Microwave and flow syntheses of Pseudomonas quinolone signal (PQS) and analogues†
James T.
Hodgkinson
a,
Warren R. J. D.
Galloway
a,
Shreya
Saraf
a,
Ian R.
Baxendale
a,
Steven V.
Ley
a,
Mark
Ladlow
b,
Martin
Welch
c and
David R.
Spring
*a
aDepartment of Chemistry, University of Cambridge, Lensfield Road, Cambridge, UK. E-mail: spring@ch.cam.ac.uk; Fax: +44 (0) 1223-336362; Tel: +44 (0) 1223-336498
bUniqsis Ltd, 29 Station Road, Shepreth, Cambridgeshire, UK SG8 6GB
cDepartment of Biochemistry, University of Cambridge, Cambridge, UK
First published on 22nd October 2010
Abstract
Expedient syntheses of Pseudomonas quinolone signal (PQS) and related structural analogues using microwave and flow methods are reported.
Quorum sensing is the intercellular signalling mechanism, mediated by small molecules, that numerous species of bacteria use to co-ordinate their gene expression in a cell-density dependent manner.1 Several clinically relevant pathogens use quorum sensing systems to regulate processes associated with virulence. However, quorum sensing is not directly involved in biological processes essential for bacterial survival.2 Thus selective disruption of quorum sensing represents a strategy to attenuate bacterial pathogenicity without imposing an intense selective pressure for the development of resistant mutants.3 Recent years have witnessed significant efforts towards identifying small molecules that can modulate quorum sensing pathways, with either agonist or antagonist activity, and thereby enhance understanding of this form of intercellular communication.4
It has emerged that some 2-alkyl-4-quinolone derivatives, most notably 2-heptyl-3-hydroxyl-4(1H)-quinolone (Pseudomonas quinolone signal, PQS), serve as signalling molecules in the quorum sensing of Pseudomonas aeruginosa (Fig. 1).5,6 This bacterium is associated with a range of life-threatening nosocomial infections and is the primary cause of mortality in cystic fibrosis (CF) patients.7
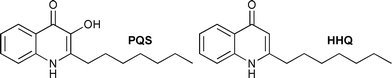 | ||
| Fig. 1 The structure of PQS together with its biosynthetic precursor 2-heptyl-4-quinolone (HHQ); both have been shown to be involved in the quorum sensing of P. aeruginosa.6 | ||
PQS has been implicated as an important factor in the virulence of P. aeruginosa. For example, PQS is known to control the production of multiple virulence determinants8 and the molecule has also been reported in the sputa of CF patients, indicating that it may play a central role in the establishment of chronic infections.9,10 Despite the biological importance of PQS, synthetic approaches towards PQS and related analogues are much less numerous than those for other quorum sensing molecules, such as the widely studied N-acyl-L-homoserine lactones (AHLs).11 Consequently there is a relative paucity of information regarding structure–activity relationships (SAR) exhibited by these quinolone-based small molecules in bacterial quorum sensing systems.12 Such data should facilitate our understanding of quorum sensing in general and consequently enhance our ability to manipulate such systems in a useful fashion. PQS analogues may prove to be valuable tools for chemical biology studies and could potentially be used in a therapeutic context for the treatment of human bacterial infections
Recently we reported the first systematic SAR analysis of PQS.10 Herein we describe how the analogues examined in that study were generated. The synthetic route centres around a novel one-pot microwave-assited procedure that allows expedient access to PQS and a range of other 2-alkyl-4-quinolone derivatives from readily available starting materials. In addition, we report the successful adaptation and optimisation of these microwave-based conditions for use in a continuous flow reaction, which has enabled the synthesis of PQS on a multi-gram scale.
The majority of PQS syntheses described in the literature are based around a method developed by Pesci et al. which involves 2-heptylquinoline (HHQ) as a key intermediate (Scheme 1).5 A Duff reaction is used to convert HHQ to 1 and subsequent Dakin oxidation furnishes PQS. However, there are several drawbacks associated with this procedure. HHQ itself is not commercially available and methods for its synthesis are somewhat lengthy.13 In addition, we have found both the Duff formylation and Dakin oxidation steps to be capricious, which significantly detracts from the efficiency of the synthesis.
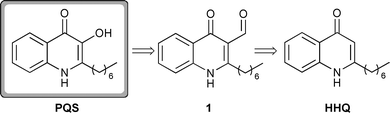 | ||
| Scheme 1 Synthesis of PQS by Pesci et al.5 proceeds viaHHQ. | ||
Hradil et al. have previously reported a two-step procedure for the synthesis of quinolinones 2 (R1 = Me or Ph) by the cyclisation of phenacyl anthranilates 3 derived from the reaction of anthranilic acids 5 and α-chloro ketones 6 (Scheme 2).14 It was envisaged that this method could be adapted for the synthesis of PQS (R1 = (CH2)6CH3), which would require the use of anthranilic acid (5) and α-chloro ketone 7.15
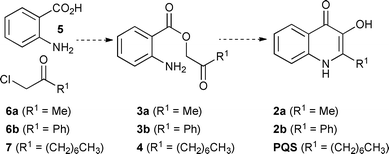 | ||
| Scheme 2 Outline of proposed synthetic route towards PQS based on methodology developed by Hradil et al.11 for the synthesis of quinolinones 2.12 | ||
Towards this end we developed a high-yielding, one-step method for the synthesis of α-chloro ketone 7 starting from readily available 2-chloro-N-methoxy-N-methylacetamide 8 and heptyl Grignard reagent9 (Scheme 3).16 In addition, we theorised that acylation of anthranilic acid and subsequent cyclisation could be carried out without isolation of the intermediate ester derivative 4.
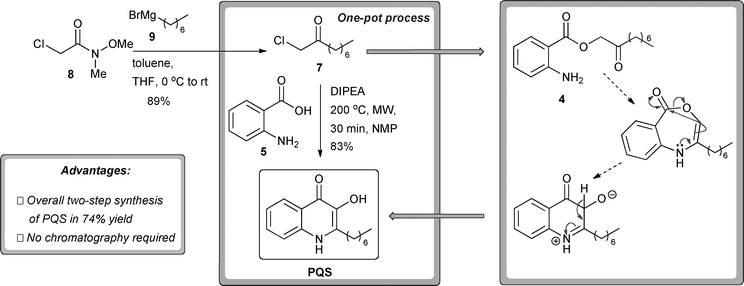 | ||
| Scheme 3 One-pot synthesis of PQS from readily synthesised α-chloro-ketone 7 and commercially available anthranilic acid (5). A tentative mechanistic proposal is shown. DIPEA = di-isopropyl-ethylamine. MW = microwave irradiation. NMP = N-methylpyrrolidone. | ||
Extensive optimisation studies culminated in the development of a highly efficient one-pot reaction protocol for the synthesis of PQS from commercially available anthranilic acid and readily synthesised α-chloro ketone 7 under microwave irradiation conditions (Scheme 3). In line with previous studies on the formation of related 3-amino systems, we propose that this one-pot process proceeds via a tandem SN2, enamine formation, nucleophilic rearrangement sequence (Scheme 3).17 Upon completion of the reaction, the desired product could be obtained in high yield simply by precipitation induced by the addition of water.18
There are several notable features associated with this protocol:
1. Both reaction set-up and purification are operationally simple.
2. The intermediate adduct 4 does not need to be isolated.
3. The starting materials for the reaction are readily available (either commerically available or easily synthesised).
4. The reaction time is relatively short (30 min).
5. The reaction is reproducible and isolated yields of pure product are usually good.
A further point worthy of note is that no purification of the intermediate α-chloro ketone 7 is required; the crude material obtained after work-up can be used directly in the next reaction step. Consequently column chromatography is avoided at all stages of PQS synthesis.
Use of this tandem reaction process allows a two-step synthesis of PQS from readily obtainable or commercially available materials with an overall yield of 74%. To the best of our knowledge this is the shortest and most efficient reported synthesis of PQS from readily available starting materials to date.19
In the context of PQS analogue synthesis, this route is also advantageous due to its inherent flexibility. For example, substitution at the 2-position and the introduction of heteroatoms around the aromatic ring could, in principle, simply be achieved through variation in the anthranilic acid and α-chloro ketone building-blocks used; as there are a wide range of such compounds available commerically (or readily synthesised), the method offers the potential for rapid modification. A variety of different α-chloro ketones were easily obtained simply by the treatment of 8 with different Grignard reagents. Reaction with 5 under the optimised one-pot conditions led to a variety of PQS analogues with different groups present at the 2-position of the quinolone ring (10, 11 and 12, Fig. 2). Similarly a range of analogues with different heteroatoms around the aryl ring (13 and 14) were obtained through the use of a variety of different anthranilic acid derivatives (Fig. 2). In all cases pure product was obtained without recourse to column chromatography. Isolated yields of the desired products were typically moderate to good.
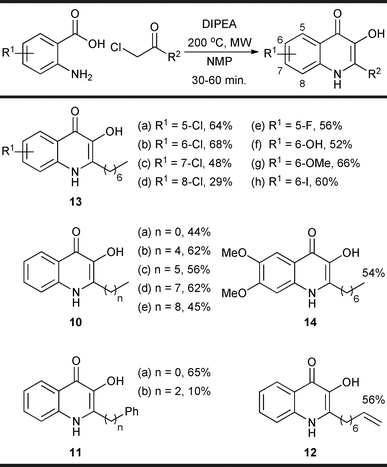 | ||
| Fig. 2 PQS analogues synthesised in this study. The quoted yields are calculated over two steps (i.e. from Weinreb amide 8 to isolated pure product). | ||
The PQS analogues described in this report were tested in a range of biological assays.10 One such assay focused upon the interaction of these quinolone molecules with the protein PqsR. PqsR (otherwise known as the multiple virulence factor regulator or MvfR) is a transcriptional regulator that plays a critical role in P. aeruginosa pathogenicity.20aPQS is known to bind to PqsR and is thought to act as a co-inducer; for example, PQS in concert with PqsR regulates the production of a variety of extracellular virulence factors such as pyocyanin and hydrogen cyanide.20a–c We were thus interested in examining how structural modifications of the PQS molecule affected its ability to bind to, and thus activate, PqsR. Towards this end the PQS analogues described in this report were tested for their ability to stimulate PqsR-dependent pqsAtranscription from the pqsA promoter in Escherichia coli strain DH5α containing the reporter plasmid pEAL08-2.10 This plasmid expresses PqsR (MvfR) and also carries a lacZ open reading frame cloned behind the pqsApromoter region.10,20b The lacZgene encodes β-galactosidase. In the presence of co-inducer, PqsR will bind to the pqsApromoter and stimulate transcription of the downstream lacZORF, thereby leading to β-galactosidase production. Therefore a comparison of the β-galactosidase activity of the E. coli cells grown in the presence of PQS with that of cells grown in the presence of a PQS analogue provides an indication of the relative ability of the different quinolone molecules to bind to the PqsR protein.10 Using this assay system a structure–activity profile of the PQS analogues for this phenotype was established.10 Some selected results are shown in Fig. 3.21 It was found that agonism was strongly dependent upon the alkyl chain length. Small modifications in chain length 10b, 10c, 10d and 10e resulted in a slight loss whereas a major reduction in chain length 10a or the substitution of the heptyl chain for a phenyl ring 11a and 11b greatly reduced activity. In addition, this analysis suggested that the electron density around the quinolone aromatic ring has an effect upon the level of PqsR binding. The introduction of electron donating groups at position 6 led to a large reduction in activity 13e, 13g and 14. For a full account of the biological assessment of the PQS analogues the reader is directed to the original report.10
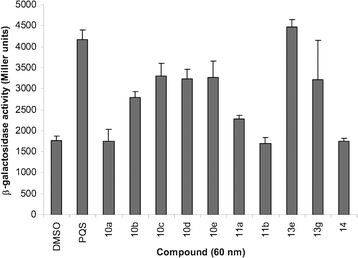 | ||
| Fig. 3 Stimulation of MvfR-dependent promoter activity (as measured by levels of β-galactosidase activity) by a selection of the PQS analogues. DMSO was added as a control. The data represent the averages and standard deviations from the results of three independent repeats.10 | ||
The microwave-based protocol outlined in Scheme 3 proved to be a highly efficient and robust method for the synthesis of a range of PQS derivatives. However, one limitation associated with this route is its amenability to scale-up. For the synthesis of large amounts (grams) of material the need to empty and refill conventional batch microwave vessels coupled with repetitive heating up and cooling down cycles is both inconvenient and decreases throughput. We were thus interested in exploring the synthesis of PQS and analogues under continuous flow conditions as this would provide a more efficient means of preparing the larger quantities of these materials required for any further, more detailed biological and pharmacological studies. Towards this end we investigated the adaptation and optimisation of our previously developed microwave-based PQS synthesis conditions for use in a Uniqsis FlowSyn™ continuous flow reactor (Scheme 4).22 Pleasingly, under slightly modified reaction conditions (reaction temperature of 220 °C, residence time of 5 min) it proved possible to synthesise PQS on a multi-gram scale and with comparable purity to the PQS generated using the microwave-based protocol (albeit in a slightly reduced yield). In principle, through the use of different anthranilic acid and α-chloro ketone building-blocks in the reaction channels, (Scheme 4) it should prove to be possible to achieve the large scale synthesis of a range of PQS analogues in-flow.23
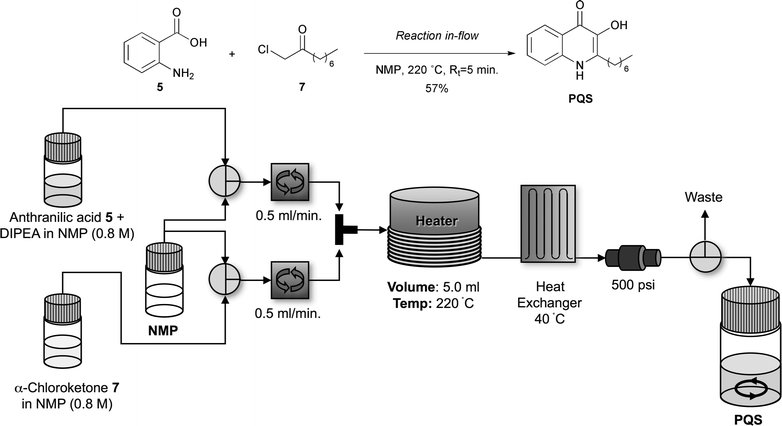 | ||
| Scheme 4 Schematic of the synthesis of PQS using a Uniqsis FlowSyn™ continuous flow reactor.22 | ||
In conclusion, we have developed an efficient microwave-based reaction process that allows expedient access to PQS and a range of novel non-natural analogues from readily available starting materials. Use of this chemistry facilitated the first systematic SAR analysis of PQS to be carried out.10 Furthermore we have described the successful adaptation and optimisation of these microwave-based conditions for use in a continous flow reaction, which has enabled the synthesis of PQS on a multi-gram scale. Additional analogue syntheses and biological studies should further enhance our understanding of PQS-mediated quorum sensing systems; this may facilitate the development of methods that can modulate these signalling networks in a useful fashion, with possible therapeutic applications in the treatment of human bacterial infections.
We gratefully acknowledge financial support from the EPSRC, BBSRC, MRC and Newman Trust.
Notes and references
- (a) W. C. Fuqua, S. C. Winans and E. P. Greenberg, J. Bacteriol., 1994, 176, 269–275 CAS; (b) B. L. Bassler and R. Losick, Cell, 2006, 125, 237–246 CrossRef CAS; (c) C. Fuqua, M. R. Parsek and E. P. Greenberg, Annu. Rev. Genet., 2001, 35, 439 CrossRef CAS; (d) M. Welch, H. Mikkelsen, J. E. Swatton, D. S. Smith, G. L. Thomas, F. G. Glansdorp and D. R. Spring, Mol. BioSyst., 2005, 1, 196–202 RSC; (e) D. Smith, J.-H. Wang, J. E. Swatton, P. Davenport, B. Price, H. Mikkelsen, H. Stickland, K. Nishikawa, N. Gardiol, D. R. Spring and M. Welch, Sci. Prog., 2006, 89, 167–211 CrossRef CAS.
- (a) T. B. Rasmussen and M. Givskov, Microbiology, 2006, 152, 895–904 CrossRef CAS; (b) M. Hentzer and M. Givskov, J. Clin. Invest., 2003, 112, 1300–1307 CrossRef CAS.
- (a) F. A. A. Amer, E. M. El-Behedy and H. A. Mohtady, Biotechnol. Molec. Biol. Rev., 2008, 3, 46–57 Search PubMed; (b) H. Suga and K. M. Smith, Curr. Opin. Chem. Biol., 2003, 7, 586–591 CrossRef CAS; (c) S. Everts, in Chem. Eng. News, 2006, 84 (43), pp. 17–26 Search PubMed.
- For recent discussions see: (a) M. E. Pomianek and M. F. Semmelhack, ACS Chem. Biol., 2007, 2, 293–295 CrossRef CAS; (b) N. Ni, M. Li, J. Wang and B. Wang, Med. Res. Rev., 2009, 29, 65–124 CrossRef CAS; (c) S. R. Chhabra, C. Harty, D. S. W. Hooi, M. Daykin, P. Williams, G. Telford, D. I. Pritchard and B. W. Bycroft, J. Med. Chem., 2003, 46, 97–104 CrossRef CAS; (d) T. Hjelmgaard, T. Persson, T. B. Rasmussen, M. Givskov and J. Nielsen, Bioorg. Med. Chem., 2003, 11, 3261–3271 CrossRef CAS; (e) G. D. Geske, J. C. O'Neil, D. M. Miller, M. E. Mattmann and H. E. Blackwell, J. Am. Chem. Soc., 2007, 129, 13613–13625 CrossRef CAS.
- E. C. Pesci, J. B. J. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg and B. H. Iglewski, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 11229–11234 CrossRef CAS.
- For a recent discussion of PQS-mediated quorum sensing see: J. F. Dubern and S. P. Diggle, Mol. BioSyst., 2008, 4, 882–888 Search PubMed.
- R. Popat, S. A. Crusz and S. P. Diggle, Br. Med. Bull., 2008, 87, 63–75 Search PubMed.
- (a) E. Deziel, S. Gopalan, A. P. Tampakaki, F. Lepine, K. E. Padfield, M. Saucier, G. Xiao and L. G. Rahme, Mol. Microbiol., 2004, 55, 998–1014 CrossRef; (b) S. P. Diggle, K. Winzer, S. R. Chhabra, K. E. Worrall, M. Camara and P. Williams, Mol. Microbiol., 2003, 50, 29–43 CrossRef CAS; (c) L. A. Gallagher, S. L. McKnight, M. S. Kuznetsova, E. C. Pesci and C. Manoil, J. Bacteriol., 2002, 184, 6472–6480 CrossRef CAS See also ref. 5.
- D. N. Collier, L. Anderson, S. L. McKnight, T. L. Noah, M. Knowles, R. Boucher, U. Schwab, P. Gilligan and E. C. Pesci, FEMS Microbiol. Lett., 2002, 215, 41–46 CrossRef CAS.
- J. Hodgkinson, S. D. Bowden, W. R. J. D. Galloway, D. R. Spring and M. Welch, J. Bacteriol., 2010, 192, 3833–3837 CrossRef CAS.
- For recent selected examples of modulating AHL-mediated quroum sensing systems see: (a) G. D. Geske, J. C. O'Neill and H. E. Blackwell, Chem. Soc. Rev., 2008, 37, 1432–1447 RSC and references therein; (b) G. L. Thomas, C. M. Bohner, H. E. Williams, C. M. Walsh, M. Ladlow, C. E. Byrant and D. R. Spring, Mol. BioSyst., 2006, 2, 132–137 RSC; (c) K. Tateda, Y. Ishii, M. Horikawa, T. Matsumoto, S. Miyairi, J. C. Pechere, T. J. Standiford, M. Ishiguro and K. Yamaguchi, Infect. Immun., 2003, 71, 5785–5793 CrossRef CAS.
- For selected examples of limited SAR studies on such molecules in the context of quorum sensing systems see: (a) S. P. Diggle, K. Winzer, S. R. Chhabra, K E. Worall, M. Camara and P. Williams, Mol. Microbiol., 2003, 50, 29–43 CrossRef CAS; (b) E. C. Pesci, J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg and B. H. Iglewski, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 11229–11234 CrossRef CAS Pesci et al. demonstrated that 3-heptyl-2-hydroxy-4(1H)-quinolone (in which the heptyl- and hydroxyl subsitutents have been switched positions relative to PQS) and 2-heptyl-4-hydroxyquinoline N-oxide (a PQS isomer which lacks the 3-hydroxy group) do not exhibit any biological activity comparable to that of PQS. In addition, Diggle et al. demonstrated that both 2-heptyl-4(1H)-quinolone and 3-formyl-2-heptyl-4(1H)-quinolone were biologically inactive in this context.
- For example, Pritchard and co-workers have reported a 3-step synthesis of HHQ from octanoic acid which proceeds in an overall yield of ∼ 48%: D. I. Pritchard, B. W. Bycroft, S. R. Chhabra and D. Hooi. Substitued 4-quinolones. PCT Int. Appl. WO/2002/047686, 2002.
- P. Hradil, J. Hlavac and K. Lemr, J. Heterocycl. Chem., 1999, 36, 141–144 CrossRef CAS.
- A similar method for synthesising PQS (via the synthesis and isolation of intermediate (4) has been disclosed: I. C. Purcell, Bacterial Autoinducer Derived 4-Quinolones as Novel Immune Modulators. PhD thesis, 2007, University of Nottingham, UK. Purcell also describes the synthesis of 5 different 3-hydroxy-4(1H)-quinolone derivatives several 2-alkyl substituted PQS derivatives.
- R. Tillyer, L. F. Frey, D. M. Tschaen and U. H. Dolling, Synlett, 1996, 225 CrossRef CAS.
- P. Hradil, M. Grepl, J. Hlavac, M. Soural, M. Malon and V. Bertolasi, J. Org. Chem., 2006, 71, 819–822 CrossRef CAS.
- The product isolated after precipitation is typically approx. 90% pure by 1H NMR analysis. Further purification, if required, can be achieved by recrystallisation from ethyl acetate.
- The synthesis of PQS achieved using the route outlined in Scheme 1 has been reported to proceed in an overall yield of ∼ 13% from commercial starting materials (see ref. 12a and ref. 13).
- (a) G. P. Xiao, J. X. He and L. G. Rahme, Microbiology, 2006, 152, 1679–1686 CrossRef CAS; (b) C. Cugini, M. W. Calfee, J. M. Farrow, D. K. Morales, E. C. Pesci and D. A. Hogan, Mol. Microbiol., 2007, 65, 896–906 CrossRef CAS; (c) F. Dubern and S. P. Diggle, Mol. BioSyst., 2008, 4, 882–888 RSC.
- The analysis of the results assumes that the activity is due to protein binding rather than cell permeability and other transport properties.
- See: http://www.uniqsis.com/ (accessed 11th August 2010). Recent papers using the Uniqsis FlowSyn continuous flow reactor include: (a) A. Palmieri, S. V. Ley, A. Polyzos, M. Ladlow and I. R. Baxendale, Beilstein J. Org. Chem., 2009, 5 DOI:10.3762/bjoc.5.23; (b) A. Palmieri, S. V. Ley, K. Hammond, A. Polyzos and I. R. Baxendale, Tetrahedron Lett., 2009, 50, 3287–3289 CrossRef CAS; (c) C. F. Carter, I. R. Baxendale, J. B. J. Pavey and S. V. Ley, Org. Biomol. Chem., 2010, 8, 1588–1595 RSC.
- The synthesis of PQS analogue 10d was also achieved in-flow (see ESI†).
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details and analytical data for all novel compounds. See DOI: 10.1039/c0ob00652a |
| This journal is © The Royal Society of Chemistry 2011 |
