A photosensitive fluorinated ionic complex with tunable surface wetting properties: mesostructure and photosensitivity
Xuemin
Lu
,
Sufang
Xiao
,
Xumeng
Chen
and
Qinghua
Lu
*
School of Chemistry and Chemical Engineering, the State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai, 200240, China. E-mail: qhlu@sjtu.edu.cn; Fax: +86-21-54747535; Tel: +86-21-54747535
First published on 1st September 2011
Abstract
Through an ionic self-assembly approach, a photosensitive complex was prepared by attaching two different matching anions, photoresponsive units of methyl orange (MO) and perfluorinated dodecanoic acid (FDA), to a poly(ionic liquid) backbone (PIL). The FTIR spectrum proved that the two anions were competitively attached to the PIL backbone. The complexes show typical liquid crystalline behavior and have a single layer lamellar mesostructure for PMOF0. The incorporation of FDA leads to different lamellar structures in casting film depending on the FDA content: when the FDA content increases to 10%, MO and FDA side chains organized into a different lamellar structure independently: MO lamellar structure favors single layer packing order and FDA favors bi-layer packing order according to XRD investigation. These complexes are effective in controlling the in-plane and out-of-plane alignment of liquid crystal molecules.
Introduction
The alignment of film with anisotropic properties plays a key role in preparing anisotropic optical elements such as retarders, polarizing gratings, and anisotropic emitters.1 With aid of this feature film, different functional molecules, such as liquid crystal molecules,2–7 fluorescent molecules,8–12 semiconductor polymers13,14 or mesoporous molecules,15–17 can be in-plane oriented in a desired direction and the orientation order can be precisely adjusted. An effective way to achieve macroscopically aligned film is the light-induced orientation of photo-chromic polymers.18,19 Photo-orientation of azobenzene is the most prominent example for this so-called command surface due to its characterized trans-cisisomerization under light illumination. Recently, azobenzen materials with fluorinated group received special attention due to its excellent effect in constructing photo-switchable surface.20,21 Under UV light illumination, the surface can be tuned from superhydrophobicity and superhydrophilicity. Such a result was related to the light-induced change of surface energy. This phenomenon would also be useful to tune out-plane orientation of functional molecules dropped atop of fluorinated photosensitive film at a molecular level.The state-of-the-art fluorinated photosensitive material was prepared through the polymerization of an azobenzene containing monomer which was covalent bonded with a fluorinated group.22,23 Such covalently functionalized polymer can assure the high concentration of fluorinated azobenzene group and provide a large response to the optical fields. However, the high concentration of the fluorinated group also leads to the difficulty in tuning the surface energy in a wide range, and on the other hand, the laborious synthesis and inefficiency in broad range tenability of surface energy make it difficult to obtain target materials.
The integration of molecular functions in supramolecular assemblies is a promising approach for the development of new materials exhibiting enhanced and dynamic functions. The non-covalent approach plays a very important role in constructing such supramolecules with multiple functions. A variety of recognition motifs are employed including hydrogen bonding, coordination binding, and columbic interaction, etc. Among these motifs, ionic self-assembly (ISA, in brief), which mainly employs the Columbic interaction to bind the oppositely charged tectonic units together to form regular nanostructure, was widely exploited in constructing functional materials with ordered structure for its high strength.24–26A series of photosensitive complexes was also reported through this approach and it was found that such photosensitive complexes show comparative or even higher photosensitivity and thermostability compared with those prepared through covalent bonding.27–31 Compared with other non-covalent approaches, especially hydrogen bonding and coordination, ionic bonding shows no selectivity and no specific design of the binding motifs. So it is possible to construct multifunctional materials by attaching two or more tectonic unit to a single polymer backbone (ABC type complex). However, few report focused on the preparation of multifunctional materials using the ionic bonding approach up to now. What kind of structure will formed when the three kinds of ions were mixed together if they can form a complex was still an ambiguous question, which would also be more interesting to construct all-in-one function materials using this approach.
In this work, we tried to prepare fluorinated azobenzene-containing polymer through an ionic self assembly approach. As a photosensitive tectonic unit, methyl orange (MO) was used to construct the photosensitive complex with poly(ionic liquid) (PIL, in brief). Perfluorinated dodecanoic acid (FDA), which was widely used in constructing polymer material with tunable wetting properties, was selected to be the second ion. PIL molecules were chosen as the backbone due to their favorable properties of ionic self-assembly and easy tuning of the structure.32,33Special attention was paid to the effect of bi-ions on the mesostructure and photosensitivity of the complex. Furthermore, as a verification of application of the photosensitive complex, we employed this complex with different groups as the aligning layer for liquid crystal (LC) molecules.
Experimental section
Preparation of the photosensitive complex
FDA was obtained from Alfa Aesar. Before use, FDA was neutralized using sodium hydroxide. MO was purchased from the Sinopharm Chemical Reagent Company and used without further purification. Poly(1-butyl-vinylpyridinium bromide) (PIL, in brief) was prepared and has a molar mass (Mw) of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and polymeric dispersity index (PDI) of 1.68 according to the literature.27 All the reagents show excellent solubility in water.
000 and polymeric dispersity index (PDI) of 1.68 according to the literature.27 All the reagents show excellent solubility in water.
For preparation of the complex, 2 mg ml−1PIL aqueous solution was mixed together with a mixture of MO and FDA aqueous solution, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar positive/negative charge ratio. The precipitated complex was filtered and washed several times with double-distilled water to remove residual salts and possible non-complexed precursors, and then dried under vacuum at 60 °C for 48 h. Complexes with different FDA molar contents were prepared by adjusting the molar ratio of MO and FDA, as shown in Scheme 1. Complexes with different FDA molar contents were designated PMOFx. Here, x is the molar percentage of FDA.
1 molar positive/negative charge ratio. The precipitated complex was filtered and washed several times with double-distilled water to remove residual salts and possible non-complexed precursors, and then dried under vacuum at 60 °C for 48 h. Complexes with different FDA molar contents were prepared by adjusting the molar ratio of MO and FDA, as shown in Scheme 1. Complexes with different FDA molar contents were designated PMOFx. Here, x is the molar percentage of FDA.
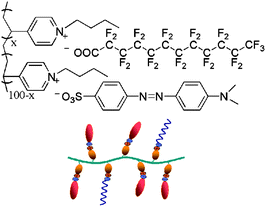 | ||
| Scheme 1 The chemical structure of the co-assembled PMOF complex and a schematic representation of the complex. | ||
PMOF10 was chosen as the sample to investigate the hierarchical assembly of the two anions to PIL backbones. 10 ml PIL aqueous solution was added dropwise to a mixture of MO and FDA aqueous solution, and then the solution was submitted to centrifugation at 4500 × g for 15 min to collect the precipitation. Another 10 ml PIL aqueous solution was then added dropwise to the residual solution and the precipitate was collected again using the same method as above. The collected precipitations were also treated with double-distilled water to remove the non-complexed precursors. After drying under vacuum at 60 °C for 48 h, the sample was submitted to FT-IR analysis.
Complex films were prepared using different methods according to experimental purpose: for XRD measurement and photosensitive test, thin films were solvent-cast from dilute solutions of the complexes in chloroform solution (30 mg ml−1) onto clean glass slides. The slides were then covered with a beaker to allow very slow solvent evaporation, followed by further drying at 60 °C for 2 h to eliminate any remaining chloroform; for UV measurement, the film was prepared by spin-coating a chloroform solution (30 mg ml−1) onto quartz plates (speed: 2000 rpm, time: 20 s).
Characterization
FTIR spectra were recorded on a Perkin Elmer Paragon 1000 FTIR spectrometer on pressed thin transparent disks of the samples mixed with KBr. UV–Vis spectra of the complex films were recorded on a Perkin Elmer lambda 20 UV-vis spectrometer. The polarized UV–Vis spectra were measured using the same device equipped with a Glan-Taylor prism. X-ray diffraction was performed on a Bruker-AXS D8 diffractometer (Cu-Kα radiation λ = 0.154 nm, U = 40 kV, I = 100 mA). Thermogravimetric analysis (TGA) was carried out using a Perkin-Elmer model 7 instrument and samples were heated at 20 °C min−1 from room temperature to 600 °C in a flowing nitrogen atmosphere. Polarized optical microscopy (POM) was performed on a Leica DMLP microscope with a Leitz 350 hot stage.Photo-induced birefringence was investigated with a He–Ne laser at 650 nm as probe light, which is far from the absorption band of the azobenzene groups, and a continuous 532 nm laser as pump light. The sample (film with a thickness about 300 nm) was placed between two crossed polarizers in the path of 650 nm laser and was irradiated by the pump light linearly polarized at ±45° with respect to the vertical axis. The birefringence (Δn) was calculated using I = I0 sin2(πΔnd/λ), where I is the intensity of the transmittance, I0 is the intensity of the probe beam, λ is the wavelength, and d is the film thickness.
Contact angle measurements were performed on a Contact Angle System OCA 20 (DataPhysics Instruments GmbH, Germany) at room temperature. Static contact angles were measured by the sessile drop method using 3 μL water drops. The contact angles reported here are the average values of at least five independent measurements. The contact angle variability was within 2°.
Laser irradiation and LC cell fabrication. Thin films of the complexes were irradiated at room temperature with linearly polarized light (Ar+ laser, λ = 488 nm). An LC cell was fabricated by sandwiching nematic LC (5CB) between a pair of irradiated PMOF-coated substrates in parallel mode. The two surfaces were separated by 10 μm spacers and held together using ethoxyline. 5CB was injected to the cell at its isotropic temperature by capillary force. The alignment of LC molecules was determined by POM and optical transmittance measurements. Optical measurements were carried out by placing LC cells between crossed polarizers and measuring the transmittance from a He–Ne laser at 628 nm using a detector coupled to an oscillograph.
Results and discussion
Synthesis and characterization of photosensitive complexes
Immediate precipitation took place when two aqueous solutions of the PIL and the mixture of MO and FDA were mixed, indicating the formation of a complex. The precipitation has survived multiple water washes that can selectively remove un-bonded MO, FDA and PIL molecules. The formation of complexes was verified by means of FTIR. As shown in Fig. 1, the absorption bands at 1600 cm−1 and 1520 cm−1 are characteristic peaks of phenyl backbone stretching vibrations, while that at 1200 cm−1 corresponds to the asymmetric stretching vibration of the sulfonic groups. A broad band at 1718 cm−1 corresponds to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in FDA. After combination with the PIL backbone, a significant shift from 1718 cm−1 to 1685 cm−1 was observed, indicating that a stoichiometric complex was formed.34
O group in FDA. After combination with the PIL backbone, a significant shift from 1718 cm−1 to 1685 cm−1 was observed, indicating that a stoichiometric complex was formed.34
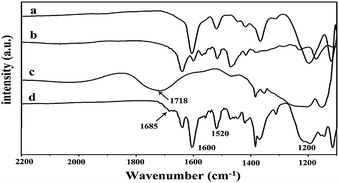 | ||
| Fig. 1 FTIR spectra of (a) MO, (b) PIL, (c) FDA, and (d) PMOF3. | ||
The competitive assembly of the two anions to the PIL backbone was also investigated by using FT-IR. As mentioned in the experimental section, we collected the complexes formed at different stages of reaction. The PIL solution was added to the aqueous mixture solutions of FDA and MO in a dropwise manner. Here, all the matched anions of FDA and MO were in excess compared to the PIL cation. Therefore, if the two anions have different affinities to PIL backbone, the complexes formed at different reaction stages should possess different amounts of FDA anions. Based on this assumption, we divided the aqueous PIL solution into three equal parts and added them to the mixture of MO and FDA in turn. After each part was added, the precipitation was collected by centrifugation, the samples were named 1∼3, respectively, afterwards submitted to FT-IR analysis. The FT-IR spectra of the three samples were shown in Fig. 2. Obvious difference was found in the spectra of the three samples: for the sample 1, strong absorption band at 1685 cm−1 appeared, indicating the existence of FDA side chain in the complex. As a comparison, no or very weak absorption band was found at this position in samples 2 and 3. This phenomenon proved that the two ions have different affinity to the PIL backbone: FDA was binding to the PIL backbone first; so most of the FDA anion was attached to the PIL backbone at the initial stage of the assembly process and a strong absorption peak appeared at 1685 cm−1.
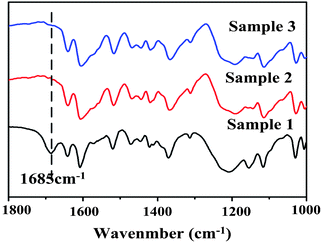 | ||
| Fig. 2 FT-IR spectrum of a hierarchical assembly of PMOF complex. Sample x is collected in turn according to the addition sequence of PIL into the mixture of MO and FDA solution. | ||
Generally, polymers containing perfluorinated long chains usually have poor solubility in various solvents. Perfluorinated acid-polyethyleneimine complexes that were previously reported can only be dissolved in 1,1,1,3,3,3,-hexafluoropropanol.35 In this study, the solubility of the powdered complexes that had different FDA contents (0%–10%) was examined, and it showed that all complexes had excellent solubility in chloroform, dimethyl sulfoxide, and dimethyl formamide. This excellent ability proved that the complex was not a simple mixture of PIL-FDA and PIL-MO complexes, but the complex of the two ions co-attaching to the PIL backbones.
The obtained powder complexes were subjected to thermal analysis to determine their stability. Thermogravimetric analysis results (Fig. 3) showed that the PMOF complexes with FDA content in the range of 0%–5% were stable up to 250 °C. When the FDA content was up to 10%, the degradation temperature was dramatically decreased to 186 °C.
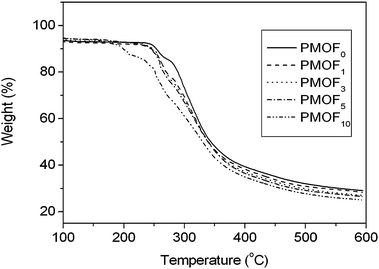 | ||
| Fig. 3 TGA of the complexes of PMOF0, PMOF1, PMOF3, PMOF5 and PMOF10. | ||
Mesostructure of the complex with different FDA content
POM observation of the PMOF0 powders found that schlieren textures appeared in the temperature range of 180–195 °C during cooling down from the isotropic state, indicating that a LC structure occurred in the complex. (Fig. 4a) However, DSC measurement shown no specific transition. This might be the result of the rigidity of the side chains.30 On the other hand, we previously reported the complexation of poly(1-ethylvinylpyridinium) with MO and no schlieren texture was found when the powder was cooled down from the isotropic state.27 This difference may come from the side alkyl chain of the PIL chain. When the side alkyl chain was too short (i.e. only two atoms in the PIL molecules chains in our previously reported work), no thermo LC properties were found. When the alkyl chain was long enough, thermo LC properties appeared. This phenomenon also indicates another approach for designing thermo LC structure in supramolecules. Further investigation of the effect of the side chain in the PIL backbone is still on-going.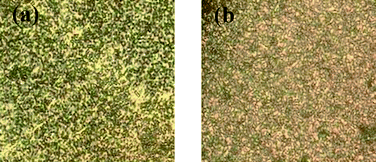 | ||
| Fig. 4 Typical texture of (a) PMOF0 powders observed at ca. 190 °C, (b) PMOF5chloroform solution as observed with a polarized optical microscope. | ||
For the FDA containing complex, similar schlieren textures appeared when the FDA content was below 1%, indicating that LC properties also existed in the PIL-FDA/AZO complexes. When the FDA content was above 1%, i.e., for PMOF3, PMOF5, and PMOF10, we could not obtain the completely isotropic state because the isotropic points of these complexes were close to or above their decomposition temperatures. It was previously reported that solution-casting followed by slow evaporation of the solvent yielded a well ordered texture of the complex.30 In this work, we dropped an approximately 30 mg mL−1chloroform solution of complex with FDA content larger than 1% on a clean glass slide and pronounced schlieren textures were observed by POM. (Fig. 4b) This result proved that the lytropic LC structure also existed in the complexes with FDA content larger than 1%.
To obtain a better insight into the packing structure of the complexes, X-ray diffraction measurement was carried out in small angle and wide angle models, respectively. In this part, we tested the complex in two forms: powder and film. Powder samples were collected directly from the precipitation after drying treatment and film samples were prepared by solvent-casting of dilute chloroform solutions of the complexes onto clean glass slides. Fig. 5 presents the SAXD profiles of complexes with different FDA content. For powders of all three complexes, only a single reflection was found. It was previously reported that such a kind of complex was organized in lamellar mesophase.27–31 The single reflection peak corresponded to the first order diffraction of this lamellar structure. The absence of other diffraction peaks proved poor order structure in the powders of the complexes. The reflex maxima positions for three complexes were 3.15 (PMOF0), 3.01 (PMOF5) and 3.00 (PMOF10), corresponding to a lateral distance of 2.80 nm, 2.94 nm and 2.95 nm, respectively. The lamellar distances in PMF0 are almost equal to the value of total length of the polymer side chains plus MO molecules in extend conformation, indicating that a single layer lamellar structure existed in these complexes. Compared with PMF0, the incorporation of the FDA component leads to the uniform increase of the lamellar distance of 0.14 nm, independent of the amount of FDA content in the complex. This value was almost equal to the length of an axial projection of a CF2 group in the fluorinated chains plane (0.13 nm).36 This result indicated that a single FDA molecular layer perpendicular to the direction of MO alignment inside the lamellar of the azobenzene side chains may exist in the complex powder (Fig. 5b).
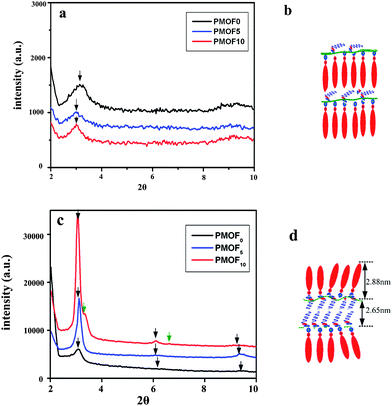 | ||
| Fig. 5 Small-angle X-ray diffraction profiles of the complex in different morphology. (a) powder, (c) casting film. (b) and (d) are the corresponding models of molecular arrangement. | ||
As a comparison, SAXD profiles of casting films of all three complexes were detected as shown in Fig. 5c. The reflex maxima positions are summarized in Table 1. The relative reflex positions were determined to be 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0
2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0. Such integral ratios are highly indicative of the lamellar structure of the complex. The two complexes have the almost the same lamellar distance (2.84∼2.87 nm), indicating a single layer lamellar structure existed. For PMOF10, two groups of reflection peaks appeared in the SAXD profiles (Table 1), indicating two kinds of lamellar structure coexisted in the complex film. The first group (3.06, 6.12, 9.29) is almost at the same position of that found in PMOF0 and PMOF5 and has the same lamellar distance (2.88 nm), corresponding the lamellar structure of azobenzene side chains. The other group (3.34, 6.62) corresponded to a lamellar distance of 2.65 nm. Thünemann systematically investigated the mesostructure of the complex of perfluorinated carboxylic acid and polyethyleneimine and found that the lamellar distance of the resulted complex depended on the packing phase and the length of the perfluorinated carboxylic chains: in a monolayer arrangement, the lamellar structure have a thickness of ca. (n − 1) (0.13 nm); and in a bilayer structure, the distance was ca. 2(n − 2) (0.13 nm).37 Here, n is the number of carbon atoms of the carboxylic acid, for example, in the case of PMOF10, the distance should be 2.6 nm due to the 12 carbon atoms, which was in accord with SAXD results. This result indicated that a much more ordered structure was formed with increasing the amount of FDA added. Addition of 10% of FDA causes a reorganization of monolayer, depicted in Fig. 5b, because of the excess amount of FDA. As a result, the formation of another new separated lamellar structure from FDA chains occurs. In the FDA lamellar, the FDA chains tend to be arranged in a tail-to-tail model and the layer distance was about 2.65 nm. The packing state of the two lamellar structures in complex PMOF10 is shown in Fig. 5d.
3.0. Such integral ratios are highly indicative of the lamellar structure of the complex. The two complexes have the almost the same lamellar distance (2.84∼2.87 nm), indicating a single layer lamellar structure existed. For PMOF10, two groups of reflection peaks appeared in the SAXD profiles (Table 1), indicating two kinds of lamellar structure coexisted in the complex film. The first group (3.06, 6.12, 9.29) is almost at the same position of that found in PMOF0 and PMOF5 and has the same lamellar distance (2.88 nm), corresponding the lamellar structure of azobenzene side chains. The other group (3.34, 6.62) corresponded to a lamellar distance of 2.65 nm. Thünemann systematically investigated the mesostructure of the complex of perfluorinated carboxylic acid and polyethyleneimine and found that the lamellar distance of the resulted complex depended on the packing phase and the length of the perfluorinated carboxylic chains: in a monolayer arrangement, the lamellar structure have a thickness of ca. (n − 1) (0.13 nm); and in a bilayer structure, the distance was ca. 2(n − 2) (0.13 nm).37 Here, n is the number of carbon atoms of the carboxylic acid, for example, in the case of PMOF10, the distance should be 2.6 nm due to the 12 carbon atoms, which was in accord with SAXD results. This result indicated that a much more ordered structure was formed with increasing the amount of FDA added. Addition of 10% of FDA causes a reorganization of monolayer, depicted in Fig. 5b, because of the excess amount of FDA. As a result, the formation of another new separated lamellar structure from FDA chains occurs. In the FDA lamellar, the FDA chains tend to be arranged in a tail-to-tail model and the layer distance was about 2.65 nm. The packing state of the two lamellar structures in complex PMOF10 is shown in Fig. 5d.
| Complex | 2θ | d-spacing |
|---|---|---|
| PMOF0 (film) | 3.08, 6.15, 9.33 | 2.87 nm |
| PMOF5 (film) | 3.11, 6.07, 9.35 | 2.84 nm |
| PMOF10 (film) | 3.06, 6.12, 9.29 | 2.88 nm |
| 3.34, 6.62 | 2.65 nm | |
| PMOF0 (powder) | 3.15 | 2.80 nm |
| PMOF5 (powder) | 3.01 | 2.94 nm |
| PMOF10 (powder) | 3.00 | 2.95 nm |
It is noteworthy that the intensity of the reflection peaks found in the SAXD profiles of the three complexes increased strongly with the increase of the FDA content. This indicated that the incorporation of FDA side chains promoted the arrangement of MO side chains in the complex. In addition, FDA side chains also formed their own lamellar layer structure when their content was more than 10% in the complex. This fact can also be further proved by detecting the molecular packing structure using WAXD. Fig. 6 shows the WAXD profile of three complex casting films. For PMOF0 and PMOF5, only a broad maximum is found, indicating an amorphous molecular chain packing in the film. For PMOF10, however, a weak peak at 17.75 is superimposed on a broad amorphous halo center at 19.80. The peak at 17.75, which correspond to spacing of about 0.50 nm, was equal to the intra-chain distance of crystalline perfluoroalkyl chains.36 This result is also consistent with that of SAXD in Fig. 5.
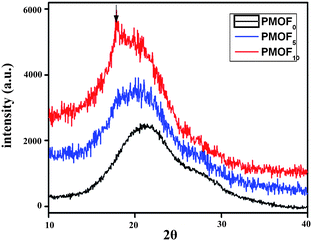 | ||
| Fig. 6 Wide angle X-ray diffraction profiles of casting film of complex. | ||
Photo-induced birefringence in complexes with different FDA content
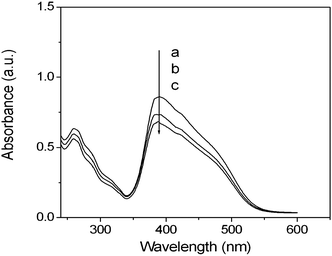
The UV-vis spectra of PMOF0, PMOF5, and PMOF10 films are presented in Fig. 7. The introduction of FDA has no effect on the UV performance of the complex films, except that there is a slight decrease in the absorbance as the FDA content increases.The real-time photo-induced birefringence was firstly investigated to evaluate the photosensitivity of the complex film. In this case, the photo-induced Δn value upon irradiation with linearly polarized 532 nm light was probed by using a weak probe light of 650 nm at room temperature. Although the pump wavelength (532 nm) lies in the tail of the film's absorption band, remarkable birefringence was induced as shown in Fig. 8. For PMOF0, the birefringence first increased rapidly with the irradiation time, then slowed down, and finally reached a saturation value after irradiation for approximately 300 s. In this case, a steady Δn value of approximately 0.067 was obtained upon irradiation with pump light with an intensity of 150 mW cm−2. After the pump light was turned off, the birefringence showed a slight decrease and then increased to a higher steady value (Δn, 0.071). The final increase of the birefringence was attributed to the thermo liquid crystalline nature of the ionic-bonding complex.38,39 After turning the light off, the segments or the chains of the liquid crystalline complexes continue to rearrange due to the self-organization ability of liquid crystal molecules. This photo-induced behavior of complex with poly(1-butyl-vinylpyridinium bromide) as backbone was different with that we previous report on complex with poly(1-ethylvinylpyridinium) as backbone. The difference may come from the effect of the side alkyl chains on the mesostructure of the complex. The complex from poly(1-ethylvinylpyridinium) only showed lytropic liquid crystalline properties. However, the butyl alkyl chain made rearrangement of the side azobenzene-containing rigid segment easily, leading to the occurrence of thermo liquid crystalline properties.
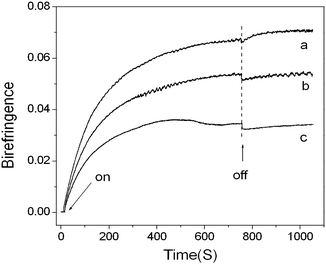 | ||
| Fig. 8 Evolution of the birefringence with time in the complex films (a) PMOF0, (b) PMOF5 and (c) PMOF10 under irradiation with 532 nm linearly polarized light at room temperature. Switching on and off of the pump light are indicated by arrows. | ||
In the case of PMOF5 (Fig. 8b), similar birefringence evolution was observed though the saturation value decreased (0.055). Even in the case of PMOF10, a pronounced birefringence with an ultimate steady value of 0.034 was observed. Another point we should pay attention to here is the relaxation behavior of the photo-induced birefringence in the complex with different FDA content. For all three complexes, the change of the photo-induced birefringence shows same tendency: slightly decreased after laser turned off, and then increased up to a steady value. However the degree of increase of birefringence decreased with the increase of the FDA content.
The orientated complex films were obtained by irradiation with linearly polarized light (Ar+ laser, λex = 488 nm) at room temperature. The polarized absorption spectra of a typical example (PMOF3), which was irradiated with a small dose of 7.2 J cm−2, is presented in Fig. 9a. The absorbance in the direction parallel to the polarization of the laser beam is obviously smaller than that in the direction perpendicular to the polarization of the laser beam, which suggests that the preferred direction of the azobenzene chromophores is perpendicular to the laser polarization direction. Changes in the photo-induced dichroic ratio at different FDA molar contents under an irradiation dose of 7.2 J cm−2 are shown in Fig. 9b (inset). It was also observed that the ultimate dichroic ratio decreased with an increase in the FDA content. This result is consistent with the change of the birefringence of the complex films with different FDA content.
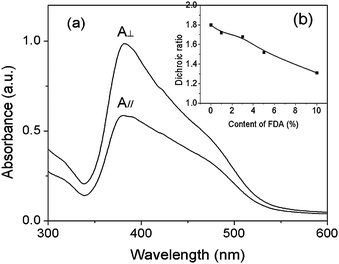 | ||
| Fig. 9 (a) Changes in the polarized absorbance spectra of the PMOF3 film irradiated with a linear polarized laser of 7.2 J cm−2 (λ = 488 nm). A// and A⊥ are absorbance that is parallel and perpendicular, respectively, to the direction of laser polarization (E). Inset graph: (b) changes in the induced dichroic ratio with different FDA molar contents after irradiation of 7.2 J cm−2. | ||
Wetting of complex with different FDA content
Fluorinated surfactants were widely used to construct low energy surface coatings for application in different fields. Here, we measured the contact angles of different liquids (water, diiodomethane and 5CB) on top of the perfluorinated ionic complex film as shown in Fig. 10a. Without the introduction of FDA, the complex film was hydrophilic: the contact angles were 65°, 36° and 27° for water, diiodomethane and 5CB, respectively. With increasing FDA content, the contact angle of the three fluids increase up to 102°, 83° and 81° for FDA content of 5%. In addition, it was observed that the values of the contact angles against three fluids became steady when the FDA content exceeded 3%.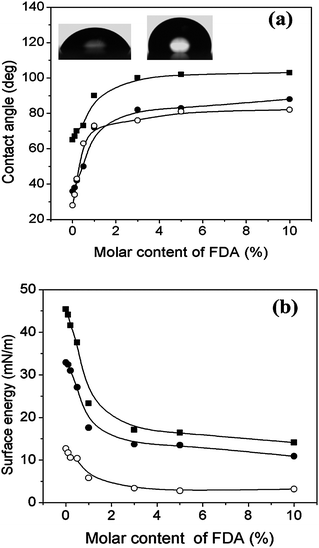 | ||
| Fig. 10 (a) Static contact angles of water (-■-), diiodomethane (-●-), and 5CB (-○-) for complex films with different FDA molar contents. The images in the inset show the changes in the water wettability of the PMOF0 and PMOF5 films. (b) Changes of surface energy (-■-), dispersive energy (-●-) and polar energy (-○-) for the complex films with different molar content of perfluorinated chain. | ||
Surface energy values as the sum of dispersion and polar components were calculated using the Owens method from the contact angles measured with water and diiodomethane. Fig. 10b shows the variations in the surface energy at different FDA molar contents. Continuous and remarkable wetting variation of the complex film occurred when the FDA content ranged between 0% and 1%. Complex films with perfluorinated chain content in excess of 1% showed stable hydrophobic wetting properties with very low surface energy. These results proved that the surface wetting properties were completely affected by the FDA content in the complex, that is to say, it is easy to tune the film wetting behavior by simply controlling the FDA content.
Liquid crystal alignment on the complex film
Thin films with the integrated function of photoresponding and controllable wetting properties are attractive for designing film-based devices. In the past ten years, azobenzene-containing thin film as a command surface was intensively investigated due to its application in liquid crystal (LC) displays. LC molecules dropped on top of the azobenzene-containing film will be aligned. The combination of FDA side chains in such a kind of photosensitive complex makes the surface wetting properties controllable. As shown in Fig. 10a, liquid crystal molecules 5-CB show controllable wetting behavior according to the amount of FDA content, so it would be possible to control the out-plane orientation of liquid crystal molecules dropped on top of complex film.40Here, we examined the alignment behavior of oriented complex films towards 5CB liquid crystal molecules. Firstly, POM observation results proved that all cells show uniform light field, indicating that LC molecules uniformly aligned on the surface of complexes film. However, when we further submitted these cells to optical transmittance measurements, different results were obtained for cells assembled with different PMOFx films (Fig. 11). All the cells show periodical changes in transmitted light intensity under crossed polarizers, that also proved the uniform LC alignment in the cells. However, the contrast between the bright and dark field decreases obviously with the increase of FDA content. When FDA content was up to 1%, the change of light intensity is subtle.
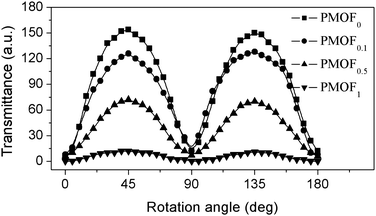 | ||
| Fig. 11 Optical transmittance of LC cells as a function of the rotation angle of the cell between crossed polarizers. The cells were assembled by irradiated PMOF substrates with different FDA content as indicated. | ||
Between crossed polarizer, the light intensity of LC cells was affected by different factors and can be calculated using following formula when the cell was rotated with respected to the cell normal:
| T = sin2(2θ)sin2(πΔnd/λ), |
Conclusion
We investigated the ionic co-assembly of both anionic MO and FDA onto a poly(ionic liquid) backbone. The two anions show hierarchical behaviors in the assembling process: FDA molecules attached to the PIL backbone more easily than MO molecules. The mesostructure of the complex with different FDA contents was investigated using POM and XRD. POM analysis proved that all complexes show LC order structure. The incorporation of the FDA side chain increased remarkably the ordered degree of MO lamellar structure in PMOF casting film. Furthermore, when the FDA content increases to 10%, bi-layer packing order of FDA formed. However, for PMOF powder, excess FDA favors a single layer perpendicular to the direction of MO arrangement. All complexes show high photosensitivity. These complexes with different function side chains show co-effect in controlling the in-plane and out-of-plane alignment of liquid crystal molecules. This study proposed a new supramolecular strategy for fabricating bi- or multi-functional materials.Acknowledgements
The authors gratefully acknowledge financial support from the National Science Fund for Distinguished Young Scholars (50925310), the National Science Foundation of China (20874059, 50902094), 973 project (2009CB93043), 863 project (2009AA03Z329) and the Shanghai Leading Academic Discipline Project (No. B202).References
- S. M. Kelly and M. O'Neil, Adv. Mater., 2011, 23, 566 CrossRef.
- O. Kawatsuki, K. Goto, T. Kawakami and T. Yamamoto, Macromolecules, 2002, 35, 706 CrossRef.
- E. Uchida and N. Kawatsuki, Macromolecules, 2006, 39, 9357 CrossRef CAS.
- X. Li, X. M. Lu, Q. H. Lu and D. Y. Yan, Macromolecules, 2007, 40, 3306 CrossRef CAS.
- R. S. Lin and J. A. Rogers, Nano Lett., 2007, 7, 1613 CrossRef CAS.
- S. Nešpùrek, Y. Zakrevskyy, J. Stumpe, B. Sapich and A. Kadashchuk, Macromolecules, 2006, 39, 690 CrossRef.
- M. Defaux, L. Vidal, M. Moller, R. I. Gearba, E. DiMasi and D. A. Ivanov, Macromolecules, 2009, 42, 3500 CrossRef CAS.
- A. E. A. Contoret, S. R. Farrar, P. O. Jackson, S. M. Khan, L. May, M. O'Neil, J. E. Nicholls, S. M. Kelly and G. J. Richards, Adv. Mater., 2000, 12, 971 CrossRef CAS.
- D. Sainova, A. Zen, H. G. Nothfoer, U. Asawapirom, U. Scherf, R. Hagen, T. Bieringer, S. Kostromine and D. Neher, Adv. Funct. Mater., 2002, 12, 49 CrossRef CAS.
- N. Kwatsuiki, R. Ando, R. Ishida, M. Kondo and Y. Minami, Macromol. Chem. Phys., 2010, 211, 1741 CrossRef.
- K. Sakamoto, K. Miki, M. Misaki, K. Sakaguchi, Y. Hijikata, M. Chikamatsu and R. Azumi, J. Appl. Phys., 2010, 107, 113108 CrossRef.
- R. Rosenhauer, J. Stumpe, R. Gimenez, M. Pinol, J. L. Serrano, A. Vinuales and D. Broer, Macromolecules, 2011, 44, 1438 CAS.
- H. Sirringhaus, R. J. Wilson, R. H. Friend, M. Inbasekaran, W. Wu, E. P. Woo, M. Grell and D. D. C. Bradley, Appl. Phys. Lett., 2000, 77, 406 CrossRef CAS.
- M. J. Lee, D. Gupta, N. Zhao, M. Heeney, I. Mcculloch and H. Sirringhaus, Adv. Mater., 2011, 21, 932 CAS.
- H. Fukumoto, S. Nagano, N. Kawatsuki and T. Seki, Adv. Mater., 2005, 17, 1035 CrossRef CAS.
- Y. Kawashima, M. Nakagawa, T. Seki and K. Ichimura, Chem. Mater., 2002, 14, 2842 CrossRef CAS.
- H. Miyata, Y. Kawashima, M. Itoh and M. Watanabe, Chem. Mater., 2005, 17, 5323 CrossRef CAS.
- A. Natansohn and P. Rochon, Chem. Rev., 2002, 102, 4139 CrossRef CAS.
- S. K. Yesodha, C. K. S. Pillai and N. Tsutsumi, Prog. Polym. Sci., 2004, 29, 45 CrossRef CAS.
- H. S. Lim, J. T. Han, D. H. Kwak, M. H. Jin and K. W. Cho, J. Am. Chem. Soc., 2006, 128, 14458 CrossRef CAS.
- N. Kano, F. Komatsu, M. Yamamura and T. Kawashima, J. Am. Chem. Soc., 2006, 128, 7097 CrossRef CAS.
- Ä. Somogyi, E. H. Elandaloussi, D. E. Hall, A. B. Padías, R. B. Bates and H. K. Hall, Jr., Macromolecules, 2007, 40, 5311 CrossRef.
- S. Chinnusamy and K. Palaninathan, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1565 CrossRef CAS.
- D. Franke, M. Vos, M. Antonietti, N. A. J. M. Sommedijk and C. F. J. Faul, Chem. Mater., 2006, 18, 1839 CrossRef CAS.
- Y. Zakrevskyy, C. F. J. Faul, Y. Guan and J. Stumpe, Adv. Funct. Mater., 2004, 14, 835 CrossRef CAS.
- C. F. J. Faul and M. Antonietti, Adv. Mater., 2003, 15, 673 CrossRef CAS.
- S. F. Xiao, X. M. Lu and Q. H. Lu, Macromolecules, 2007, 40, 7944 CrossRef CAS.
- Q. Zhang, C. G. Bazuin and C. J. Barrett, Chem. Mater., 2008, 20, 29 CrossRef CAS.
- S. F. Xiao, X. M. Lu and Q. H. Lu, Macromolecules, 2008, 41, 3884 CrossRef CAS.
- Q. Zhang and C. G. Bazuin, Macromolecules, 2009, 42, 4775 CrossRef CAS.
- O. Kulikovska, L. M. Goldengerg, L. Kulikovsky and J. Stumpe, Chem. Mater., 2008, 20, 3528 CrossRef CAS.
- J. Lu, F. Yan and J. Texter, Prog. Polym. Sci., 2009, 34, 431 CrossRef CAS.
- K. Vijayakrishna, D. Mecerreyes, Y. Gnanou and D. Taton, Macromolecules, 2009, 42, 5167 CrossRef CAS.
- A. F. Thünemann and K. H. Lochhaas, Langmuir, 1998, 14, 4898 CrossRef.
- A. F. Thünemann, Polym. Int., 2000, 49, 636 CrossRef.
- C. W. Bunn and E. R. Howells, Nature, 1954, 174, 549 CrossRef CAS.
- A. F. Thünemann, Langmuir, 2000, 16, 824 CrossRef.
- E. Uchida, T. Shiraku, H. Ono and N. Kawatski, Macromolecules, 2004, 37, 5282 CrossRef CAS.
- F. Puntoriero, P. Ceroni, V. Balzani, G. Bergamini and F. Vogtle, J. Am. Chem. Soc., 2007, 129, 10714 CrossRef CAS.
- D. R. Chiou and L. J. Chen, J. Phys. Chem. C, 2009, 113, 9797 CAS.
| This journal is © The Royal Society of Chemistry 2011 |