Investigation of Hg species binding biomolecules in dolphin liver combining GC and LC-ICP-MS with isotopic tracers†
Zoyne
Pedrero
,
Sandra
Mounicou
,
Mathilde
Monperrus
and
David
Amouroux
Laboratoire de Chimie Analytique Bio-Inorganique et Environnement, CNRS UMR 5254 IPREM, Université de Pau et des Pays de l'Adour, Pau, France
First published on 30th November 2010
Abstract
Hg stable isotopically labeled species were used to assist the investigation of Hg species (i.e. IHg and MeHg) binding biomolecules in the aqueous soluble fraction of white-sided dolphin (Lagenorhynchus acutus) liver homogenate (QC04LH4). By using stable enriched isotopic tracers (199IHg and 201MeHg), the transformation yields of both mercury species (i.e. methylation and demethylation) were evaluated during the different sample preparation steps, such as the tissue lyophilization and the extraction by ultrasonication. Hg species partitioning after fractionation of the sample (cytosolic fraction and residual fraction) was also studied and showed that MeHg is mainly present in the cytosol (60%), while IHg is preferentially associated to the solid residue (88%). The analysis of the water-soluble fraction by size exclusion chromatography-ICP MS revealed the association of Hg species with biomolecules in a wide molecular weight range. The ratio of IHg and MeHg was successively determined in each SEC fraction by GC-ICP-MS. Furthermore the use of isotopic tracers allowed demonstration of specific Hg species affinity with different biomolecule molecular weight fractions. The results indicate a specific affinity of the IHg with a biomolecule at an elution time corresponding to a metallothionein whereas MeHg is associated to a biomolecule of larger molecular weight.
Introduction
The toxicity of mercury is well recognized, especially when it is present as methylmercury (MeHg).1,2 The major source of this species in aquatic ecosystems is the biotic methylation of inorganic mercury (IHg), mainly in sediments. This organomercurial species can be bioaccumulated and its negative effect biomagnified through the aquatic food chain, where marine mammals represent the top position.Despite mercury being well recognized as an important pollutant in marine animals, the metabolism of this metal is not fully elucidated. Little is known about the mechanisms that enable Hg uptake into the cells, and its subsequent bioaccumulation in organisms. It has been suggested that Hg can bind thiol containing proteins/biomolecules.2,3 The study of mercury containing biomolecules is thus essential for the assessment of its metabolic pathways and toxicological impact. Most of the speciation studies have been carried out in muscles tissues because of its consumption by humans4,5 and its regulation by the World Health Organization (WHO).6 However, speciation of this heavy metal in vital organs such as liver and kidney7 has not been extensively studied.
Most of Hg speciation studies are mainly limited to the simplistic discrimination between IHg and MeHg in various samples. It is commonly performed by the hyphenation of a chromatographic separation with a highly sensitive mass spectrometric detection. The most popular chromatographic separation of mercury species includes gas chromatography (GC)8,9 and high-performance liquid chromatography (HPLC)10,11 which require extraction, preconcentration and derivatisation steps for sample preparation. Because of the great analytical challenge, the interaction of Hg with biomolecules has been less studied from the structural identification point of view. Some recent studies deal with this analytical challenge, like the investigations of interactions of thimerosal with human serum albumin and β-lactoglobulin A,12 the identification of Hg species-antidote adducts in plasma surrogate13 and the structural identification of Hg bound to several biothiols using ESI-ion-trap-MS.14
In general terms, one of the main problems associated with the speciation of metals binding biomolecules is to guarantee the species integrity during the whole analytical procedure. Several steps of the analytical procedure can induce modifications on the initial species distribution by degradation and/or transformation. Regarding mercury, the evaluation of the impact of the sample treatment, has been mainly limited to the transformation of Hg species (IHg and MeHg), i.e. methylation and demethylation reactions.15,16 The nature and origin of such transformation is a result of a complex interaction of the different steps of the analytical procedure and the chemical composition of the matrix.15,17,18 Several studies report the abiotic Hg species transformation reactions during the sample treatment and its correction by using species specific isotope dilution.4,9,16,19 The use of isotopically labeled species greatly assists not only on the correction but also on the determination of the extent of all these transformations that can potentially affect the initial composition of the species in the sample.
The influence of lyophilization, as one of the first steps of the sample treatment for speciation studies, and ultrasonication, as a protein extraction procedure, on the Hg species integrity are poorly documented. Despite lyophilization being commonly used to preconcentrate the element by drying, this process can produce protein denaturation by inducing structural perturbations like unfolding and conformation changes, as well as oxidation of some radical bonds like sulfhydryl.20 Investigations performed by Horvat et al. suggested that the freeze drying process can affect the stability of MeHg in biological samples.21 More recently, Point et al. compared two sets of biological samples differentially processed, one freeze dried and one cryogenized and stored fresh frozen, by two different isotope dilution approaches.15 The obtained results show that by using single spike species isotope dilution the results of both sample preparation methodologies were not commutable. Regarding ultrasonication, it is a simple and fast method effectively used for speciation of different elements in several matrices22,23 and for protein extraction.24 Nevertheless, the success of its application depends on the operational conditions and the type of sample.25
The main aim of this work was to develop an analytical method to determine Hg species (i.e. IHg and MeHg) binding biomolecules in the water-soluble fraction of white-sided dolphin (Lagenorhynchus acutus) liver homogenate (QC04LH4). By using stable enriched isotopic tracers (199IHg and 201MeHg), the extent of species transformation during lyophilization and protein extraction by ultrasonication was evaluated by GC-ICPMS. The fractionation of Hg binding biomolecules was investigated by size exclusion chromatography-ICP MS. Isotopically labeled Hg species were not only used as tracers to validate the sample treatment, but also as a valuable tool to demonstrate the specific Hg species affinity with different biomolecules.
Experimental
Reagents and standards
All solutions were prepared using ultrapure water (18 MΩ cm, Millipore). For the preparation of samples, standards and blanks, trace metal grade acids (HNO3 and HCl) were purchased from Fisher Scientific (Illkirch, France). Stock solutions (1000 mg (Hg) L−1) of inorganic mercury (IHg) and methylmercury (MeHg) of natural isotopic composition were prepared by dissolving mercury(II) chloride (Strem Chemicals, USA) in 1% HNO3 and methylmercury chloride (Strem Chemicals, USA) in methanol (Sigma Aldrich, France), respectively. Working standard solutions were prepared daily by appropriate dilution of the stock standard solutions in 1% HNO3 and stored at 4 °C until use. 199HgCl2 was prepared by dissolving 199HgO (Oak Ridge National Laboratory, USA) in HCl (12 mol L−1). 201MeHg was synthesized from methylcobalamin and 201HgO obtained from Oak Ridge National Laboratory (USA) according to the procedure previously described.26 The isotopic abundance of isotopes 199 and 201 in the 199IHg and 201MeHg tracers was 98 and 92%, respectively. In both cases the abundance of 202Hg was lower than 1.5%. The exact concentrations of 199IHg and 201MeHg of the working solutions were determined by reverse isotope dilution analysis. IHg and MeHg were derivatized by using NaBPr4 purchased from Merseburger Spezialchemikalien (Germany).The rabbit liver metallothionein-2 isoform standard was purchased from Enzo life sciences (Villeurbanne, France) and contains approximately 9% of metal (Cd and Zn) and 67% of protein. The stock solution (1 mg mL−1) was prepared by dissolving 1 mg of metallothionein in 1 mL of water, subdivided in 10-μl aliquots to avoid multiple thawing and freezing, and frozen at −20 °C. Working solutions were prepared daily by dilution with water and stored at 4 °C before analysis. White-sided dolphin (Lagenorhynchus acutus) liver homogenate (QC04LH4) were provided by NIST (consensus mean Hg total value 3.60 μg g−1).27
Instrumentation
Liquid chromatographic separations were carried out with an Agilent 1100 liquid chromatograph (Agilent, Wilmington, DE, USA) equipped with a binary HPLC pump, an autosampler and a diode array detector. An Agilent ICP MS 7500ce (Yokogawa Analytical Systems, Tokyo, Japan) was used as liquid chromatographic detector.A Thermo Electron GC (Focus) coupled to a Thermo Electron ICP-MS (X7 X series) was used for the determination of total concentration of each mercury species. The experimental conditions are described elsewhere.28 For GC-ICP-MS analysis, samples were digested by using an analytical microwave (CEM), following the methodology proposed in previous experiments.28
Samples were lyophilized by using a Cryotec lyophilizer (Saint Gely du Fesc, France) and an Ultrasonic probe (USP) - Vibracell 75115 (Bioblock Scientific, Illkirch) instrument (3 mm diameter) offering a nominal power of 500 W was used for water soluble biomolecules extraction. The obtained extracts were centrifuged by using an ultracentrifuge HimaCs 120GX model (Hitachi, Tokyo, Japan).
Sample preparation procedure
The analytical approach is illustrated in Fig. 1. Water soluble biomolecules were extracted from lyophilized samples (approximately 0.7 g) by ultrasonication (30 s at 20% (100 W), 30% (150 W) and 40% (200 W), and 60 s at 40%) in 2 mL 200 mM ammonium acetate pH 7.5. In all cases the sonication was performed in pulse mode to avoid overheating (which was also reduced by immersing the flask in ice). The ultrasound probe was immersed 1 cm and these conditions were kept constant for all the extractions. The obtained extracts were centrifuged at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g during 20 min, at 4 °C to separate residue and cytosol, which represent the non-particle containing soluble fraction of the cellular material. The solid residue was extracted with 3 mL 4% (w/v) of SDS in water by shaking 1 h at room temperature. The SDS soluble and SDS non-soluble fractions were separated by centrifugation (4000 rpm, 10 min).
000 g during 20 min, at 4 °C to separate residue and cytosol, which represent the non-particle containing soluble fraction of the cellular material. The solid residue was extracted with 3 mL 4% (w/v) of SDS in water by shaking 1 h at room temperature. The SDS soluble and SDS non-soluble fractions were separated by centrifugation (4000 rpm, 10 min).
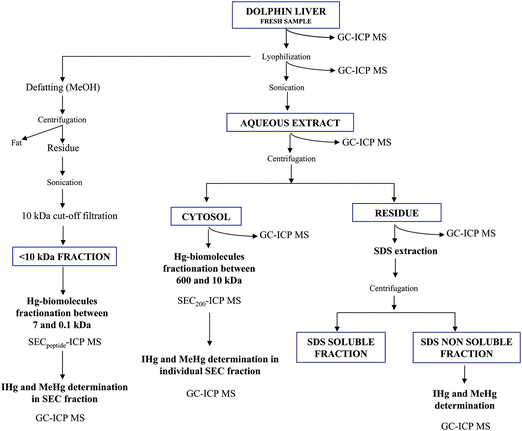 | ||
| Fig. 1 Scheme of the analytical procedure used for Hg speciation in the different tissue fractions. | ||
At the same time, an aliquot of the lyophilized sample (0.7 g) was defatted by shaking with 3 mL MeOH (5 min) and after centrifugation, the water soluble fraction was recovered by sonication (20%, 30s). The obtained solution was filtered by 10 kDa cut-off filters and directly chromatographed in a Superdex peptide chromatographic column coupled to ICP-MS.
Concentrations of IHg and MeHg species were determined by GC-ICP-MS in the fresh and lyophilized liver sample. This analysis was also performed in the aqueous extract after sonication, the cytosol and both residual fractions (after aqueous and SDS extraction) as shown in Fig. 1.
For fractionation of the cytosolic fraction, 100 μL were directly injected in the Superdex 200 chromatographic column coupled to ICP-MS. In order to determine the ratio of IHg and MeHg, the different SEC fractions (between: 10–12 min, 12–16 min, 16–18 min, 18–19 min, 19–23 min, 23–25 min) were individually collected after injection of 100 μL of the protein extracts and analyzed by GC-ICP-MS. The <10 kDa fraction was directly analyzed by HPLC-ICP-MS using a Superdex peptide chromatographic column. The Hg containing fraction eluting between 15 and 18 min was collected and analyzed by GC-ICP-MS.
Mercury speciation analysis by GC-ICPMS
As previously mentioned, aliquots of all studied fractions were digested with 6 N HNO3, following the methodology proposed in previous experiments.28 All the samples were previously derivatized and adequately diluted for GC analysis in order to quantify Hg species. IHg and MeHg were propylated by using NaBPr4 and extracted in isooctane by manual shaking during 5 min. Extracts were stored at −20 °C before analysis by GC-ICP MS.The experimental data was mathematically processed by applying isotope pattern deconvolution approaches previously developed in our laboratory.29 This technique allows the quantification of both mercury species concentrations and transformation factors (i.e. methylation and demethylation) affecting the two isotopic tracers during the analytical procedure.
Hg species containing biomolecules by SEC-ICP MS
ICP MS was daily optimized for maximum sensitivity using a tuning solution containing 1 μg L−1 Y, Tl, Li, Ce. For size exclusion chromatography experiments, a Superdex 200 HR (10 × 300 mm × 13 μm) and a Superdex peptide HR 10/30 (10 × 300 mm × 13 μm), (GE Healthcare, Uppsala, Sweden) with an operation range of 10 to 600 and 0.1 to 7 kDa, respectively, were used. A Superdex 200 SEC column was calibrated with thyroglobulin (670 kDa), transferrin (81 kDa), carbonic anhydrase (29 kDa) and myoglobulin (16.7 kDa), meanwhile the Superdex peptide column was calibrated by using myoglobulin (16.7 kDa), MT (6–7 kDa), vitamin B 12 (1.3 kDa) and selenocystine (0.3 kDa). Before sample analysis, these columns were cleaned by flushing with the mobile phase containing 20 mM EDTA during 60 min and conditioned (60 min) with mobile phase. Supernatant was fractionated by injection of 100 μL and isocratic elution at 0.7 mL min−1 with 100 mM ammonium acetate pH 7.4. Column contamination by Hg species was controlled by injection of 10 mM cysteine solution. The reproducibility of the chromatograms was verified by triplicate injection of the different extracts. Chromatographic data were processed using Microsoft Excel software.Results and discussion
Evaluation of Hg species transformations during the sample preparation procedure by GC-ICPMS
In this work the effect of lyophilization and sonication on Hg species transformation was evaluated by exploiting the advantages of the isotopically enriched species. The isotopically labeled species (199IHg and 201MeHg) were added before the lyophilization and ultrasonication steps, with the aim to estimate simultaneously the yields of methylation and demethylation and their respective concentration for both Hg species.Although the analysis by isotope pattern deconvolution showed that there are no transformations of IHg into MeHg or vice versa for any of the tested conditions, the impact of these different conditions on the interactions between Hg species and biomolecules was nevertheless studied by SEC-ICP-MS. In Fig. 2a, the SEC-ICP MS profiles of Hg-binding biomolecule, corresponding to the softest and strongest extraction conditions are compared. Noticeable differences regarding the chromatographic pattern can be observed, with the disappearance of the major peaks, suggesting Hg-biomolecule entity degradation when the highest amplitude is used for 60 s. Similar trends were obtained for other elements (Zn, Se and Cd) binding biomolecules (data not shown). In case of Hg, the influence of sonication is mainly observed for compounds eluting in the void volume of the column and between 19 and 23 min (ca 60–20 kDa). Therefore the investigation of Hg containing biomolecules requires the assessment of the analytical procedure impact, on both methylation and demethylation process, as well as on Hg-biomolecules integrity. Considering that the recovery of Hg species was quantitative for all the tested conditions and that an increase on the sonication amplitude produces Hg-biomolecules degradations, the softest conditions (30 s, 20%) were selected for further experiments.
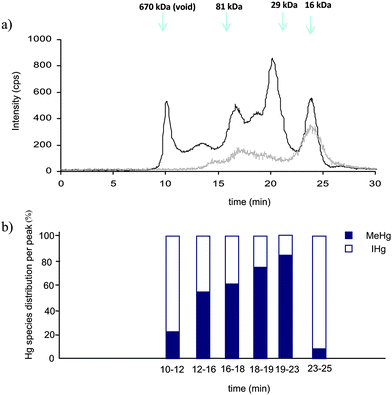 | ||
| Fig. 2 a) Typical 202Hg SEC200-ICP MS chromatograms of the water-soluble extracts obtained by sonication at 20% during 30 s (dark line) and at 40% during 60 s (grey line), b) Hg species distribution into the different size exclusion chromatographic fractions. | ||
Partitioning of IHg and MeHg into the different tissue fractions
The extract obtained by sonication was centrifuged and both, supernatant and solid residue, were analyzed by GC-ICP MS. The distribution of Hg species, between these two fractions was noticeably different (Table 1). The global mass balance was quantitative for both species. Approximately, 60% of the total MeHg is present in the cytosolic fraction, while IHg is mainly found in the solid residue (88%). To improve the Hg-biomolecules extraction yield, the residue was extracted with SDS which favors protein solubilization notably from membranes. An additional 49 and 14% of IHg and MeHg species were respectively recovered from the solid residue, most likely arising from residual cytosolic fraction and part of membrane solubilization.| Experimental conditions | IHg/μg g−1 | MeHg/μg g−1 | Methylation yield (%) | Demethylation yield (%) |
|---|---|---|---|---|
| a Results expressed as mean value ± S.D. (n = 3). DL is 2% for methylation and 3% for demethylation. | ||||
| Fresh tissue | 2.8 ± 0.1 | 0.74 ± 0.03 | <D.L. | <D.L. |
| Lyophilized tissue | 2.8 ± 0.1 | 0.77 ± 0.03 | <D.L. | <D.L. |
| USP 20% 30s | 2.7 ± 0.3 | 0.71 ± 0.05 | <D.L. | <D.L. |
| USP 30% 30s | 2.7 ± 0.3 | 0.72 ± 0.08 | <D.L. | <D.L. |
| USP 40% 30s | 2.7 ± 0.2 | 0.72 ± 0.02 | <D.L. | <D.L. |
| USP 40% 60s | 2.7 ± 0.1 | 0.74 ± 0.02 | <D.L. | <D.L. |
Unlike in other fish tissues, i.e. muscle, the information about Hg species distribution among tissue fractions is quite reduced. Previous investigations of the total Hg distribution in liver tissue have been done regarding the total content of Hg into the cytosol and the solid residue.30,31,32 They show that only a minor part of the total concentration of Hg in liver and kidney organs of different dolphin species is present in the cytosol.30,31,32 The same behaviour has been reported in mussel hepatopancreas,33 in porcine liver and kidney in which respectively 13 and 4% of total mercury were found in the cytosolic fraction.34 The preferential distribution of Hg in the insoluble fraction has been related by several authors with the formation of tiemannite (HgSe) which is assumed as a detoxification mechanism.35 Taking into account that IHg represent 75% of total Hg, it can be concluded that the obtained results are in good agreement with those previously published.
Hg species binding biomolecules in the cytosolic fraction
In order to obtain more information about Hg species the composition of the size exclusion chromatographic fractions containing Hg, all of them were individually collected, digested as mentioned above, and analyzed by GC-ICP MS. The ratio of IHg and MeHg was different depending on the size exclusion fraction (Fig. 2b). The obtained results evidence a specific Hg species affinity, which is mainly remarkable in three fractions. Around 80% of IHg was determined in the high molecular weight fraction (void volume). The fraction that elutes between 19 and 23 min is mainly constituted by MeHg (80%), while IHg represent 90% in the fraction (23–25 min) of lowest molecular weight (<10 kDa).
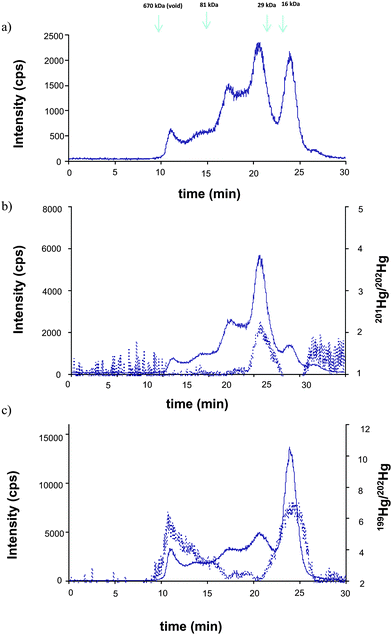 | ||
| Fig. 3 Typical SEC200-ICP-MS chromatograms corresponding to the different Hg isotopes a) 202Hg, b) 201Hg and c) 199Hg) after simultaneous incubation of the water extract with isotopic tracers (solid line). b) 201Hg/202Hg ratio and c) 199Hg/202Hg (dotted line) after individual incubation of the water extract with 199IHg and 201MeHg, respectively. | ||
The individual incubation of the extract with the isotopic labeled species shows the same result. In this case the intensity ratio 201Hg/202Hg and 199Hg/202Hg are compared (Fig. 3b and c, respectively). It confirms that the incorporation of IHg is in the peaks that elute at the void (≥670 kDa) and 23–25 min (<20 kDa), meanwhile MeHg is mainly accumulated in the peak eluting at 19–23 min (66–30 kDa), without any apparent competition. The obtained results demonstrate the unambiguous specific affinity of some biomolecules to either IHg or MeHg. At that level of investigation, identification of these biomolecules is not possible and a more advanced analytical methodology (multidimensional HPLC coupled to mass spectrometry) is required.
Understanding interaction of Hg species with biomolecules is compulsory to elucidate the metabolism of this element in living organisms as it can provide key information regarding the transport, transformation and detoxification pathways, etc. Unfortunately, there is a data lack of the literature related to this topic and the discrimination between IHg and MeHg is even scarcer. One study performed in milk and plasma samples reports that both Hg species are bound to albumin.36 A deeper study carried out on human serum albumin suggested that MeHg binds to the sulfhydryl group of the free Cys34, while IHg is bound to the same sulfhydryl group of two human serum albumin (HSA) molecules.37 Another discrimination between Hg species was also stated as thiol group is the unique target of organomercurial species (MeHg, EtHg, PhHg) while IHg can also access N and O atoms of amino acids residues to form coordination complex.37 The investigation of interactions of Hg species and DNA reported that Hg coordinates covalently to endocyclic and exocyclic N sites of DNA bases. They also show that organomercurial species, present stronger affinity than IHg for DNA.38
Hg species binding biomolecules in the 10 kDa fraction
The identification of biomolecules containing Hg is certainly a challenge. As an initial step towards the characterization of Hg-biomolecules, we focus on the LMW fraction. For that purpose the sample preparation protocol was modified as two steps were added: 1) defatting by treatment of the sample with MeOH and 2) removal of high molecular weight compounds using 10 kDa cut-off filters. Therefore the defatted and filtered sample was fractionated using a chromatographic column with a narrower molecular weight separation range (0.1–7 kDa) chromatographic column. As observed in Fig. 4, only one Hg peak is detected in the size exclusion chromatogram. This fraction which has been collected and analyzed by GC-ICP MS, consists of 95% IHg and its elution time matches the one of metallothionein-2 standard. This fact and the observed co-elution of Hg with Zn, Cd and Cu, elements known to complex metallothionein, support the hypothesis of the presence of metallothionein isoforms binding Hg.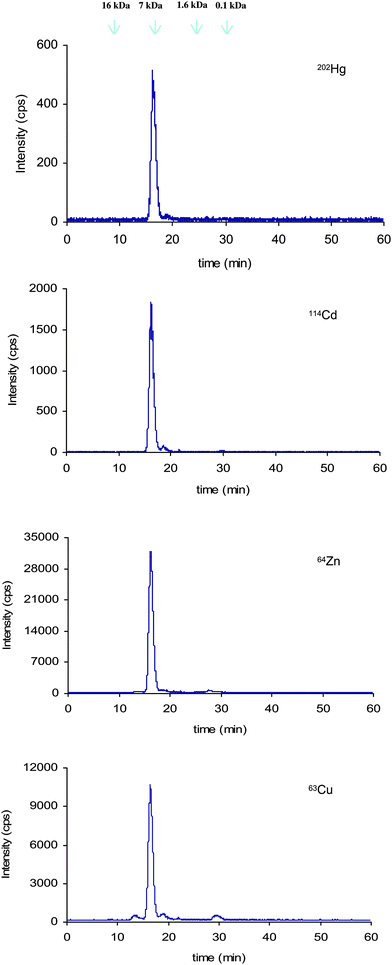 | ||
| Fig. 4 Size exclusion chromatogram (Superdex peptide) corresponding to the fraction lower than 10kDa. | ||
Metallothioneins (MT) are a superfamily of proteins of approximately 6∼7 kDa, that contains in general 61 or 62 amino acids residues. Due to their high sulfur content (30%), metallothioneins are characterized by their ability to bind a wide variety of metals.39 The biological role of the MT is not fully elucidated but it is suggested that they are involved in the fixation and homeostasis of essential elements like Zn and Cu, and in detoxification mechanisms of Cd40 and Hg.7,41 In spite of various studies, less attention has been paid to Hg binding MT. Some studies suggest the interaction of Hg with MT in liver of marine mammals. However, most of these results are based on the correlation of the concentration of Hg and MT7,31,42,43 or on the coelution in size exclusion chromatography of a Hg containing fraction and a MT standard.32,33 However the structural elucidation is needed, since the identification of biomolecules, cannot be based only in retention time matching of standards and samples. Therefore confirming the identity of this Hg binding biomolecule in this dolphin liver by molecular mass spectrometry technique represents the next perspective of this work.
The characterization of Hg containing MT standards will be useful for further investigations of these biomolecules in the real sample. Unfortunately, these standards are not commercially available. In the scope to synthesize it, a commercially available MT containing Cd, Cu and Zn as characterized by Mounicou et al.,44 was incubated individually and simultaneously with 199IHg and natMeHg at different Hg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MT molar ratios (3
MT molar ratios (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 7
1 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), chosen based on the ability of the MT to bind seven atoms. The mixtures were incubated at 37 °C during 2 h, adequately diluted and analyzed by SEC peptide-ICPMS. As shown in Fig. 5, the increase of the molar ratio 199Hg
1), chosen based on the ability of the MT to bind seven atoms. The mixtures were incubated at 37 °C during 2 h, adequately diluted and analyzed by SEC peptide-ICPMS. As shown in Fig. 5, the increase of the molar ratio 199Hg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MT produces a rise on the intensity of 199Hg and a decrease on the signal of Cd. It can be understood as a replacement of Cd in the Cd7-MT. In contrast, when incubations were performed with MeHg, a weak complexation of this Hg species with the MT is observed. The intensity of Hg isotope is not increasing with its concentration and Cd is not replaced. This demonstrates that MeHg+ does not compete with Cd2+ for complexation with MTs. Similar behaviors were observed when Cd7-MT was incubated simultaneously or individually with the Hg species. These results evidence the high specific affinity of the MT by IHg.
MT produces a rise on the intensity of 199Hg and a decrease on the signal of Cd. It can be understood as a replacement of Cd in the Cd7-MT. In contrast, when incubations were performed with MeHg, a weak complexation of this Hg species with the MT is observed. The intensity of Hg isotope is not increasing with its concentration and Cd is not replaced. This demonstrates that MeHg+ does not compete with Cd2+ for complexation with MTs. Similar behaviors were observed when Cd7-MT was incubated simultaneously or individually with the Hg species. These results evidence the high specific affinity of the MT by IHg.
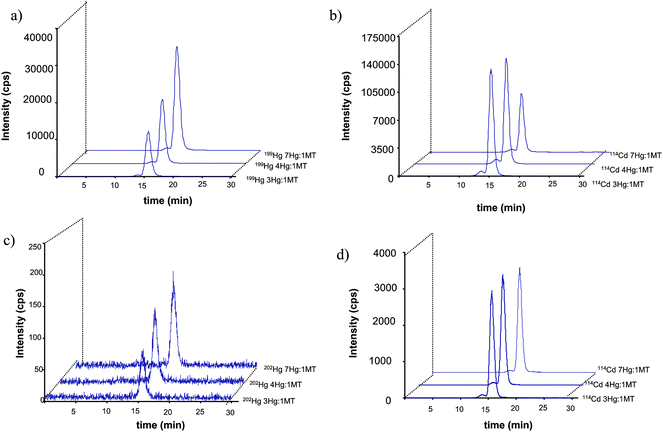 | ||
| Fig. 5 Size exclusion chromatogram (Superdex peptide) corresponding to the incubation of Cd7-MT with 199IHg (a and b) and natMeHg (c and d). | ||
Conclusion
In this work Hg isotopically labeled species isotopes have been used for the evaluation of sample treatment for Hg containing biomolecules of white-sided dolphin liver homogenate. Despite abiotic methylation and demethylation not being significant during the tested steps, the Hg-biomolecules entities can be degraded under strong sonication conditions. The partition of Hg into the different tissue fractions demonstrated the weak proportion of Hg in the cytoplasm, and this distribution pattern was different depending on the Hg species (60% of the MeHg is present in the cytoplasm, in contrast with 88% of IHg present in the residue). In the cytoplasm, several Hg-biomolecules were detected, in a wide molecular weight range. The analysis by GC-ICP-MS of the different size exclusion chromatography fractions has shown different ratios of IHg and MeHg. The incubation of the protein extract with Hg isotopically labeled species isotopes evidenced the unambiguous specific affinity of the different biomolecules by IHg and MeHg.Acknowledgements
Financial support from ANR (Agence National de la Recherche) through project IDEA (Isotopic Deconvolution for Environmental and Analytical Chemistry of Organometallic Pollutants) is acknowledged. We thank Clay W. Davis (NIST, Hollings Marine Laboratory, 331 Ft. Johnson Rd., Charleston, SC, 29412, United-States) for providing the white-sided dolphin liver homogenate material (QC04LH4).References
- T. W. Clarkson and L. Magos, Crit. Rev. Toxicol., 2006, 36, 609–622 CrossRef CAS.
- I. Onyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911–5929 CrossRef CAS.
- H. H. Harris, I. J. Pickering and G. N. George, Science, 2003, 301, 1203 CrossRef CAS.
- L. Hinojosa Reyes, G. M. Mizanur Rahman and H. M. Skip Kinston, Anal. Chim. Acta, 2009, 631, 121–128 CrossRef CAS.
- P. Krysteck and R. Ritsema, Anal. Bioanal. Chem., 2005, 381, 354–359 CrossRef CAS.
- WHO Environmental health criteria 101: methylmercury. 1990. World Health Organization, Geneva.
- K. Das, V. Debacker and J. M. Bouquegnau, Cell Mol. Biol., 2000, 46, 283–294 CAS.
- R. C. Rodriguez Martin Doimeadios, E. Krupp, D. Amouroux and O. F. X. Donard, Anal. Chem., 2002, 74, 2505–2512 CrossRef CAS.
- L. H. Reyes, G. M. M. Rahman, T. Farenholz and H. M. S. Kingston, Anal. Bioanal. Chem., 2008, 390, 2123–2132 CrossRef CAS.
- Y. W. Chen and N. Belzile, Anal. Chim. Acta, 2010, 671, 9–26 CrossRef CAS.
- J. G. Chen, H. W. Chen, X. Z. Jin and H. T. Chen, Talanta, 2009, 77, 1381–1387 CrossRef CAS.
- S. Trumpler, W. Lohmann, B. Meerman, W. Buscher, M. Sperling and U. Karst, Metallomics, 2009, 1, 87–91 RSC.
- S. Trumpler, S. Nowak, B. Meermann, G. A. Wiesmuller, W. Busher, M. Sperling and U. Karst, Anal. Bioanal. Chem., 2009, 395, 1929–1935 CrossRef.
- E. M. Krupp, B. F. Milne, A. Mestrot, A. A. Meharg and J. Feldmann, Anal. Bioanal. Chem., 2008, 390, 1753 CrossRef CAS.
- D. Point, W. C. Davis, J. I. Garcia Alonso, M. Monperrus, S. J. Christopher, O. F. X. Donard, P. R. Becker and S. A. Wise, Anal. Bioanal. Chem., 2007, 389, 787–798 CrossRef CAS.
- J. Qvarnstrom and W. Frech, J. Anal. At. Spectrom., 2002, 17, 1486–1491 RSC.
- H. Hintelmann, R. Falter, G. Ilgen and R. D. Evans, Fresenius J. Anal. Chem., 1997, 358, 363 CrossRef CAS.
- D. Point, J. I. Garcia Alonso, W. C. Davis, S. J. Christopher, A. Guichard., O. F. X. Donard, P. R. Becker, G. C. Turk and S. A. Wise, J. Anal. At. Spectrom., 2008, 23, 385–396 RSC.
- G. M. Mizanur, T. fahrenholz and H. M. Skip Kingston, J. Anal. At. Spectrom., 2009, 24, 83–92 RSC.
- W. Wang, Int. J. Pharm., 2000, 203, 1–60 CrossRef CAS.
- M. Horvat and R. Byrne, Analyst, 1992, 117, 665–668 RSC.
- M. Gallego-Gallegos, M. Liva, R. M. Olivas and C. Camara, J. Chromatogr., A, 2006, 1114, 82–88 CrossRef CAS.
- G. Vale, R. Rial-Otero, A. Mota, L. Fonseca and J. L. Capelo, Talanta, 2008, 75, 872–884 CrossRef CAS.
- S. Roe, in Protein purification techniques: a practical approach, Second Edition, Oxford University Press, 2001 Search PubMed.
- Z. Pedrero, Y. Madrid, C. Camara, E. Schram, J. B. Luten, I. Feldmann, L. Waentig, H. Hayen and N. Jakubouski, J. Anal. At. Spectrom., 2009, 24, 775–784 RSC.
- R. C. R. Martin-Doimeadios, T. Stoichev, E. Krupp, D. Amouroux, M. Holeman and O. F. X. Donard, Appl. Organomet. Chem., 2002, 16, 610–615 CrossRef.
- S. J. Christopher, S. R. Pugh, M. B. Ellisor, E. A. Mackey, R. O. Spatz, B. J. Porter, K. J. Bealer, J. R. Kucklick, T. K. Rowles and P. R. Becker, Accredit. Qual. Assur., 2007, 12, 175–187 CrossRef CAS.
- M. Ranchou-Peyruse, M. Monperrus, R. Bridou, R. Duran, D. Amouroux, J. C. Salvado and R. Guyoneaud, Geomicrobiol. J., 2009, 26, 1–8 CrossRef CAS.
- P. Rodríguez-González, M. Monperrus, J. I. García Alonso, D. Amouroux and O. F. X. Donard, J. Anal. At. Spectrom., 2007, 22, 1373 RSC.
- M. M. Storelli and G. O. Marcotrigiano, Mar. Pollut. Bull., 2002, 44, 71–81 CrossRef.
- K. Das, V. Jacob, J. M. Bouquegneau. Comparative Biochemistry and Physiology Part C, 2002, 131, pp. 245–251 Search PubMed.
- K. Das, A. De Groof, T. Jauniaux and J. M. Bouquegnau, BMC Ecol., 2006, 6, 2 CrossRef.
- M. Tusek Znidarick, I. Falnoga, M. Skreblin, V. Turk. Biological Trace Element Research, 2006, 111, pp. 121–135 Search PubMed.
- C. Chen, L. Qu, J. Zhao, S. Liu, G. Deng, B. Li, P. Zhang and Z. Chai, Sci. Total Environ., 2006, 366, 627–637 CrossRef CAS.
- F. Palmisano, N. Cardellicchio and P. G. Zambonin, Mar. Environ. Res., 1995, 40, 109–121 CrossRef CAS.
- J. Sundberg, B. Ersson, B. Lonnerdal and A. Oskarsson, Toxicology, 1999, 137, 169–184 CrossRef CAS.
- Y. Li, X. P. Yan, C. Chen, Y. L. Xia and Y. Jiang, J. Proteome Res., 2007, 6, 2277–2286 CrossRef CAS.
- Y. Li, Y. Jiang and X. P. Yan, Anal. Chem., 2006, 78, 6115–6120 CrossRef CAS.
- Metallothioneins;Stillman, M. J.; Shaw, C. F. I.; Suzuki, K. T., ed.; VCh: New York, 1992 Search PubMed.
- I. Paul-Pont, P. Gonzalez, M. Baudrimont, H. Nili and X. de Montaudouin, Aquat. Toxicol., 2010, 97, 260–267 CrossRef CAS.
- G. Henkel and B. Krebs, Chem. Rev., 2004, 104, 801–824 CrossRef CAS.
- T. Endo, K. Haraguchi and M. Sakata, Sci. Total Environ., 2002, 300, 15–22 CrossRef CAS.
- C. Sonne, O. Aspholm, R. Dietz, S. Andersen, M. H. G. Berntssen and K. Hylland, Sci. Total Environ., 2009, 407, 6166–6172 CrossRef CAS.
- S. Mounicou, L. Ouerdane, B. L'Azou, I. Passagne, C. Ohayon-Courtés, J. Szpunar and R. Lobinski, Anal. Chem., 2010, 82, 6947–6957 CrossRef CAS.
Footnote |
| † This article is part of a themed issue highlighting outstanding and emerging work in the area of speciation. |
| This journal is © The Royal Society of Chemistry 2011 |
