryszewska@p.lodz.pl
bUniversidad Complutense Madrid, Facultad de Ciencias Químicas, Dpto. Química Analítica, Ciudad Universitaria s/n, 28040, Madrid, Spain. E-mail: rimunoz@quim.ucm.es; ccamara@quim.ucm.es; Fax: (+34) 91 3944329; Tel: (+34) 913944318
cUniversidad de Salamanca, Departamento de Biología Animal, Facultad de Farmacia, 5a planta, Campus Miguel de Unamuno, 37007, Salamanca, Spain
First published on 30th November 2010
Abstract
This study examined the effects of arsenic exposure and the biotransformation capacity of developing Iberian green frogs (Rana perezi) with the main aim of finding valid biomarkers to monitor pollution and toxicological problems. Total arsenic concentration and arsenic species distribution were determined using liquid chromatography coupled with inductively coupled plasma-mass spectrometry (LC-ICP-MS). The level of accumulation of arsenic and its species distribution differed depending on the metamorphosis stage and level of exposure. Tetramethylarsonium cation was the major compound detected and continuously increased in concentration during frog development. Results suggest that rather low As accumulation is obtained and that Rana perezi metabolised arsenic during embryonic and larval development.
1. Introduction
Arsenic is widely distributed in the environment and has both natural and anthropogenic sources. Industrial applications, such as the smelting of metal ores, and the use of arsenical pesticides and wood preservatives may release arsenic directly into the environment. Inorganic species have been measured predominantly in water and soil, whereas organic compounds are mainly found in living organisms. Acute and chronic exposure to arsenic is a growing problem in the world.1 The toxicity of some arsenic compounds is well established and is critically dependent on the chemical nature of the compound. Some forms, such MMAV (monomethylarsinic acid) and DMAV (dimethylarsenic acid), have been identified as carcinogens.2,3Recently, arsenic metabolism in animals and humans has been intensively studied and researchers have documented significant variations in the metabolic conversion of inorganic arsenic to methylated derivatives.4 Dogs and mice methylate inorganic arsenicals rapidly and excrete at least 80% as DMAV in urine and very little MMA.5 Gnawings excrete only 1–5% arsenic as MMAV, while humans excrete 10–20% as MMAV which suggests that the methylation of inorganic arsenic occurs more slowly than for other mammal species.6–8 In fish and crustaceans, arsenobetaine (AsB) is the dominant species, usually comprising more than 80% of the total arsenic.9–11 In marine algae, arsenosugars (dimethylarsinoyl- and trimethylarsonioribosides) were found to be the dominant compounds.12–17 Production of arsenolipids by seaweed18 and marine animals19 has also been reported.
Currently, metabolic pathways of arsenic are only partially identified. The processes involved in the production of MMAV and DMAV are relatively well established, while processes occurring during the synthesis of trimethylarsonium species, arsenobetaine, and arsenocholine (AsC) are not yet well understood. Metabolism is generally thought to involve consecutive enzyme-catalysed steps that reduce AsV to AsIII, followed by oxidative methylation of AsIII. Historically, DMAV has been considered to be less acutely toxic than inorganic As species, and this observation led to the thesis that the methylation of inorganic As serves as a detoxification mechanism. However, technological progress and analytical improvements have allowed for the detection of MMAIII and DMAIII in the urine of individuals exposed to inorganic As,20 and these trivalent methylated compounds were shown to be even more toxic than inorganic As species.21,22 Thus, trivalent organic arsenic compounds serve as intermediates in pathways that both activate and mitigate As toxicity.
Variability in the sensitivity of organisms to a pollutant is generally a consequence of differences in morphology, physiology, and environmental settings. Animals with complex life cycles, such as amphibians, undergo drastic rearrangements in morphology, physiology and habitat requirements and the toxicity of a pollutant may thus vary throughout their development. The use of amphibians as toxicological biomarkers is well established and takes advantage of their position in the trophic chain, the combination of aquatic and terrestrial habitats that may be used to study changes in both ecosystems, and the well-known biology of these animals.23 Different patterns of retention and elimination of elements during amphibian development have been associated with the structural reorganization and remodelling of organ systems that occur during the metamorphosis.24,25
The aim of this work was to examine the bioaccumulation and biotransformation of arsenic by one of this amphibians, Iberian green frog (Rana perezi). The main goals of this study were: i) to develop a method for the separation of the different arsenic compounds in only one run ii) to estimate the As accumulation in the different development stages of the Iberian green frog; iii) to evaluate arsenic biotransformation by identifying and quantifying As species during the metamorphosis process.
2. Materials and methods
2.1 Reagents and standards
All sample and standard solutions used in the experiments were prepared using high-purity deionised water (Milli-Q Element system, Millipore, USA). Mobile phases for high pressure liquid chromatography (HPLC) were prepared using formic acid (85%, Panreac, Barcelona, Spain), ammonium formate (95% Panreac, Barcelona, Spain), 1,2 benzene disulfonic acid, dipotassium salt (99%, Aldrich, Munich, Germany), and methanol (99.8%, Carlo Erba-SDS, Milan, Italy). Concentrated nitric acid (Merck, Darmstadt, Germany) was further purified in a Teflon sub-boiling distillation unit.The following arsenic standards were used: monomethylarsonic acid and dimethylarsinic acid (98% purity), As2O5·2H2O (98.5%) were purchased from Merck (Darmstadt, Germany); As2O3 (99.5%) was purchased from J.T.Baker (Deventer, Holland); arsenobetaine and arsenocholine (both 99%) were prepared from Tri Chemical Laboratory Inc. (Yamanashi, Japan). Trimethylarsine oxide (TMAO) and tetramethylarsonium cation (TMA+) were kindly donated by Prof. Dr Kevin A. Francesconi (Karl Franzens University, Graz, Austria). Stock solutions of standards were prepared in water at concentrations of one gram per litre and stored at 4 °C in high density polyethylene (HDPE) bottles until use. Working solutions were prepared daily.
Certified Reference Material 627, arsenic (total arsenic, arsenobetaine and dimethylarsenic acid) in tuna fish tissue, was purchased from IRMM - Institute for Reference Materials and Measurements (Geel, Belgium)
2.2 Instrumentation
An ultrasonic homogenizer, model Sonopuls HD 2200 (Bandelin, Germany), equipped with a converter UW 2200, a SH 213 G horn as amplifier, and sonotrode MS 73 (3 mm titanium microtip) was used for sample treatment. A centrifuge (model 5415 R Eppendorf, Hamburg, Germany) was used to separate the aqueous fraction from insoluble pellets after the extraction step.The chromatographic system consisted on a LC pump (model PU-2080 Plus, JASCO Corporation, Tokyo, Japan) and analytical columns IonPac AS-7 (Dionex, Olten, Switzerland) with a IonPac AG7 guard column (Dionex), and PRP-X200 (Hamilton, Bonaduz, Switzerland). The column effluent was directly introduced into the nebulizer of the ICP-MS. The samples were injected through a six-port valve (Rheodyne 9125, Connecticut, USA).
The ICP-MS system used for As determination was an HP-4500 Plus (Agilent Technologies, Analytical System, Tokyo, Japan), equipped with a Babington nebulizer, Fassel torch, double pass Scott-type spray chamber cooled by a Peltier system. Single ion monitoring at m/z 75 was performed. Monitoring of Cl (m/z 35) species was carried out in order to evaluate possible ArCl (m/z 75) polyatomic interference. The ICP-MS operating conditions used for total As determination and for arsenic speciation along with chromatographic parameters are summarised in Table 1.
| ICP-MS | |
|---|---|
| RF power: | 1280 W |
| Ar flow rate plasma gas: | 15.3 L min−1 |
| Nebulizer gas: | 1 L min−1 |
| Auxiliary gas: | 0.9 L min−1 |
| Isotope monitored: | 75As |
| Chromatography | ||
|---|---|---|
| Anion-exchange chromatography | Cation-exchange chromatography | |
| Column | Dionex: IonPac AS7 (4 × 250 mm) | Hamilton: PRP-X200 (10 × 250 mm) |
| Injection volume | 100 μL | 100 μL |
| Flow rate | 1 mL min−1 | 1 mL min−1 |
| Mobile phase composition | A: pH 3.4; 0.4 mM HNO3; 0.5% methanol (v/v) | pH 2.8; 36 mM formic acid, 2 mM ammonium formate, benzene disulfonic acid, dipotassium salt, 2% methanol (v/v). |
| B: pH 1.3; 50 mM HNO3; 0.5% methanol (v/v) | ||
| Program | Gradient mode: | Isocratic mode |
| 0–2 min: 100% A | ||
| 2–7 min: change to B with linear slope | ||
| 7–15 min. 100% B | ||
| 15–20 min: change to A with linear slope | ||
| 22: 100% A | ||
2.3 Arsenic exposure study design
Fresh eggs (<24 h) from five different clutches of R. perezi were collected in Prado de las Pozas (Sierra de Gredos, Central Spain). This mountainous and pollutant free location was selected because of the expected low arsenic content and the high density of R. perezi. The arsenic content in natural water samples was measured and found below 10 μg L−1 (the allowed level in tap water). The exposure study was conducted in the laboratory, under static conditions, at the ambient temperature (22 °C) and normal sunlight photoperiod. One hundred eggs (20 per clutch × 5 clutches) were introduced to one of the nine independent tanks filled with 7 L of carbon-filtered water. The tanks were randomly assigned as control (no arsenic added), and two exposure experiments (50 μg As L−1, and 100 μg As L−1). Each exposure experiment was performed in triplicate. These exposure levels were selected considering the OCDE Bioconcentration Test (OCDE/305) which sets the highest concentration of the test substance at about 1% of LC50.26,27 This test evaluates the accumulation of a dissolved chemical in adult fish, by measuring its concentration in both the fish and the surrounding media after an equilibration time (bioconcentration factor, BCF). Literature gave us an LC50 interval for amphibians tadpoles of 5–100 mg L−1.23 Then, the two As concentrations selected are included in the interval.During the experiments, water in the tanks was renewed and recharged with the same As concentration every 12 days. The pH of the tanks were controlled by daily measuring. Taking into consideration the possible As instability in the exposure solution and how important it is to monitor the concentration for the bioconcentration factor (OCDE 305 test), the concentration of arsenic was determined at the different sampling times.
Beginning from the day of the hatch, tadpoles were fed with 5 mg of cooked lettuce per individual until the 36th day, when the lettuce amount was increased to 10 mg per individual. The lettuce As content was 106 ± 4 ng g−1. Samples were collected at six time points during the experiments and correspond to the six R. perezigrowth phases as suggested by Gossner's developmental stages:28phase 1 (day 5)—hatchlings; phase 2 (day 20)—complete operculum and gills internalized; phase 3 (day 45)—hind limbs emerged; phase 4 (day 55)—knee joint formed; phase 5 (day 66)—forelimbs emerged; phase 6 (day 72)—tail reabsorbed, metamorphosis finished. The experiments were completed after day 72, when metamorphosis is considered to be completed. The number of dead individuals was annotated during each time to monitor the lethal effects of As over time. The arcosin of square root-transformed mortality rates were analysed by ANOVA to assess differences between exposure and control experiments.
On the day of sample collection, the animals were removed and washed with distilled water and excess water was removed with paper towels. For euthanasia, they were immersed in ethanol absolute. Samples were frozen (−20 °C) and stored until the analyses were performed. Defrosted samples were dried in an oven at 55 °C, then manually ground and homogenised prior to analysis.
2.4 Determination of arsenic by ICP-MS
Arsenic content was measured in the acidic digests and in the aqueous extracts of tadpole by flow injection analysis coupled to ICP-MS (FIA-ICP-MS) using 2% distilled nitric acid as carrier. The As concentration was calculated using the standard addition calibration method.The procedure of acidic digestion consisted of the addition of 0.75 mL of distilled nitric acid and 0.3 mL H2O2 to 10 mg of the grounded sample. Then closed teflon reactors were kept at room temperature for 2 h for pre-digestion and then overnight at 110 °C in an oven. After cooling, the digests were transferred to 5 mL volumetric flasks and diluted with Milli-Q water. This procedure was performed in triplicate for each sample. Two samples of the certified reference material CRM-627, tuna fish tissue, were also included in each acid digestion batch along with procedural blank.
Arsenic determination in the aqueous extracts was performed by suspending 10–15 mg of the sample in 1 mL of Milli-Q water, and the vials with diluted samples were then sonicated in an ice water bath to prevent samples from overheating. The ultrasonic probe was then introduced into the solution and a single sonication for 30 s and a 20% amplitude was applied. The vials were then centrifuged at 4000 rpm for 20 min, at 10 °C. Supernatant fractions were separated from pellets and filtered through a 0.22 μm nylon syringe filter before analysis.
2.5 Determination of arsenic species by LC-ICP-MS
Aliquots of the aqueous extracts described above were used for As speciation analysis. A LC-ICP-MS method was developed, described in the results and discussion, by using the As-7 Dionex anion exchange column. For those samples containing high levels of cationic species poorly resolved by the previous column were separated also by cation exchange chromatography employing a PRP-X200 column. In order to verify the reproducibility of the procedure, the extraction was performed in duplicate, and repeated experiments were performed on different days. The extracted samples were analysed by LC-ICP-MS on the same day due to the possible instability of As compounds in the extracts.2.6 Quality assurance
Once the separation was optimized, repeatability and reproducibility of quantitative analysis at three concentration levels (0.01; 0.05 and 0.1 ng g−1 of each compound) was evaluated. The relative standard deviation data were calculated based on retention time and peak area obtained after five injections spread over one day measurement (repeatability) or four injections done on different days. The obtained results are summarised in Table 2. Column recovery calculated for Dionex IonPac AS7 column was 95%–116% and 92–101% for Hamilton PRP-X200.| Dionex IonPac As7 | Hamilton PRP-X200 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| iAsIII | MMA | DMA | iAsV | AsB | TMAO | AsC | TMA+ | TMA+ | |
| Retention time | |||||||||
| Repeatability | 0.7 | 1.2 | 0.8 | 0.3 | 0.7 | 0.6 | 0.6 | 0.7 | 2.3 |
| Reproducibility | 9.1 | 9.6 | 9.4 | 11.4 | 6.9 | 5.4 | 9.9 | 8.9 | 9.7 |
| Peak area | |||||||||
| Repeatability | 0.7 | 8.6 | 13.6 | 2.0 | 6.8 | 7.0 | 7.8 | 8.5 | 9.2 |
| Reproducibility | 8.4 | 11.5 | 13.2 | 8.3 | 7.8 | 7.6 | 8.7 | 7.3 | 9.5 |
The detection limit calculated as three times the standard deviation of the blank divided by the calibration slope, was, under these conditions 14.0, 18.9, 19.7, 14.3, 17.8, 14.4, 7.8, and 15.8 ng L−1 for AsB, iAsIII, DMA, MMA, iAsV, TMA+, AsC and TMAO, respectively. Quantification was based on peak area measurements.
Arsenic concentration found in the CRM 627 Tuna Fish Tissue was within the 94.3% confidence interval of certified values (certified value: 4.8 ± 0.3 μg g−1, determined value: 4.5 ± 0.2 μg g−1), demonstrating the good accuracy of the proposed method.
3. Results and discussion
3.1 Arsenic accumulation
The concentration of arsenic found in R. perezi samples is summarised in Table 3. Animals showed the ability to absorb the element from the exposure solution, and the As content in the animal bodies was strongly dependent on the developmental stage. Analysis of tadpoles after 5 days of exposure revealed very low content of arsenic (ranging from 200–300 ng g−1). Samples began to exhibit significant differences during the second developmental phase observing the highest accumulation after 45 days of exposure, being the arsenic concentration 9.7 and 15 times higher than control for exposure to 50 and 100 ng g−1 tanks, respectively. In addition, the accumulation of As in tadpole tissue was linearly related to exposure time up to the third phase and drastically decreased after this point for both exposure levels. This accumulation tendency may suggest that absorption of As was not passive and that some mechanisms regulate the uptake of arsenic from the exposure solution. Additionally, increased concentration of As in the exposure solution resulted in increased accumulation of arsenic. However, the bioconcentration factors (BCF's) gave very similar values in both experiments. It is relevant to mention here that the analysis of the exposure solution at the different sampling times remained constant (±10% of the nominal level), accordingly with OCDE 305 Test requirements.| AsV Exposure dose/ng g−1 | Exposure days | |||||
|---|---|---|---|---|---|---|
| 5 (phase 1) | 20 (phase 2) | 45 (phase 3) | 55 (phase 4) | 66 (phase 5) | 72 (phase 6) | |
| a BCF: As tadpole concentration/As exposure solution concentration. | ||||||
| 0 | 250 ± 5 | 240 ± 8 | 230 ± 9 | 220 ± 2 | 100 ± 2 | 190 ± 8 |
| 50 | 200 ± 9 | 900 ± 10 | 1800 ± 11 | 1060 ± 10 | 608 ± 10 | 440 ± 8 |
| 100 | 310 ± 20 | 1500 ± 19 | 3200 ± 24 | 2440 ± 32 | 890 ± 11 | 920 ± 45 |
| BCF (50) | 4 | 18 | 34 | 22 | 11 | 10 |
| BCF (100) | 3 | 15 | 31 | 24 | 10 | 9 |
| F | 0.528 | 18.645 | 4.336 | 12.566 | 129.921 | 11.306 |
| df | 2.6 | 2.5 | 2.6 | 2.6 | 2.6 | 2.6 |
| P | 0.615 | 0.005 | 0.068 | 0.007 | <0.001 | 0.009 |
Mortality rates at the end of experiments were 1.7% in the control, 4.7% at concentration of 50 ng g−1 and 9.7% at 100 ng g−1. Despite these slight differences, lethality caused by As was not statistically significant (ANOVA: F2,6 = 3.189; P = 0.114). Apart from deaths, no sub-lethal effects that altered individual behaviour or development were observed.
The most striking observation of the exposure experiment was that As accumulation can be considered in a low ratio (maximum BCF = 36). These data are in good agreement with those summarized in the database METI-NITE Japan BCF (<4 at concentration 50 μg L−1 and BCF < 38 at concentration 5 μg L−1)29 and by the results obtained recently by other authors (BCF = 15–17 for Corbicula Fluminea30 and maximum values of 35 for ten freshwater fish species31). These data were also obtained according to OCDE 305 Guidelines for bioaccumulation in fish species. It is relevant as well to underline that BCF's obtained in this work fitted well with the low Kow of arsenic (Kow = 0.74).
Despite the low accumulation of the animals, they were able to eliminate As from their tissue as they approached the end of the larval stage, and this may have resulted from differences in surface area to volume ratio and skin permeability. Our results are similar to those reported by Roe et al.,32 who observed a lower concentration of As in Rana sphenocephala and Bufo terrestris metamorphic animals compared to that of larvae. Decreases in the accumulation of As in exposed animals during larval development and metamorphosis could have resulted from a lower absorption of As from the exposure solution or from an enhanced ability to eliminate the element. The latter hypothesis was tested by analysing the biotransformation of As species throughout development of R. perezi.
3.2 Arsenic speciation analysis
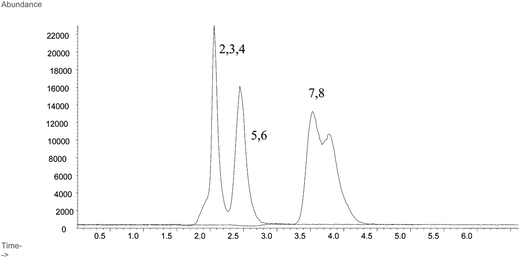 | ||
| Fig. 1 LC-ICP-MS chromatograms for the separation of arsenic standards at 1 ng As g−1 using Shodex Asahipak GS-220 HQ column. Peaks representing As standard compounds are labelled as: 1. iAsIII; 2. MMA; 3. DMA; 4. iAsV; 5. AsB; 6. TMAO; 7. AsC; 8. TMA+. | ||
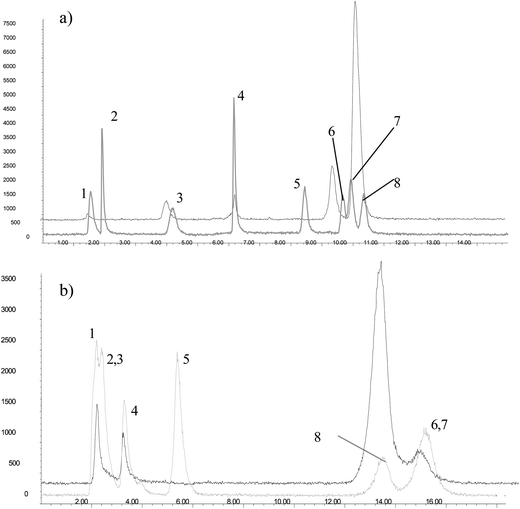 | ||
| Fig. 2 LC-ICP-MS chromatograms for the separation of arsenic standards at 1 ng As g−1 (grey colour) and As compounds from aqueous extracts of R. perezi at phase 6 and As exposure: 50 ng As g−1 (black colour) using a) a Dionex AS-7 column, or b) a cation exchange column, Hamilton PRP-X200. Peaks representing As standard compounds are labelled as: 1. iAsIII; 2. MMA; 3. DMA; 4. iAsV; 5. AsB; 6. TMAO; 7. AsC; 8. TMA+. Conditions applied for the separation are described inTable 1. | ||
Based on the Dionex IonPac AS7 column composition: strong anion-exchange and medium-high hydrophobicity, 2% cross-linking and an alkyl quaternary ammonium functional group, the order of elution of the different species (iASIII, MMA, DMA, iAsV, AsB, TMAO, AsC and TMA+) should be attributed to a combination of ionic and hydrophobic interactions with the stationary phase. In the pH range of the mobile phase, between 3.4 and 1.4, arsenious acid (pKa = 9.2) is not charged, thus being the first compound eluted. iAsV and DMA are present as anions (first pKa = 2.3 and 1.6, respectively) at the beginning of the gradient so they should be strongly retained in the stationary phase due to the anion-exchange mechanism. MMA exists as either the neutral or the anionic species (pKa 3.6) so it is slightly retained. AsB (pKa = 2.18) is a zwiterionic species so at pH = 3.4 can interact with both, the ionic exchange and the reversed-phase mechanisms. The last compounds to elute are the methylated arsenic species: AsC, TMAO and TMA+, which suggest that the reversed-phase mechanism is controlling the separation. Both AsC and TMAO contain the (CHs)3As moiety. AsC having aliphatic hydrocarbon chain instead of oxygen, is stronger retained, thus indicating a stronger hydrophobic interaction. TMA+ in the presence of nitrate ions from the mobile phase, can undergo a neutralization reaction, forming (CHs)4As+ X−. This “neutral” molecule is able to interact with the hydrophobic phase of the column.
These experiments raised doubts for identifying cationic As compounds by retention time, as the matrix of the sample extract appeared to alter retention times of As compounds. This was especially true for TMA+, which was present at a relatively high concentration in most samples, and the shape of this peak indicated that it contained more than one compound. Londesborough et al.34 showed that the addition of benzene-1,2-disulfonic acid as an ion-pair agent can significantly improve the resolution of AsC and TMA+. However, this strategy was not successful and did not appear to improve chromatographic separation. The co-eluting compounds mentioned above were finally separated after optimization of cation exchange chromatography, employing a Hamilton PRP-X200 column in an isocratic mode (Table 1). Using these conditions, three of the four anionic As species eluted close to the dead volume, and AsB coelutes with TMAO (Fig. 2b). Taking into account that the main goal of this cationic chromatographic separation was to clearly identify TMA+, the most concentrated species in these samples, application of this second chromatographic system was satisfactory for our purposes.
| AsV exposure dose/ng g−1 | Exposure days | AsV | AsIII | DMAV | TMA+ | AsC | UNC 1 |
|---|---|---|---|---|---|---|---|
| 50 | 5 (phase 1) | 50 ± 1 (33.7%) | 20 ± 7 (13.5%) | nd | nd | nd | 78 ± 13 (52.8%) |
| 20 (phase 2) | 60 ± 3 (11.5%) | 40 ± 5 (7.6%) | 19 ± 4 (3.6%) | 254 ± 147 (48.6%) | nd | 150 ± 56 (28.7%) | |
| 45 (phase 3) | 496 ± 161 (23.8%) | 99 ± 24 (4.8%) | 106 ± 4 (5.1%) | 979 ± 174 (47.0%) | 115 ± 36 (5.5%) | 289 ± 14 (13.9%) | |
| 55 (phase 4) | 150 ± 148 (16.2%) | 39 ± 19 (3.8%) | 158 ± 153 (16.7%) | 451 ± 118 (40.3%) | 102 ± 47 (9.9%) | 130 ± 25 (12.6%) | |
| 66 (phase 5) | 28 ± 12 (4.7%) | 23 ± 20 (3.9%) | 46 ± 37 (7.8%) | 378 ± 65 (67.0%) | 37 ± 19 (6.3%) | 79 ± 22 (13.4%) | |
| 72 (phase 6) | 11 ± 2 (4,.2%) | 49 ± 39 (10.7%) | 46 ± 14 (10.1%) | 251 ± 37 (55.0%) | 41 ± 5 (9.0%) | 58 ± 10 (12.7%) | |
| 100 | 5 (phase 1) | 95 ± 19 (45.0%) | 23 ± 10 (10.9%) | nd | nd | nd | 93 ± 17 (44.0%) |
| 20 (phase 2) | 98 ± 14 (14.2%) | 26 ± 3 (3.8%) | 60 ± 13 (8.7%) | 302 ± 34 (43.9%) | nd | 202 ± 33 (29.4%) | |
| 45 (phase 3) | 1015 ± 20 (35.3%) | 159 ± 59 (10.9%) | 141 ± 32 (6.2%) | 731 ± 31 (32.1%) | 30 ± 2 (1.3%) | 200 ± 38 (8.8%) | |
| 55 (phase 4) | 507 ± 136 (21.1%) | 75 ± 21 (4.0%) | 80 ± 71 (6.3%) | 1164 ± 107 (48.4%) | 92 ± 8 (3.8%) | 485 ± 58 (20.2%) | |
| 66 (phase 5) | 194 ± 31 (19.5%) | 7 ± 2 (0.7%) | 15 ± 6 (7.2%) | 574 ± 59 (57.6%) | 150 ± 15 (15.1%) | 56 ± 14 (11.2%) | |
| 72 (phase 6) | 364 ± 42 (4.5%) | 21 ± 27 (2.6%) | 48 ± 41 (6.0%) | 503 ± 39 (77.0%) | 12 ± 1 (1.5%) | 179 ± 56 (22.4%) |
These speciation studies revealed that AsV was absorbed by R. perezi from the exposure solution and was transformed into a variety of arsenicals. Fig. 3 shows the distribution of As species in the different development phases of the frogs. The maximum amount of the dimethylarsinic acid relative to total As was 17%, and its relative abundance was not significantly different in any of the samples analysed. Arsenocholine was also detected but only represented a small fraction of total detected arsenic (up to 16%). Arsenite concentration ranged from 23 to 99 ng g−1, and 7 to 159 ng g−1 in the 50 μg L−1 and 100 μg L−1 exposure experiments, respectively. Arsenate was detected in all of the samples analysed. Changes in the concentration the AsV in developing larvae followed a similar pattern as that for total As. Arsenate concentration increased up to the 45th day of the experiments, then dropped, then stayed at a similar level till the end. The relative percentage of arsenate to total arsenic in the samples ranged from 20 to 45% from the beginning of the exposure up to phase 4, then dropped to 4%. The content of AsV in extracts determined in this study is higher than those reported by other authors38 while AsIII found in such study constituted 30% of extracted arsenicals, higher than the value obtained in the present work.
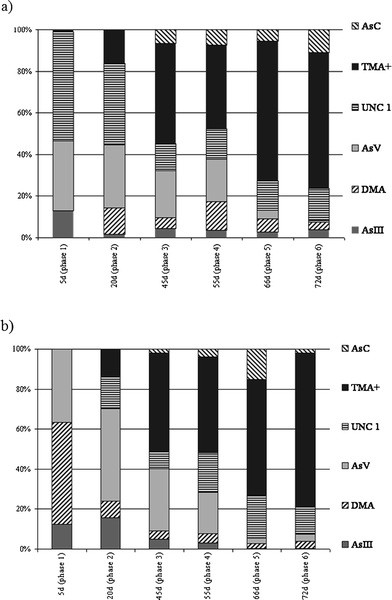 | ||
| Fig. 3 Distribution of the As compounds found in the aqueous extracts in the two exposure experiments a) R. perezi exposed to 50 ng As g−1. b) R. perezi exposed to 100 ng As g−1. UNC1 represents an unidentified As compound that eluted from the column (Dionex AS-7) between iAsVand AsB. | ||
The major arsenic compound found in the extracts from the exposure experiments was TMA+. The highest levels of TMA+, relative to total absorbed As, was found in the extracts of samples collected on the 66th and 72nd day of the experiment. TMA+ comprised 67% in the extracts of frogs in the 5th phase of development of the 50 μg As L−1 exposure, and 77% of total detected As in the extracts of frogs in phase 6th of the 100 μg As L−1 exposure experiment. Selected chromatograms illustrating the changes in the TMA+ content of sample extracts are shown in Fig. 4. These results together with the quantified As species content presented in Table 4 indicate high variability in the composition of water soluble arsenic compounds, especially when they reach phases 4 and 5 of the morphological development of R. perezi. This may be associated with the metamorphosis of a tadpole to an adult that occurs during these stages. The observed pattern of water soluble As compounds revealed that the metabolism of As in R. perezi is complicated. Presence of AsIII and dimethylarsenic acid in the sample extracts suggests that As was metabolised by the production of methylated derivatives. Transformation of inorganic As compounds to the methylated metabolites MMA and DMA has been reported in numerous studies. The mechanism for As methylation was thought to be oxidative methylation followed by reduction.40 More recently an alternative model of methylation was proposed by Hayakawa et al.41 In this model, methylation occurs by sequential reactions, in which thiol-containing complexes of arsenicals are fundamental, and the presence of products such as MMA or DMA is attributed to the decomposition of the arsenical complexes and oxidation of the methylated arsenicals containing AsIII. In this study, the final product of the methylation processes appears to be TMA+, the most abundant As species in the final stages of R. perezi development. Tetramethyl-biomethylation of As has been previously reported in marine animals. For example, the major metabolite of As detected in the polychaetes Nereis diversicolor and Neris virens was TMA+, which comprised around 70% of the accumulated As for a seawater concentration of 50 and 100 ng g−1. TMA+ relative abundance increased to approximately 85% when the As concentration was increased to 1000 ng g−1.42 Additionally, increased concentration of iAsv in the exposure solution was reflected by increased TMA+ accumulation, the arsenic exposure dose may influence biomethylation. Increased TMA+ content in the extracts matched with decreases in total As content, which may suggest that production of highly methylated compounds is a mechanism that increases resistance to As in the exposure solution. This suggestion is supported by an experiment that exposed cells to AsIII for an extended period, in which a decrease in the total amount of As in the culture was accompanied by increased production of volatilised trimethylated arsenicals.43
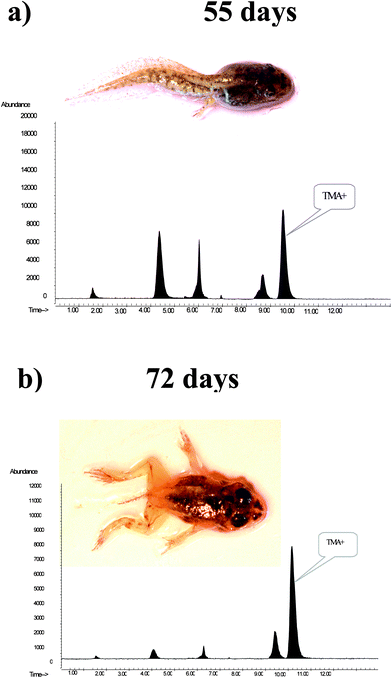 | ||
| Fig. 4 HPLC separation of water soluble As compounds extracted from R. perezi grown in a medium containing 100 ng As g−1 after a) 55 days and b) 72 days. Separation was performed using anion exchange column - Dionex AS7. Life developmental stages are marked as they coincided with changes in the tetramethylarsonium cation content in sample extracts. | ||
4. Conclusions
Rana perezi individuals grown in the presence of different concentrations of arsenate absorbed the ion from the exposure solution, but accumulation factors were low: from 4 to 34 for the to 50 ng g−1 exposure, and from 3 to 31 for the 100 ng g−1 exposure. Arsenic exposure resulted in no significant effects on mortality or on development of R. perezi, which suggests that individuals are relatively resistant to this environmental pollutant at the levels tested (1% LC50). Absorption of the element from the exposure solution was strongly dependent on the development stage and was probably associated with morphological and anatomical differences. Speciation analysis revealed the presence of a complicated distribution of arsenicals that likely arise due to metabolic changes in the biotransformation of the initial arsenic species associated with larval development. The distribution of As compounds R. perezi extracts varied significantly, depending on the frogs metamorphosis stage and iAsV exposure. Finally, methylation is the dominant biotransformation process as reflected by the relatively high levels of TMA+ in the final developmental stages. It was the major compound detected and continuously increased in concentration during frog development.Acknowledgements
The authors thank the Department of Environment of the Regional Government of Castilla y León for permitting animal collection. This work has been undertaken under the financial support of the CICYT project Ref. CTQ2008-01031/BQU and the Spanish Ministry of Environment for the Project Ref. 046/PC08/214.4. Malgorzata A. Bryszewska would like to acknowledge Spanish Ministry of Education and Science for the financial supportReferences
- J. A. Centeno, F. G. Mullick, L. Martinez, N. P. Page, H. Gibb, D. Longfellow, C. Thompson and E. R. Ladich, Pathology related to chronic environmental arsenic exposure, Environ. Health. Perspect., 2002, 110, 883–886 CAS.
- R. R. Walvekar, S. V. Kane, M. S. Nadkarni, I. N. Bagwan, D. A. Chaukar and A. K. D'Cruz, Chronic arsenic poisoning: a global health issue – a report of multiple primary cancers, J. Cutaneous Pathol., 2007, 34, 203–206 Search PubMed.
- S. M. Cohen, T. Ohnishi, L. L. Arnold and X. C. Le, Arsenic-induced bladder cancer in an animal model, Toxicol. Appl. Pharmacol., 2007, 222, 258–263 CrossRef CAS.
- J. Qin, B. P. Rosen, Y. Zhang, G. Wang, S. Franke and C. Rensing, Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase, Proc. Natl. Acad. Sci. U. S. A., 2006, 14, 2075–2080 CrossRef.
- M. Vahter and E. Marafante, Intracellular interaction and metabolic fate of arsenite and arsenate in mice and rabbits, Chem.-Biol. Interact., 1983, 47, 29–44 CrossRef CAS.
- G. K. H. Tam, S. M. Charbonneau, F. Bryce and G. Lacroix, Separation of arsenic metabolites in dog plasma and urine following intravenous injection of 74As, Anal. Biochem., 1978, 86, 505–511 CrossRef.
- M. Vahter, Genetic polymorphisms in the biotransformation of inorganic arsenic and its role in toxicity, Toxicol. Lett., 2000, 112, 209–217 CrossRef.
- H. V. Aposhian and M. M. Aposhian, Arsenic toxicology: five questions, Chem. Res. Toxicol., 2006, 19, 1–15 CrossRef CAS.
- K. Ackley, C. B'Hymer, K. Sutton and J. Caruso, Speciation of arsenic in fish tissue using microwave-assisted extraction followed by HPLC-ICP-MS, J. Anal. At. Spectrom., 1999, 14, 845–850 RSC.
- K. A. Francesconi, D. Kuehnelt, Arsenic Compounds in the Environment, in: W. T. Frankenberger (ed.), Environmental Chemistry of Arsenic, Marcel Dekker Inc., New York, 2000, pp. 51–95 Search PubMed.
- M. A. Suner, V. Devesa, M. J. Clemente, D. Velez, R. Montoro, I. Urieta, M. Jalón and M. L. Macho, Organoarsenical species contents in fresh and processed seafood products, J. Agric. Food Chem., 2002, 50, 924–932 CrossRef CAS.
- A. Madsen, W. Goessler, S. Pedersen and K. Francesconi, Characterization of an algal extract by HPLC-ICPMS and LC electrospray MS for use in arsenosugar speciation studies, J. Anal. At. Spectrom., 2000, 15, 657–662 RSC.
- S. McSheehy, P. Pohl, D. Velez and J. Szpunar, Multidimensional liquid chromatography with parallel ICP MS and electrospray MS/MS detection as a tool for the characterization of arsenic species in algae, Anal. Bioanal. Chem., 2002, 372, 457–466 CrossRef CAS.
- R. Tukai, W. A. Maher, I. J. McNaught and M. J. Ellwood, Measurement of arsenic species in marine macroalgae by microwave-assisted extraction and high performance liquid chromatography–inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 2002, 457, 173–185 CrossRef CAS.
- M. Amran, F. Lagarde and M. Leroy, Determination of arsenic species in marine organisms by HPLC-ICP-OES and HPLC-HG-QFAAS, Microchim. Acta, 1997, 127, 195–202 CAS.
- V. W. M. Lai, W. R. Cullen and S. Ray, Arsenic speciation in scallops, Mar. Chem., 1999, 66, 81–89 CrossRef CAS.
- E. H. Larsen, C. R. Quetel, R. Muñoz, A. Fiala-Medioni and O. F. X. Donard, Arsenic speciation in shrimp and mussel from the Mid-Atlantic hydrothermal vents, Mar. Chem., 1997, 57, 341–346 CrossRef CAS.
- J. S. Edmonds and K. A. Francesconi, Transformations of arsenic in the marine environment, Experientia, 1987, 43, 553–557 CAS.
- J. S. Edmonds, Y. Shibata, K. A. Francesconi, J. Yoshinaga and M. Morita, Arsenic lipids in the digestive gland of the western rock lobster Panulirus cygnus: an investigation by HPLC ICP-MS, Sci. Total Environ., 1992, 122, 321–335 CrossRef CAS.
- H. V. Aposhian, E. S. Gurzau, X. C. Le, A. Gurzau, S. M. Healy, X. Lu, M. Ma, L. Yip, R. A. Zakharyan, R. M. Maiorino, R. C. Dart, M. G. Tircus, D. Gonzalez-Ramirez, D. L. Morgan, D. Avram and M. M. Aposhian, Occurrence of monomethylarsonous acid in urine of humans exposed to inorganic arsenic, Chem. Res. Toxicol., 2000, 13, 693–697 CrossRef.
- J. S. Petrick, F. Ayala-Fierio, W. R. Cullen, D. E. Carter and H. V. Aposhian, Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes, Toxicol. Appl. Pharmacol., 2000, 163, 203–207 CrossRef CAS.
- D. J. Thomas, M. Styblo and L. Shan, Cellular methabolism and systemic toxicity of arsenic, Toxicol. Appl. Pharmacol., 2001, 17, 127–144 CrossRef.
- D. W. Sparling, K. O. Richter, A. Callhoun, M. Micacchion, 2002, United States Environmental Protection Agency, Washington, DC, USA.
- J. W. Snodgrass, W. A. Hopkins, J. Broughton, D. Gwinn, J. A. Baionno and J. Burger, Species-specific responses of developing anurans to coal combustion wastes, Aquat. Toxicol., 2003, 66, 171–182.
- C. J. DeGarady and R. S. Halbrook, Using anurans as bioindicators of PCB contaminated streams, J. Herpetol., 2006, 40, 136–139 CrossRef.
- Test No 305: Bioconcentration: Flow-through Fish Test. OECD Series on Testing and Assessment. http://www.oecdilibrary.org/oecd/content/book/9789264070462-en, Organisation for Economic Co-operation and Development (OECD), 1996. DOI:10.1787/9789264070462-en.
- S. K. Acharyya and B. A. Shah, Groundwater arsenic contamination affecting different geologic domains in India - a review: influence of geological setting, fluvial geomorphology and Quaternary stratigraphy, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2007, 42, 1795–1805 Search PubMed.
- K. L. Gosner, A simplified table for staging anuran embryos and larvae with notes on identification, Herpetologica., 1960, 16, 183–190.
- Japanese Ministry of Economy, Trade and Industry (METI) - National Institute of Technology and Evaluation (NITE). 2006. Biodegradation and Bioconcentration Database of the Existing Chemical Substances. http://www.safe.nite.go.jp/english/kizon/KIZON_start_hazkizon.html Search PubMed.
- C. M. Liao, S. F. Jau, W. Y. Chen, C. M. Lin, L. J. Jou and C. W. Liu, Acute toxicity and bioaccumulation of arsenic in freshwater clam Corbicula fluminea, Environ. Toxicol., 2008, 23, 702–711 CrossRef CAS.
- A. Qadir Shah, K. T. Gul, M. Balal Arain, M. K. Jamali, H. I. Afridi, N. Jalbani, J. A. Baig and G. A. Kandhro, Accumulation of arsenic in different fresh water fish species – potential contribution to high arsenic intakes, Food Chem., 2009, 112, 520–524 CrossRef CAS.
- J. H. Roe, W. A. Hopkins and B. P. Jackson, Species- and stage-specific differences in trace element tissue concentrations in amphibians: implications for the disposal of coal-combustion wastes., Environ. Pollut., 2005, 136, 353–363 CrossRef CAS.
- L. S. Milstein, A. Essader, E. D. Pellizzari, R. A. Fernando, J. H. Raymer, K. E. Levine and O. Akinbo, Development and Application of a Robust Speciation Method for determination of Six Arsenic Compounds Present in Human Urine, Environ. Health Perspect., 2003, 111, 293–296 CAS.
- S. Londesborough, J. Mattusch and R. Wennrich, Separation of organic and inorganic arsenic species by LC-ICP-MS, Fresenius J. Anal. Chem., 1999, 3636, 577–581 CrossRef.
- S. Simon, H. Tran, F. Pannier and M. Potin-Gautier, Simultaneous determination of twelve inorganic and organic arsenic compounds by liquid chromatography–ultraviolet irradiation–hydride generation atomic fluorescence spectrometry, J. Chromatogr., A, 2004, 1024, 105–113 CrossRef CAS.
- K. A. Francesconi and D. Kuehnelt, Determination of arsenic species: A critical review of methods and applications, Analyst, 2004, 129, 373–395 RSC.
- C. Dietz, J. Sanz, E. Sanz, R. Muñoz-Olivas and C. Cámara, Current perspectives in analyte extraction strategies for tin and arsenic speciation, J. Chromatogr., A, 2007, 1153, 114–129 CrossRef CAS.
- R. Schaeffer, K. A. Francesconi, N. Kienzl, C. P. Soeroes, F. L. Varadi, R. Raml, W. Goessler and D. Kuehnelt, Arsenic speciation in freshwater organisms from the river Danube in Hungary, Talanta, 2006, 69, 856–865 CrossRef CAS.
- R. Schaeffer, C. Soeroes, I. Ipolyi, P. Fodor and N. S. Thomaidis, Determination of arsenic species in seafood samples from the Aegean Sea by Liquid Chromatography-(Photo Oxidation)-Hydride Generation - Atomic Fluorescence Spectrometry, Anal. Chim. Acta, 2005, 547, 109–118 CrossRef CAS.
- D. J. Thomas, S. B. Waters and M. Styblo, Elucidating the pathway for arsenic methylation, Toxicol. Appl. Pharmacol., 2004, 198, 319–326 CrossRef CAS.
- T. Hayakawa, Y. Kobayashi, X. Cui and S. Hirano, A new metabolic pathway of arsenite: arsenite-glutathione complexes are substrates for human arsenic methyltransferase Cyt19, Arch. Toxicol., 2005, 79, 183–191 CrossRef CAS.
- A. E. Geiszinger, W. Goessler and K. A. Francesconi, Biotransformation of arsenate to the tetramethylarsonium ion in the marine polychaetes Nereis diversicolor and Nereis virens, Environ. Sci. Technol., 2002, 36, 2905–2910 CrossRef CAS.
- J. Qin, B. P. Rosen, Y. Zhang, G. Wang, S. Franke and C. Rensing, Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase, Proc. Natl. Acad. Sci. U. S. A., 2006, 14, 2075–2080 CrossRef.
Footnote |
| † This article is part of a themed issue highlighting outstanding and emerging work in the area of speciation. |
| This journal is © The Royal Society of Chemistry 2011 |
