Mercury speciation and mercury-binding protein study by HPLC-ICP-MS on the estimation of mercury toxicity between maternal and infant rats†
Feng
Weiyue
*a,
Wang
Meng
a,
Guan
Ming
b,
Hui
Yuan
a,
Shi
Junwen
a,
Wang
Bing
a,
Zhu
Motao
a,
Ouyang
Hong
a,
Zhao
Yuliang
a and
Chai
Zhifang
a
aCAS Key Laboratory of Nuclear Analytical Techniques, CAS Key Laboratory for Bio-Medical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing, 100049, China. E-mail: fengwy@mail.ihep.ac.cn
bSchool of Pharmaceutical Sciences, Jilin University, Changchun, Jilin Province 130022, China
First published on 18th November 2010
Abstract
A comparative study of methylmercury (MeHg) metabolism and its conversion into inorganic mercury (I-Hg) in organic tissues (brain, liver and kidney) and the subcellular fractions (nucleus, mitochondrion, lysosome, microsome and cytosol) of maternal and infant rats after infant in utero and lactational exposure to methylmercury was achieved by extraction preconcentration with HPLC-ICP-MS determination. The quantification of Hg-containing proteins in brain cytosol was studied by HPLC hyphenated on-line isotope dilution ICP-MS and the proteins were analyzed by SDS-PAGE and MALDI-TOF-MS. The results demonstrated that the methods could be successfully used in the study of mercury species in sub-compartments of organ cells. The study showed that the distribution patterns of MeHg and I-Hg in the levels of organs, the subcellular fractions of organs and proteins are all significantly different between mothers and their offspring, indicating their significantly different metabolism, transportation and accumulation behavior of mercury species, which may be all involved in the toxic mechanism of mercury for adults and young children.
Introduction
Mercury (Hg) is a ubiquitous contaminant in the global environment. Generally, there are three forms of mercury existing in the environment: elemental Hg (Hg0), inorganic mercury (Hg+ and Hg2+) and organic Hg (methylmercury, ethylmercury, etc.). Among the mercury species, methylmercury (MeHg) is the most common and most toxic in the environment and organisms. Methylmercury can permanently damage the children's developing brains, even at low levels of exposure. Following the episodes of mercury poisoning in Japan and Iraq, many fetuses exposed to MeHg through the placenta of the exposed mother showed severe cerebral-palsy-like symptoms, while their mothers had mild or even no manifestation of the poisoning.1 In the late 1990s, several epidemiological studies showed that the developing nervous system of unborn babies and young children might be harmed after their mothers' consumption of fish with relatively high methylmercury levels.2,3 The Faroe Islands study showed that the nursing mothers who consumed a high content of MeHg in seafood had increased Hg content in their hair with the length of the nursing period, which suggested the transportation of Hg from mothers to their fetus.4 Based on the epidemiological and animal studies, in 2001 the U.S. EPA provided a reference dose (RfD) of 0.1 μg/kg body weight/day as a safety limit on methylmercury exposure to women who are pregnant or nursing mothers,5 which was two times lower than the standard level recommended by the WHO for adults. However, up to now, the exact toxic and transformation mechanisms of mercury from mother to fetus that induce the significant toxic difference between them have not clearly been known.Human breast milk contains both methylmercury and inorganic mercury. Inorganic mercury may source from metabolically converted methylmercury. It is known that methylmercury can easily transport across the placenta and accumulate in the fetal brain; in contrast, inorganic mercury has a low transport into the fetus.6 However, for the opposite lactational transportation, inorganic mercury is more efficiently transferred into milk than methylmercury.7 Though the toxicity of mercury species is already known, the depletion of methylmercury in organs, especially in the brain, and the difference of accumulation and excretion patterns of mercury species between mothers and their offspring have been insufficiently studied to date.
Recently, though large-scale proteomics programs are still promising, many laboratories began to apply traditional subcellular fractionation procedures within proteome studies.8,9 Subcellular proteomics has the advantage of simplifying the complexity of crude cell or tissue extracts; thus it is thought to be the available approach to study proteins. Identifying biological changes in organelles is considered to be an important step towards understanding the molecular mechanisms governing the functions of cells and organs.8 In our previous work, by using an animal model, it was found that the quantities of mercury-binding proteins between maternal and infant rats were significantly different: more high-molecular-weight masses accumulated in the cytosol of the infant rat brain; in contrast, more low-molecular-weight mass (metallothionein, etc.) accumulated in the maternal brain, which indicated a different metabolism and toxicity between them.10 In this study, a further comparative study of methylmercury metabolism and conversion into inorganic mercury in organic tissues (brain, liver and kidney) and in the subcellular fractions, including the nucleus, mitochondrion, lysosome, microsome and cytosol, in maternal and infant rats was studied.
We have successfully developed a mild, efficient and convenient extraction method of using 2-mercaptoethanol-containing extractant solution for the simultaneous determination of mercury species (CH3Hg+ and Hg2+) in biological samples by HPLC-ICP-MS.11 The limit of detection for methyl (CH3Hg+) and inorganic mercury (Hg2+) by this method were both 0.2 μg L−1. Additionally, a quantification method for mercury-binding protein study in brain cytosol by HPLC combined with post-column isotope dilution ICP-MS was developed previously.12,13 In this study, the methods were proven to be successfully used in the study of mercury species in organic tissues and their subcellular fractions. The results could be useful in the understanding of MeHg metabolic and toxic mechanisms in mothers and their offspring after infant in utero and lactational exposure to methylmercury.
Materials and methods
Reagents
Methylmercury chloride was supplied by Riedel-de Haën, Germany, and prepared to 1000 μg mL−1 as stored solution. The enzyme inhibitor including leupeptin, pepstatin and trypsin inhibitor were obtained from Sigma (St. Louis, MO, USA). Tris(hydroxymethyl)aminomethane was purchased from Roche (Germany); sucrose and phenylmethylsulfonyl fluoride (PMSF) was from Amresco (USA) and DTT (dithiothreitol) was from Merck (Germany).The guaranteed grade hydrochloric acid, HPLC grade methanol, analytical grade potassium chloride and ammonium acetate were obtained from Beijing Chemical Reagents Company (Beijing, China). The guaranteed grade 2-mercaptoethanol was supplied by Nacalai Tesque Inc. (Kyoto, Japan). Ultra-pure water (18.2 MΩ cm−1) from a Milli-Q water purification system (Millipore, MA, USA) was used.
A standard solution of 1000 mg Hg L−1 of Hg2+ in 10% (v/v) HNO3 was purchased from AccuStandard Inc. (Connecticut, USA). The methylmercury chloride (CH3HgCl) was supplied by Riedel-de Haën (Seelze, Germany) and a stock solution of 1000 mg Hg L−1 was prepared by dissolving CH3HgCl in 10% (v/v) HNO3. All the stock standard solutions were protected from light and stored at 4 °C. The mercury working standards were prepared daily by proper dilution with the extraction solution.
The mercury oxide (HgO) containing enriched stable isotopes of 198Hg (isotope abundance: 196Hg 13.94%, 198Hg 38.91%, 199Hg 17.99%, 200Hg 13.92%, 201Hg 5.25%, 202Hg 8.69%, 198Hg 1.30%) was purchased from Isotope Production & Distribution, U.S. Department of Energy.
Other reagents (unless otherwise indicated) were obtained from the Second Chemical Factory of Beijing, China and were all in analytical purity.
Animals
Healthy adult male and female Sprague-Dawley rats were purchased from the Center of Experimental Animals of Peking Union Medical College. After 1 week adapting feeding, male and female rats were put together at the mating ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at six in the evening. The gestational day 0 of female rats was defined as the day when vaginal plug was confirmed. The pregnant rats whose weights were about 290 ± 10 g were chosen as the experimental animal. Twenty-four pregnant rats were divided into 2 groups randomly. One group of rats were exposed to 0.3 mg Hg kg−1 d−1 bw (as CH3HgCl, 12 rats/group) in drinking water from pregnancy day 0 continuing to lactational day 20. The animal experiments were carried out in accordance with the Guiding Principles in the Use of Animals in Toxicology adopted by Society of Toxicology.
1 at six in the evening. The gestational day 0 of female rats was defined as the day when vaginal plug was confirmed. The pregnant rats whose weights were about 290 ± 10 g were chosen as the experimental animal. Twenty-four pregnant rats were divided into 2 groups randomly. One group of rats were exposed to 0.3 mg Hg kg−1 d−1 bw (as CH3HgCl, 12 rats/group) in drinking water from pregnancy day 0 continuing to lactational day 20. The animal experiments were carried out in accordance with the Guiding Principles in the Use of Animals in Toxicology adopted by Society of Toxicology.
Sample preparation
At day 20 of lactation, the maternal and infant rats were sacrificed and the liver, kidney and brain were collected. For total and MeHg determination, some brain samples were further dissected into cerebrum and cerebellum.The brain tissue was suspended with 3 volumes (w/v) of 6.0 mL 0.02 mol L−1 Tris-HCl buffer (pH 7.4) in the presence of 0.3 mol L−1 sucrose, 5 mmol L−1 DTT and the mixture protease inhibitors: 40 μg mL−1 PMSF, 5 μg mL−1 leupeptin, 0.7 μg mL−1 pepstatin and 5 μg mL−1 trypsin inhibitor. The liver and kidney samples were homogenized with 4 volumes of 0.02 mol L−1 Tris-HCl buffer (pH 7.4). The homogenate was first centrifuged at 50 × g for 7 min to remove non-disrupted cell and then the supernatant was centrifuged successively at 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g × 10 min, 9000
g × 10 min, 9000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g × 10 min, 30
g × 10 min, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g × 25 min, and 100
g × 25 min, and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g × 2 h in a Beckman model 7L ultracentrifuge to obtain nucleic, mitochondrial, lysosomal, microsomal, and cytosolic fractions. All of the above operations were carried out at 4 °C. Then the homogenate and subcellular fractions of organic samples were freeze dried and stored at −70 °C until use.
g × 2 h in a Beckman model 7L ultracentrifuge to obtain nucleic, mitochondrial, lysosomal, microsomal, and cytosolic fractions. All of the above operations were carried out at 4 °C. Then the homogenate and subcellular fractions of organic samples were freeze dried and stored at −70 °C until use.
Instrumentation
The HPLC system used for the separation of mercury species consisted of a Waters metal-free 626 gradient pump, a Rheodyne 7725 injector with a 50 or 100 μL sample loop. A reverse phase column (Symmetry Shield RP18 column, 150 × 3.9 mm, 5 μm, Waters) was used for inorganic and methylmercury separation. The HPLC mobile phase contained 5% (v/v) methanol, 0.1% (v/v) 2-mercaptoethanol and 0.06 mol L−1 ammonium acetate. The elution flow rate was 1 mL/min.The TSK-GEL G3000SWxl gel filtration column (7.8 × 300 mm id, TOSOH, Japan) was used for isolation of mercury-containing proteins. The 0.1 mol L−1 Tris-HCl (pH 7.4) was used as the elution solution. The mobile phase flow rate was 0.5 mL/min. The outlet of the column was directly connected to the nebulizer of an ICP-MS system by PEEK tubing (∅ = 0.13 mm).
The determination of mercury was carried out on a Thermo X7 ICP-MS instrument (Thermo Electron Corp. Waltham, USA). The mass calibration of the ICP-MS instrument was tuned daily with a tuning solution of 5 μg L−1 Be, Co, In and U in 5% (v/v) methanol for speciation or total mercury analysis. The optimal operation conditions and data acquisition parameters are given in Table 1.
| MeHg and I-Hg analysis | Hg-binding protein analysis | |
|---|---|---|
| SEC chromatographic conditions | ||
| Waters 626 HPLC system | ||
| Analytical column | Waters Symmetry Shield RP18 column (150 × 3.9 mm, 5 μm) | TSK-GEL G3000SWxl column (7.8 mm × 300 mm) |
| Mobile phase | 0.06 mol L−1 ammonium acetate, 5% (v/v) methanol, 0.1% (v/v) 2-mercaptoethanol | 0.1 mol L−1 Tris-HAc |
| Flow rate (mL min−1) | 1 | 0.5 |
| Injection volume (μL) | 50 | 100 |
| ICP-MS conditions | ||
| Spray chamber | Quartz impact bead | Quartz impact bead |
| Nebulizer | Glass concentric | Glass concentric |
| Interface | Xi cones | Xi cones |
| Chamber temperature (°C) | 5 | 2 |
| Forward power (W) | 1300 | 1350 |
| Acquired mode | Time resolved analysis | Time resolved analysis |
| Isotopes monitored | 202Hg | 198Hg, 202Hg |
| Dwell time per points (ms) | 100 | 100 |
| Total analytical time (s) | 800 | 1500 |
MS measurements were performed on an autoflex MALDI-TOF-MS (Bruker Daltonics, Germany) with a 337 nm nitrogen laser. Spectra were recorded in the positive linear mode.
Total, inorganic and methylmercury determination
The detailed method of simultaneous analysis of inorganic and methylmercury in biological samples was described in our previous work.11 The method was using efficient extraction method combined with RP-HPLC-ICP-MS. Briefly, 5 mL extraction solution and 100 mg sample was mixed and shaken overnight (about 12 h) at room temperature. Afterwards, the supernatant was collected and then passed through a 0.45 μm filter prior to analysis. The concentration of total mercury in the extracted solution was analyzed by ICP-MS in a continuous mode. The Hg2+ and CH3Hg+ were analyzed by RP-HPLC (Waters Symmetry Shield RP18 column, 150 × 3.9 mm, 5 μm particle size) hyphenated with ICP-MS in time resolved analysis (TRA) mode. The analytical conditions of the chromatographic separation are presented in Table 1. The detection limits of Hg2+ and CH3Hg+ are both as low as 0.2 μg/mL.Mercury-containing protein determination in brain samples
The brain cytosol was directly injected into the gel column. The protein peaks were monitored by UV/VIS detector at 225 and 280 nm. A species-unspecific mercury spike solution was continuously added into the eluate from the HPLC column, and then the isotope-diluted fractions were introduced into the nebulizer of the ICP-MS. The schematic diagram of the analytical system was shown in our previous work.12,13 The signals of 198Hg and 202Hg were on-line monitored with the elution time.The quantification of mercury in the different chromatographic fractions was performed by post-column isotope dilution analysis.14 The equation for mercury calculation is described as follows:
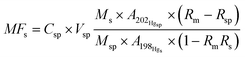 | (1) |
Identification of metallothionein in brain cytosol by SDS-PAGE and MALDI-TOF-MS
The metallothionein (MT) in organic samples was purified by a method of acetonitrile precipitation.16 In order to purity the MT fraction in the samples, an equal volume of acetonitrile was mixed with the samples and vortexed. After the protein precipitation was complete, the sample was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g for 45 min. The supernatant was collected and concentrated with a 5 kDa molecular mass cutoff concentrator.
g for 45 min. The supernatant was collected and concentrated with a 5 kDa molecular mass cutoff concentrator.
The proteins in the samples were separated and identified by SDS-PAGE. For SDS-PAGE, samples were mixed with 5× loading buffer, boiled for 5 min at 95 °C, and 15 μL applied onto a discontinuous 5% loading gel and 15% separating polyacrylamide gel. After running, the gels were stained with Coomassie blue. The molecular mass standards (Sigma) used in SDS-PAGE contained rabbit muscle phosphorylase b (97.4 kDa); bovine serum albumin (66.2 kDa); rabbit skeletal muscle, 43.0 kDa; bovine carbonic anhydrase (31 kDa); hen egg white lysozyme (14.3 kDa) and rabbit liver MT I and MT II.
The MALDI-TOF-MS measurements were achieved using 4-hydroxy-3,5-dimethoxycinnamic acid, i.e., sinapinic acid (Bruker Daltonik, Germany) as matrix. Acetonitrile treated samples were mixed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with a supersaturated matrix solution (20 mg sinapinic acid in 50% v/v aqueous acetonitrile, 0.1%), spotted onto a stainless 384 well hydrophobic MALDI-TOF plate and dried in room temperature. The MS was analyzed in linear mode.
3) with a supersaturated matrix solution (20 mg sinapinic acid in 50% v/v aqueous acetonitrile, 0.1%), spotted onto a stainless 384 well hydrophobic MALDI-TOF plate and dried in room temperature. The MS was analyzed in linear mode.
Results and discussion
Distribution patterns of mercury species in organic tissues of maternal and infant rats
The comparative distribution patterns of total mercury (T-Hg) and methylmercury (MeHg) in the liver, kidneys, cerebrum and cerebellum between maternal and infant rats after pre-natal exposure of methylmercury are presented in Fig. 1. The data showed that mercury in the observed tissues mainly existed as MeHg for both maternal and infant rats. In maternal rats, mercury was highly accumulated in the kidneys, which were the primary excretion organ of T-Hg and MeHg. However, comparatively, the highest contents of T-Hg and MeHg in infant rats were in the cerebellum, more than 2 times higher than those in other organs. The ratios of MeHg to T-Hg in the organs of maternal rats and their offspring clearly showed that, except for the liver, infant rats contained a higher percentage of MeHg in the kidneys, cerebrum and cerebellum than their mothers. However, in the liver, which is the most important organ for detoxification, a lower percentage of MeHg was observed in infant rats than in maternal ones, indicating the lower capacity of MeHg degradation or depletion in infants than adults. The results demonstrate that MeHg is the main species in infants that is responsible for the toxicity in the kidneys, and especially in brain, and furthermore, the results demonstrate the more severe damage of MeHg to young children than adults when the fetus in utero is exposed to MeHg via their mother. Such results also support the epidemiological observations that the fetus and infant are sensitive to methylmercury exposure.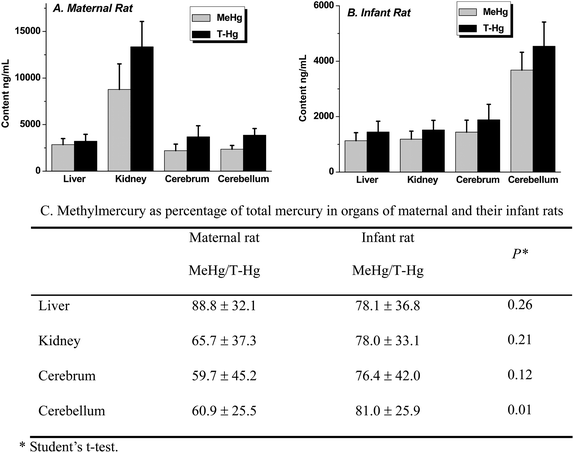 | ||
| Fig. 1 Total mercury (T-Hg) and methylmercury (MeHg) in liver, kidney, cerebrum and cerebellum of maternal and infant rats. (A) T-Hg and MeHg in the samples of maternal rats; (B) T-Hg and MeHg in the samples of infant rats; (C) the calculated data of MeHg as percentage of total mercury in organs of maternal and infant rats. | ||
Comparative study of intracellular distributions of mercury species in tissues between maternal and infant rats
Furthermore, the distributions of T-Hg, inorganic mercury (I-Hg) and MeHg were compared in subcellular fractions, including the nucleus, mitochondrion, lysosome and microsome and cytosol of brain, liver and kidney between the maternal and infant rats (Fig. 2–5). It was found that in both maternal and infant rats, the highest concentration of Hg likely existed in the nuclei, then in lysosomes and microsomes, and mitochondria of brain and liver, while as in the kidneys, the highest mercury was in lysosomes and microsomes, then in mitochondria of both maternal and infant rats (Fig. 2). The top curves show the relative percentage of T-Hg accumulation in the intracellular fractions. It should be noticed that about 55% and 44% of the Hg was accumulated in the brain nucleic fraction of maternal and infant rats, respectively (Fig. 2), and almost 80% of Hg exists in the cytosol of kidneys of both rats, which indicates the excretion of Hg from urine. Lysosome has been proven to be the major Hg storage sites in cells, where Hg inhibits the lysosomal marker enzyme acid phosphatase to some extent.17 Mercury is to some degree known that at the molecular level it has genotoxic effects including inducing DNA single-strand breaks or alkali labile sites following acute exposure to eucaryotic cells at low Hg levels.18 Thus, in the work the different distribution patterns of mercury in organs and subcellular fractions may indicate the different toxic mechanism between mothers and their offspring.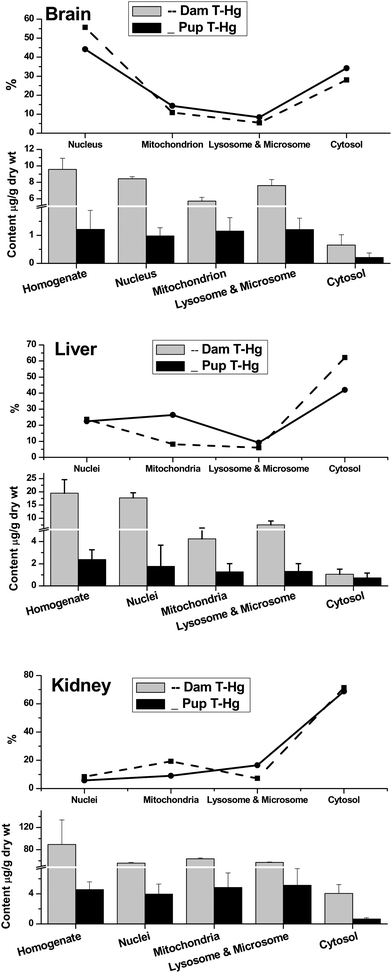 | ||
| Fig. 2 Comparison of total Hg (T-Hg) in homogenate, nuclei, mitochondria, lysosomes & microsomes and cytosol of brain, liver and kidneys between maternal and infant rats. The top broken lines present the relative percentage of T-Hg in the subcellular fractions of the whole tissues. | ||
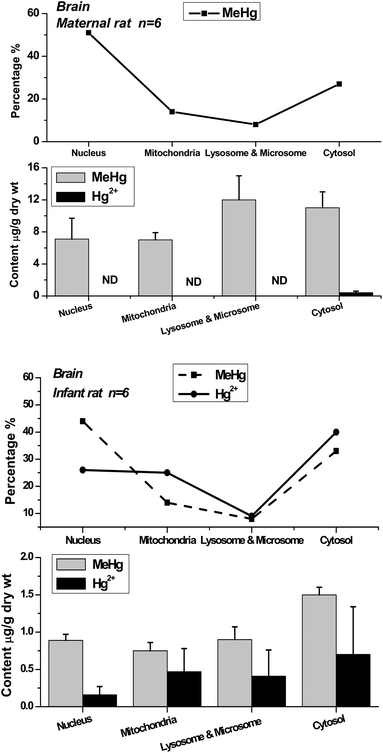 | ||
| Fig. 3 Methylmercury (MeHg) and inorganic mercury (Hg2+) in nuclei, mitochondria, lysosomes & microsomes and cytosol of maternal and infant rat brain. The top broken lines present the relative percentage of MeHg and Hg2+ in the subcellular fractions of brain. | ||
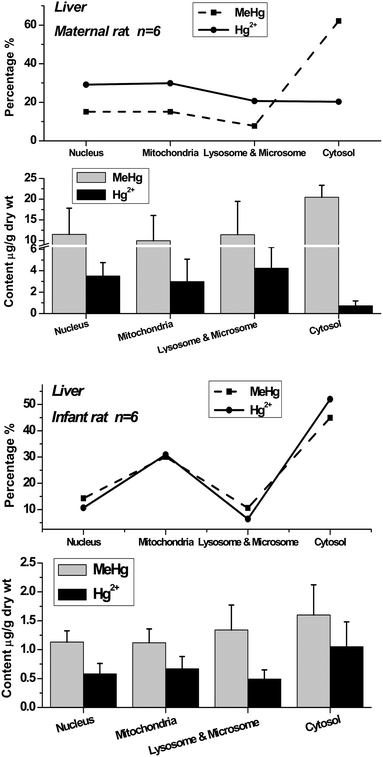 | ||
| Fig. 4 Methylmercury (MeHg) and inorganic mercury (Hg2+) in nuclei, mitochondria, lysosomes & microsomes and cytosol of maternal and infant rat liver. The top broken lines present the relative percentage of MeHg and Hg2+ in the subcellular fractions of liver. | ||
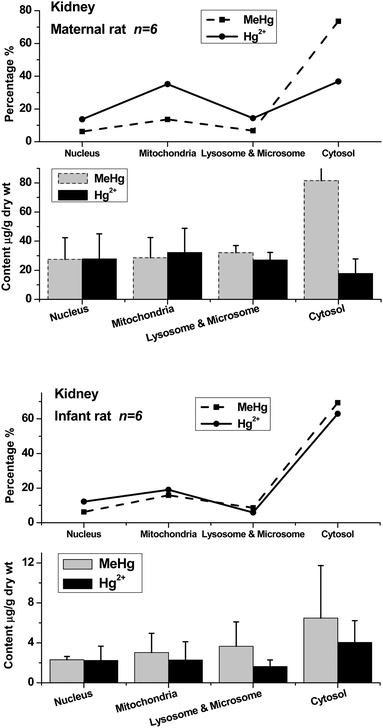 | ||
| Fig. 5 Methylmercury (MeHg) and inorganic mercury (Hg2+) in nuclei, mitochondria, lysosomes & microsomes and cytosol of maternal and infant rat kidneys. The top broken lines present the relative percentage of MeHg and Hg2+ in the subcellular fractions of kidneys. | ||
The distribution patterns of mercury species in intracellular fractions may help to explore the metabolic pathway and toxicity of methylmercury in organs. The data show that the distribution patterns of Hg species in the brains of maternal and infant rats are significantly different. In the maternal rat, most Hg existed as MeHg in the brain, and almost no inorganic mercury (I-Hg) was found in almost all of the intracellular fractions except for very low content in cytosol. Comparatively, I-Hg was detected in all the intracellular fractions of the infant rat brain, among which the cytosol contained the highest concentrations of I-Hg and MeHg, followed by mitochondria and lysosomes & microsomes (Fig. 3). The top curves present the percentage of mercury species in each subcellular fraction. In the liver and kidneys, most of the MeHg existed in the cytosolic fraction of both maternal and infant rats, and about one-third to one-half of the MeHg in the nucleic, mitochondrial, lysosomal & microsomal fractions was converted to I-Hg after 20 days of exposure.
It has been known that only a minor demethylation of methylmercury takes place in the brain,19 which is in great accordance with the results in adult rats (Fig. 3). However, as in the case of infant subjects, the mercury species in their body, and especially in the brain, are still seldom known. In the study of the infant rats exposed to mercury during the pre-natal and post-natal periods, the mercury accumulation in their bodies may all come from their mothers, either via placental transportation or breast milk digestion. The I-Hg in the infant body may be the sources from MeHg conversion or demethylation by the metabolism of maternal rats or infant rats themselves. For example, a considerable portion of I-Hg could be determined in the placenta following pregnant women's exposure to MeHg.20 Studies on rats and monkeys indicate that inorganic mercury penetrates the blood–brain barrier only to a very limited extent.19 Recently, an in vitro study has demonstrated that MeHg can be converted to I-Hg or increases demethylation with oxidative stress in astrocyte cells.21 In conclusion, the metabolism of mercury species in organisms is very complex; nevertheless, no matter how the process is accomplished, the distribution patterns of mercury species in maternal and infant rats show the differences of mercury metabolic behavior and toxic mechanism between them.
The organelles are all sub-compartments in cells with specialized functions. For example, lysosomes are subcellular organelles containing a battery of hydrolytic enzymes that are capable of degrading exogenous macromolecules.22 Lysosome accumulation of chemical contaminants can lead to membrane damage resulting in leakage of their contents into the cytosol and damage to cells.22 Lysosomes are proved to be the major mercury storage sites in the Aedes cells after 30 min of inorganic mercury treatment.17 At sub-lethal concentrations, Hg was found to be inhibiting the lysosomal marker enzyme acid phosphatase to some extent. However, no effect of MeHg was observed on this enzyme.17 In other studies, the highest ratios of inorganic to total mercury (about 50% of the total as inorganic) were observed in the mitochondria and microsomes of the kidney at days 26–37 of post-injection of MeHg in rats.23 In the liver, the ratio was strikingly low in the cytosol and microsomes as compared to the mitochondria, where about 40% of the total existed as inorganic species at day 26.17 Toxic exogenous contamination in different organelles always leads to differently harmful effects, such as oxidative stress, membrane damage, cell apoptosis, genotoxicity and so forth.
Comparative study of mercury-containing proteins in brain cytosol between maternal and infant rats
Fig. 6 presents the SEC-ICP-MS chromatograms of mercury-containing proteins in the brain cytosol of the maternal and infant rats. There are three mercury-containing proteins found in the maternal and infant samples in this study. In the maternal sample, the relative molecular weights of the proteins from peak I to peak III are calibrated as 480, 288 and 13 kDa, respectively, and in the infant sample, peaks I–III are calibrated as 100, 79 and 12.6 kDa, respectively. Based on the isotopic dilution equation described in the method, the quantitative amounts of mercury could be obtained. Peak III was found to contain the highest content of Hg, i.e. 1.55 ng/g concentration with a relative percentage of 53% of the total amount of mercury-containing proteins in the brain cytosol of the maternal rat; in contrast, in the infant rat, the concentration of Hg in the similar molecular weight fraction was about 5.7 times lower than in the maternal sample. Approximately 34% Hg existed in relatively high-molecular-weight proteins in maternal samples, in comparison to the 47% Hg presented in the infant samples. The results are congruent with our previous study.10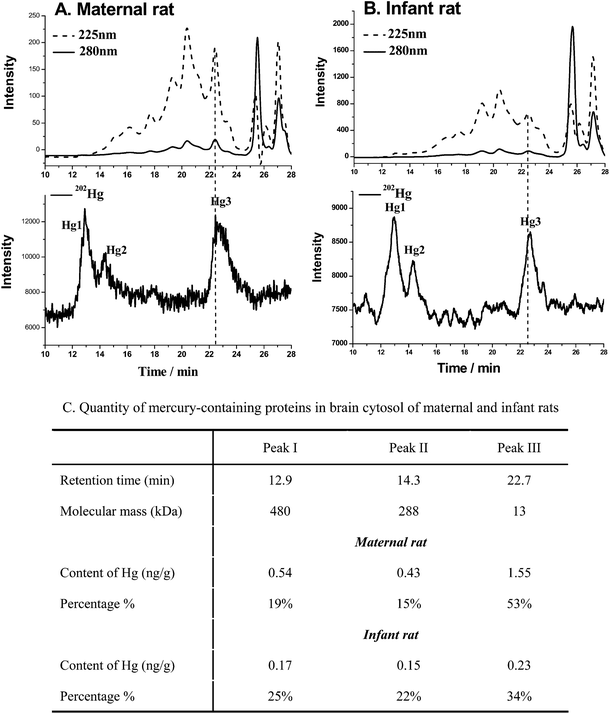 | ||
| Fig. 6 Hg-containing proteins in cytosol of rat brain by SEC-ICP-MS. (A) Hg-containing proteins in cytosol of maternal brain; (B) Hg-containing proteins in cytosol of infant brain; (C) quantity of mercury-containing proteins in brain cytosol of maternal and infant rats by SEC-ID-ICP-MS. | ||
It is known that metallothioneins (MTs) likely participate in the accumulation, transport, and metabolism of heavy metals in biological systems. In particular, the MT-3 (growth inhibitory factor, GIF), a specific member mainly presenting in brain, has been demonstrated in response to brain injury or during neurodegenerative disease progression, such as Alzheimer's disease.24 In order to investigate if there was differential expression of MTs in the organs after MeHg exposure, the proteins in the brain, liver and kidney cytosol of maternal samples were firstly purified by acetonitrile precipitation and then separated by SDS-PAGE (Fig. 7). Fig. 7 shows that there are ca. 4–5 lines of brain, liver and kidney samples presenting in the gel, in which the lines 1, 3, 4, and 5 coexist in the brain, liver and kidney samples, whereas, line 2 is only present in the brain sample. The MALDI-TOF-MS measurements (Fig. 8) show that in the range of ca. 6000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, all of the present proteins are similar among the brain, liver and kidneys, except for Pro.2 at m/z = 6810 in the brain sample. The molecular weight of this protein accords exactly with the molecular weight of MT-3 (6809 Da) in Swiss-Prot. The data by MALDI-MS verify the results of SDS-PAGE. In comparison, the levels of MTs in the infant rat were very low. The results suggest the MTs may relate with the toxicity of mercury in mother and offspring.
000 Da, all of the present proteins are similar among the brain, liver and kidneys, except for Pro.2 at m/z = 6810 in the brain sample. The molecular weight of this protein accords exactly with the molecular weight of MT-3 (6809 Da) in Swiss-Prot. The data by MALDI-MS verify the results of SDS-PAGE. In comparison, the levels of MTs in the infant rat were very low. The results suggest the MTs may relate with the toxicity of mercury in mother and offspring.
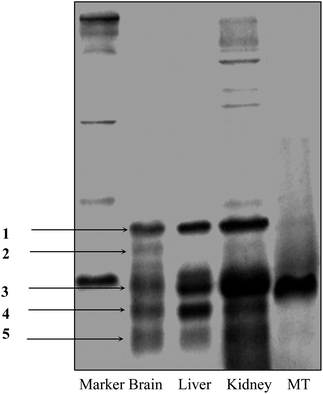 | ||
| Fig. 7 SDS-PAGE analysis of proteins in cytosol of rat brain, liver and kidney after acetonitrile precipitation. There are five lines present in brain samples: lines 1, 3, 4, and 5 coexist in brain, liver and kidney samples; line 2 is only in the brain sample. | ||
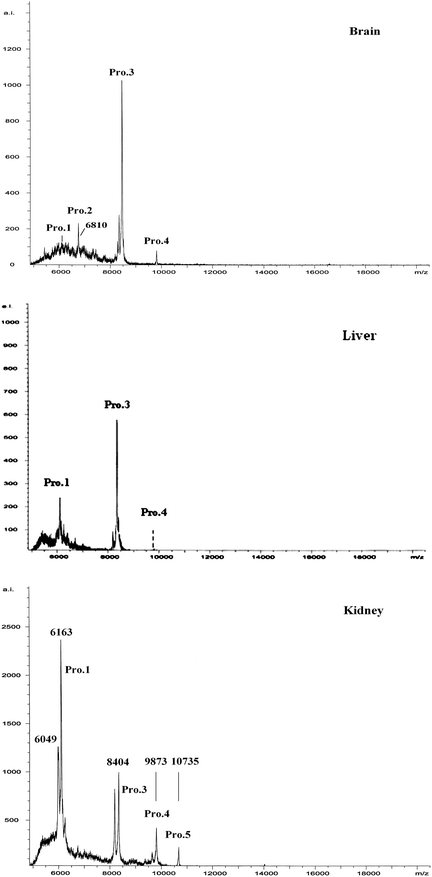 | ||
| Fig. 8 MALDI-TOF-MS measurements of proteins in cytosol of maternal rat brain after acetonitrile precipitation purification. The molecular weight of Pro.2 is accordance with the molecular weight of MT-3 (6809 Da). | ||
Conclusions
In this study, we compare the distribution patterns of mercury species, including methylmercury and inorganic mercury, and mercury-containing proteins in brain, liver and kidneys, and the subcellular fractions (nucleus, mitochondrion, lysosome, microsome and cytosol) of maternal and infant rats after infant rat in utero and lactational exposure to methylmercury by the methods of extraction preconcentration with HPLC-ICP-MS for MeHg and I-Hg determination, the HPLC-ICP-ID (Isotope Dilution)-MS for Hg-containing proteins quantification, and SDS-PAGE and MALDI-TOF-MS for protein identification. The results demonstrated the distribution patterns of MeHg and I-Hg of mothers and their offspring either in the levels of organs, the subcellular fractions or proteins are significantly different, indicating the significantly different metabolism, transportation and accumulation of mercury species between them, which may be all involved in the toxic mechanism of the toxic metal. Further, the results show that the identification of metallic speciation changes in organelles can be an important step towards understanding the metal's physiological functions in cells and organs.Acknowledgements
This work is funded by the National Basic Research Program (2011CB933400), the Knowledge Innovation Program of Chinese Academy of Sciences (KJCX3.SYW.N3) and the National Natural Science Foundation of China (20805048, 10975148 and 10905064).References
- M. Harada, Teratology, 1978, 18, 285–288 CrossRef CAS.
- P. Grandjean, P. Weihe, R. F. White, F. Debes, S. Araki, K. Yokoyama, K. Murata, N. Sørensen, R. Dahl and P. J. Jørgensen, Neurotoxicol. Teratol., 1997, 19, 417–428 CrossRef CAS.
- P. W. Davidson, G. J. Myers, C. Cox, C. Axtell, C. Shamlaye, J. Sloane-Reeves, E. Cernichiari, L. Needham, A. Choi, Y. Wang, M. Berlin and T. W. Clarkson, JAMA, J. Am. Med. Assoc., 1998, 280, 701–707 CrossRef CAS.
- P. Grandjean, P. J. Jorgensen and P. Weihe, Environ. Health Perspect., 1994, 102, 74–77.
- U.S. EPA, Methylmercury Reference Dose for Chronic Oral Exposure. U.S. Environmental Protection Agency, Integrated Risk Information System (IRIS), Washington, 2001, DC, U.S. Environmental Protection Agency; available: http://www.epa.gov/iris/subst/0073.htm [accessed 22 April 2004].
- Y. Kajiwara, A. Yasutake, T. Adachi and K. Hirayama, Arch. Toxicol., 1996, 70, 310–314 CrossRef CAS.
- J. Sundberg, A. Oskarsson and L. Albanus, Bull. Environ. Contam. Toxicol., 1991, 46, 255–262.
- L. Huber, K. Pfaller and I. Vietor, Circ. Res., 2003, 92, 962 CrossRef CAS.
- S. Brunet, P. Thibault, E. Gagnon, P. Kearney, J. Bergeron and M. Desjardins, Trends Cell Biol., 2003, 13, 629–638 CrossRef CAS.
- J. Shi, W. Feng, M. Wang, F. Zhang, B. Li, B. Wang, M. Zhu and Z. Chai, Anal. Chim. Acta, 2007, 583, 84–91 CrossRef CAS.
- M. Wang, W. Feng, J. Shi, F. Zhang, B. Wang, M. Zhu, B. Li, Y. Zhao and Z. Chai, Talanta, 2007, 71, 2034–2039 CrossRef CAS.
- M. Wang, W. Feng., W. Lu, B. Li, B. Wang, M. Zhu, Y. Wang, H. Yuan, Y. Zhao and Z. Chai, Anal. Chem., 2007, 79, 9128–9134 CrossRef CAS.
- M. Wang, W. Feng, H. Wang, Y. Zhang, J. Li, B. Li, Y. Zhao and Z. Chaia, J. Anal. At. Spectrom., 2008, 23, 1112–1116 RSC.
- P. Rodriguez-Gonzalez, J. M. Marchante-Gayón, J. I. G. Alonso and A. Sanz-Medel, Spectrochim. Acta, Part B, 2005, 60, 151–207 CrossRef.
- M. Wang, Y. Zhang, W. Y. Feng, M. Guan, B. Wang, J. W. Shi, M. T. Zhu, B. Li, Y. L. Zhao and Z. F. Chai, Chin. J. Anal. Chem., 2007, 35, 945–948 CAS.
- A. Prange, D. Schaumlöffel, P. Brätter, A. Richarz and C. Wolf, Fresenius J. Anal. Chem., 2001, 371, 764–774 CrossRef CAS.
- B. Braeckman and H. Raes, Tissue Cell, 1999, 31, 223–232 CrossRef CAS.
- L. Bucio, C. Garcia, V. Souza, E. Hernandez, C. Gonzalez, M. Betancourt and M. C. Gutierrez-Ruiz, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1999, 423, 65–72 CrossRef CAS.
- L. Friberg and N. Mottet, Biol. Trace Elem. Res., 1989, 21, 201–206 CrossRef CAS.
- K. Ask, A. Akesson, M. Berglund and M. Vahter, Environ. Health Perspect., 2002, 110, 523 CAS.
- A. Shapiro and H. Chan, Chemosphere, 2008, 74, 112–118 CrossRef CAS.
- D. M. Lowe and V. U. Fossato, Aquat. Toxicol., 2000, 48, 75–85 CrossRef CAS.
- S. Omata, M. Sato, K. Sakimura and H. Sugano, Arch. Toxicol., 1980, 44, 231–241 CrossRef CAS.
- Y. Uchida, K. Takio, K. Titani, Y. Ihara and M. Tomonaga, Neuron, 1991, 7, 337–347 CAS.
Footnote |
| † This article is part of a themed issue highlighting outstanding and emerging work in the area of speciation. |
| This journal is © The Royal Society of Chemistry 2011 |
