Submicro-polymer particles bearing imidazoline-2-selenone: dual mode adsorbents with color-sensing for halogens and mercury ions†
Jaewon
Choi
a,
So Yeon
Park
a,
Hye Yun
Yang
a,
Hae Jin
Kim
b,
Kyuwook
Ihm
c,
Jeong Ho
Nam
d,
Joung Real
Ahn
*d and
Seung Uk
Son
*a
aDepartment of Chemistry and Department of Energy Science, Sungkyunkwan University, Suwon, 440-746, Korea. E-mail: sson@skku.edu; Fax: +82-031-290-4572; Tel: +82-031-299-5932
bKorea Basic Science Institute, Daejeon, 350-333, Korea
cPohang Accelerator Laboratory, Pohang University of Science and Technology, Pohang, 790-784, Korea
dBK21 Physics Research Division and SAINT, Sungkyunkwan University, Suwon, 440-746, Korea. E-mail: jrahn@skku.edu
First published on 20th September 2011
Abstract
Submicron-sized polymer particles (PSE) containing imidazoline selenones were prepared by co-polymerization of styrene derivative (MSE) bearing an imidazoline selenone moiety with 1,4-divinylbenzene (DVB). The size and chemical composition of PSE were controlled by changing the stoichiometric ratios of MSE to DVB. The physical and chemical properties of PSE were characterized by SEM, EDS and elemental analysis. PSE showed an interesting reactivity towards halogens with vivid color-change from white to red-orange, which is attributed to the reaction of selenium in imidazoline-2-selenone with halogens. Acid treatment of PSE generated the hydrophilic red-orange colored particles (PSEA) which showed very selective adsorption properties towards mercury ions with color change to pale yellow. To figure out the origin of color change, model studies were conducted using 1,3-dimethyl-imidazoline-2-selenone. The dimerization of 1,3-dimethyl-imidazoline-2-selenone through Se–Se bond formation by acid-treatment resulted in color change from colorless to red-orange. The coordination-induced cleavage of the Se–Se bond of the dimerized species by mercury ions resulted in color change from red-orange to pale yellow. These observations indicate that hydrophobic PSE and hydrophilic PSEA are efficient systems for adsorption of halogens and mercury ions with a vivid color-detection.
Introduction
Selenium chemistry has attracted the continuous attention of not only synthetic chemists but also biochemists and material scientists.1–3Selenium and its derivatives can be used as catalysts or reagents in the development of new synthetic methodologies1 and selenoproteins play an important role in diverse biosystems.2 Additionally, selenium and selenium-containing inorganic materials have been applied to energy and display devices.3 Concomitantly, there have been extensive studies on organoselenium chemistry.4As a recent example of an organoselenium system, 1,3-disubstituted imidazoline-2-selenones have shown very interesting reactivity.5 The selenium can be easily introduced at the 2-position of the imidazole ring via treatment of disubstituted imidazolium salts with a base and sequential reaction with elementary selenium (Scheme 1). The selenium in imidazoline-2-selenone is quite nucleophilic and exhibits good reactivity towards electrophiles.5 In addition, it can be used as a coordination mode for transition metals.6 Moreover, this imidazoline-2-selenone chemistry has been applied for specific purposes. For example, our research group has studied the reactivity of imidazoline-2-selenone as a selenide source in the synthesis of semiconducting metal selenide nanomaterials.7 In recent literature, imidazoline-2-selenones have been applied to biochemical systems with focus upon thyroid hormone synthesis as potential anti-hyperthyroid drugs.5d
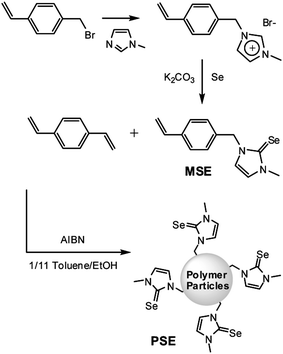 | ||
| Scheme 1 Synthesis of monomer (MSE) and polymer particles (PSE) having imidazoline-2-selenone moieties. | ||
Recently, there has been great progress in size-control technology and now, many nano- or micro-material scientists are recognizing that more effort should be put forth for new practical applications based on the accumulated knowledge. Our research group has studied the development of functional nano- or micro-materials bearing the molecular units.8 In this work, we report on the synthesis, control of chemical properties of the submicron-sized polymer particles having imidazoline-2-selenones, and their applications in color sensing of halogens or metal ions in aqueous solution.
Experimental section
Instruments
The SEM images and EDS data were obtained by FE-SEM (JSM6700F). 1H NMR and 13C NMR spectra were recorded on Varian (300 MHz) spectrophotometers. Elementary analysis was performed on a CE EA1110 elementary analysis instrument. X-Ray photoelectron spectra (XPS) were measured with an incident beam energy of 400 eV and an emission angle of 0° at a vacuum ultraviolet beamline (BL-4B1) connected to an undulator of the synchrotron radiation source at the Pohang Light Source (PAL) in Korea. The end station is equipped with a high-resolution electron analyzer (SES-R3000, Gamma Data, Sweden). ICP-atomic emission spectroscopy (AES) experiment was performed by Shimadzu ICPS-1000IV. The single-crystal X-ray diffraction data were collected with Bruker SMART APEX II.Synthetic procedure for the preparation of monomer MSE
In a 50 mL Schlenk tube, 4-vinylbenzylbromide (4.2 mL, 30 mmol) and N-methylimidazole (2.0 mL, 25 mmol) were dissolved in chloroform (10 mL). After refluxing overnight, the product was isolated by extraction with water. Evaporation of water resulted in formation of a pale-yellow solid. 1H NMR (300 MHz, D2O) δ = 8.69 (s, 1H), 7.47 (d, J = 8.1 Hz, 2H), 7.38 (s, 2H), 7.31 (d, J = 8.1 Hz, 2H), 6.72 (dd, J = 18, 10 Hz, 1H), 5.81 (d, J = 18 Hz, 1H), 5.30 (d, J = 10 Hz, 1H), 5.28 (s, 2H), 3.81 (s, 3H) ppm match well with those in literature.9 The salt (5.0 g, 21 mmol) was dissolved in methanol (50 mL). Then, K2CO3 (14.7 g, 106 mmol) and selenium powder (5.0, 64 mmol) were added and the reaction mixture was stirred at room temperature overnight. After evaporating the methanol, the product (MSE) was extracted using methylene chloride. After evaporating the solvent, the white product was purified by recrystallization using hexane and methylene chloride. 1H NMR (300 MHz, CDCl3) δ = 7.37 (d, J = 8.1 Hz, 2H), 7.31 (d, J = 8.1 Hz, 2H), 6.85 (d, J = 2.4 Hz, 1H), 6.75 (d, J = 2.4 Hz, 1H), 6.70 (dd, J = 18, 11 Hz, 1H), 5.72 (d, J = 18 Hz, d), 5.30 (s, 2H), 5.21 (d, J = 11 Hz, 1H), 3.67 (s, 3H) ppm 13C NMR (75 MHz, CDCl3) δ = 155.7, 137.0, 135.7, 134.6, 128.1, 126.2, 119.9, 118.1, 114.1, 52.4, 36.8 ppm. Elementary analysis, calcd (%) for C13H14N2Se: C 56.32, H 5.09, N 10.10; found: C 56.33, H 5.09, N 10.13.Preparation of polymer particles PSE
MSE (0.90 g, 3.3 mmol) and 1,4-divinylbenzene (DVB, 0.20 mL, 1.4 mmol) were dissolved in toluene (6 mL). Ethanol (60 mL) was then added to the reaction mixture and the solution was vigorously stirred at 60 °C for half an hour to form a submicroemulsion, followed by addition of AIBN (0.16 g, 0.95 mmol) in 5 mL ethanol. After 24 hours, a white precipitate was formed in the solution, retrieved by centrifugation, washed with ethanol several times, and dried under vacuum.Adsorption of halogens by PSE
PSE3 (0.10 g) was treated with 0.10 M halogen (10 mL) in hexane for an hour. The resultant particles were washed with hexane five times and dried under vacuum.Preparation of hydrophilic particles PSEA
PSE3 (0.60 g) was treated with 1.0 M HCl (20 mL) for 24 hours at room temperature. The resultant product was retrieved by centrifugation, washed using water and dried under vacuum.Adsorption of transition metal ions by PSEA
PSEA (50 mg) was added to the model solution (33 mL) containing Cr3+ (119 ppm), Mn2+ (132 ppm), Fe3+ (124 ppm), Co2+ (130 ppm), Ni2+ (128 ppm), Cu2+ (127 ppm), Zn2+ (131 ppm), Pb2+ (133 ppm) and Hg2+ (88 ppm). The solution was stirred for 3 hours at room temperature. The particles were retrieved by centrifugation and the remaining solution was analyzed by ICP–AES.Preparation of model compounds
M2 (0.10 g, 0.57 mmol) was prepared by the method reported by our group,8d dissolved in water (10 mL) and then treated with aqueous solution of HgCl2 (0.40 g, 1.48 mmol in 10 mL water) to form crystalline solids (Hg–SE). 1H NMR (300 MHz, DMSO-d6) δ = 7.60 (s, 4H), 7.69 (s, 12H) ppm; 13C NMR (75 MHz, DMSO-d6) δ = 138.1, 123.9, 37.6 ppm. Elementary analysis, calcd (%) for C10H16N4Se2Hg2Cl4: C 13.45, H 1.81, N 6.27; found: C 13.45, H 1.76, N 6.22. Benzyl analogue of Hg–SE in Fig. 6c: 1H NMR (300 MHz, DMSO-d6) δ = 7.76 (s, 2H), 7.74 (s, 2H), 7.36–7.33 (brs, 10H), 5.42 (s, 4H), 3.80 (s, 6H) ppm; 13C NMR (75 MHz, DMSO-d6) δ = 140.6, 135.3, 128.8, 128.2, 127.9, 124.3, 122.7, 52.9, 37.6 ppm; Crystallographic data: CCDC# 745623, monoclinic, P21/c, a = 8.858(4), b = 16.147(8), c = 10.691(4) Å, β = 111.088(17)○, V = 1427(1) Å3, ρcalc = 2.433 mg m−3, completeness 97.3%, GOF = 0.910 T = 293(2) K; Rint = 0.1355, final R1 (I > 2σ(I)) = 0.0536, wR2 = 0.1052.Results and discussion
For the synthesis of submicron-polymer particles containing the 1,3-disubstituted imidazoline-2-selenone, we designed and prepared the monomer MSE, as shown in Scheme 1.N-Methylimidazole was heated with 4-vinylbenzylbromide to form the imidazolium salt that was further reacted with excess selenium powder after treatment with base at room temperature. White monomeric MSE was isolated by column chromatography in high yield. Recently, there have been great advances in the size-controlled synthesis of polystyrene particles.10 Usually, the relatively less polar organic monomers form the microemulsions in polar solvents such as water and alcohols. The polymerization can be initiated by a radical generator such as azobisisobutyronitrile (AIBN). In a typical synthetic procedure of polymer particles bearing imidazoline-2-selenone (PSE), the monomer MSE was dissolved in toluene. To this solution was added 1,4-divinylbenzene (DVB) to induce cross-linking. Then, ethanol was added to the reaction mixture and the solution was stirred vigorously to form microemulsions at 60 °C. Finally, AIBN in ethanol was added. After 24 hours, a white precipitate was retrieved by centrifugation, washed several times with ethanol and dried under vacuum (see the Experimental section for detailed procedure).
To control the size and composition of the polymer particles, the relative ratios of monomer MSE to DVB were screened. Table 1 summarizes the reaction conditions used. The sizes of particles could be controlled with a narrow size distribution by screening the amount of DVB with a fixed amount of MSE, whereby the amount of DVB was gradually reduced from 0.40 (PSE1) to 0.10 mL PSE4) by 0.10 mL increments. Fig. 1 shows typical scanning emission microscopy (SEM) images of the resultant polymer particles PSE1, PSE2, PSE3 and PSE4.
| MSE/g | DVB/mL | Sizeb/μm | Se c (wt%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: the reaction mixture of MSE, DVB, toluene (6 mL), AIBN (50 mg) and ethanol (65 mL) was stirred at 60 °C for 24 hours. b Average sizes were obtained by measuring 372, 372, 215 and 218 particles. c Weight% values were calculated based on the nitrogen contents obtained from elementary analysis. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSE1 | 0.90 | 0.40 | 0.71 | 20.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSE2 | 0.90 | 0.30 | 0.74 | 21.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSE3 | 0.90 | 0.20 | 0.89 | 22.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSE4 | 0.90 | 0.10 | 1.01 | 24.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
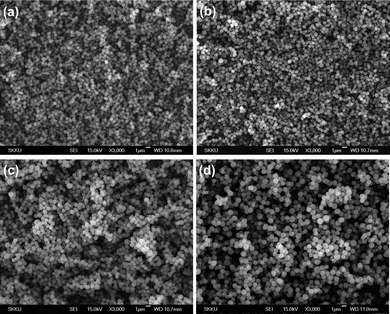 | ||
| Fig. 1 SEM images of polymer particles (PSE) prepared using 3.3 mmol MSE and 0.40 (a), 0.30 (b), 0.20 (c) and 0.10 mL (d) of DVB. | ||
As the amount of DVB increased, average size of polymer particles decreased from 1.01 μm to 710 nm, possibly due to the shrinking of polymer chains by the increased cross-linking (Table. 1).11 The molar contents of selenium in polymer particles gradually increased by using the monomer solutions with higher molar ratios of MSE to DVB. Qualitatively, the trend of selenium content in polymer particles was analyzed by energy dispersive X-ray spectroscopy (EDS) analysis. Quantitatively, based on the elementary analysis of nitrogen content in polymer particles, the molar percent of imidazoline-2-selenone moieties could be calculated as 53 (PSE1), 57 (PSE2), 63 (PSE3) and 77 mol% PSE4) respectively.12
Selenium in 1,3-dimethylimidazoline-2-selenone has a good reactivity toward halogens to form T-shaped halogen adducts with color change from colorless to red-orange.5a Also, halogen can induce the dimerization of the selenone moiety to form ionic species.5c To test the reactivity of PSE toward halogens, the prepared particles, PSE3 (0.10 g) was treated with 10 mL of 0.10 M I2, Br2 in hexane and 1 atm Cl2 gas for an hour and washed with organic solvent. The color of particles was changed from white to red (iodine) and yellow (bromine and chlorine) (Fig. 2). In a control experiment, the 750 nm sized particles prepared using styrene and DVB maintained the original white color after the same treatment of iodine and washing. The amount of adsorbed halogens was estimated up to 0.730 g (2.87 mmol) of I2, 0.539 g (3.37 mmol) of Br2 and 0.196 g (2.76 mmol) of Cl2 per gram of PSE3 by measuring the mass change of the particles after washing with hexane five times and drying under vacuum for several hours. The results indicate that the prepared PSEs are a highly efficient system for color-detection of halogens and adsorption for environmental reasons.
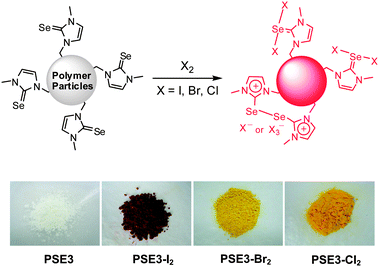 | ||
| Fig. 2 The reaction of PSE3 with halogens and their photographs before and after treatment of halogens. | ||
Next, we tested the polymer particles as adsorbents for transition metals in aqueous solution. The polymer particles (PSE) showed relatively poor adsorption behavior for transition metal ions in water, possibly due to the hydrophobic properties of particles. It was recently reported by our group that the reaction of 1,3-dimethylimidazoline-2-selenone (M1) with acids such as hydrochloric acid resulted in formation of the red-orange colored dimerized salt (M2, see the compound in Fig. 3b).8d Thus, it can be speculated that acid-treatment of PSE would imbue the polymer particles with hydrophilic properties due to the generated ionic character (Fig. 3). In this regards, polymer particles (PSE3) were treated with 1.0 M hydrochloric acid solution for 24 hours to form water-dispersible red-orange colored particles (PSEA) (see the Experimental section for detailed procedure). The change in the electronic surroundings of the selenium atom by reaction could be monitored by X-ray photoelectron spectroscopy (XPS) using synchrotron radiation. Fig. 4 compares the XPS spectra of the original polymer particles (PSE) and acid-treated particles (PSEA) with model compounds, 1,3-dimethylimidazoline-2-selenone (M1) and imidazolium diselenide compound (M2). After treatment of polymer particles with acid, the Se 3d orbital peak was shifted from 54.4 to 55.2 eV, which matched well with those of model compounds (Fig. 4).
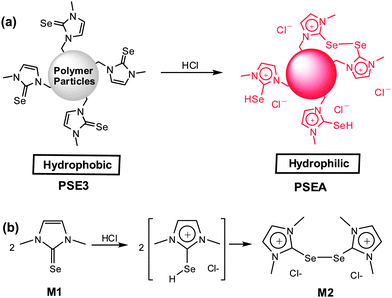 | ||
| Fig. 3 The reaction of polymer particles bearing imidazoline-2-selenone moieties (PSE3) with acid to form hydrophilic polymer particles (PSEAs) (a) and its mode reaction with 1,3-dimethylimidazoline-2-selenone (b). | ||
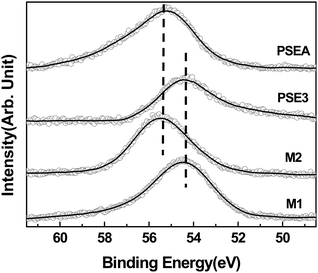 | ||
| Fig. 4 X-Ray photoelectron (Se 3d orbital) spectra of PSEA, PSE3, M1 (1,3-dimethyl-imidazoline-2-selenone) and M2 which were measured using synchrotron radiation with an incident beam energy (hν) of 400 eV and an emission angle (θe) of 0°. The black solid lines denote the smoothed spectra as a guide. | ||
The water-dispersible polymer particles PSEA were applied as adsorbents for the selective removal or recovery of transition metal ions in aqueous solution. It was speculated that the soft selenium coordination mode would be suitable for adsorption for chalcogenphilic metals such as toxic Hg2+ or Pb2+. The adsorption selectivity for Hg2+ and Pb2+ was tested in a mixture of 3rd row transition metals (Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+). The model solution containing Cr3+ (119 ppm), Mn2+ (132 ppm), Fe3+ (124 ppm), Co2+ (130 ppm), Ni2+ (128 ppm), Cu2+ (127 ppm), Zn2+ (131 ppm), Pb2+ (133 ppm) and Hg2+ (88 ppm) was prepared by dissolving diverse metal chlorides in water. This solution (33 mL) was then treated with 50 mg of the PSEA for 3 hours at room temperature and directly analyzed viainductively coupled plasma atomic emission spectroscopy (ICP-AES). As shown in Fig. 5a, PSEA showed excellent adsorption selectivity toward toxic Hg2+ ions. In addition, according to the energy dispersive X-ray spectroscopy (EDS) analysis, the Hg was only detected in the resultant particles. The maximum absorption amount for Hg2+ was estimated up to 0.110 g (Hg2+) per gram of PSEA.13
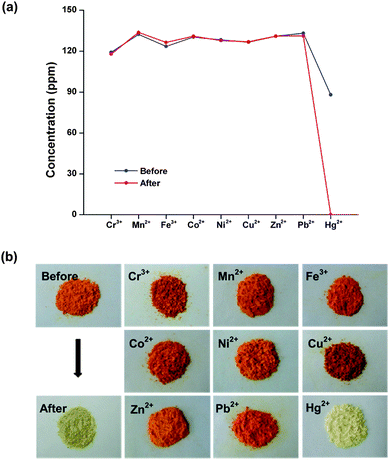 | ||
| Fig. 5 Selective adsorption of Hg2+ from a mixture of transition metal ions (Cr3+ (119 ppm), Mn2+ (132 ppm), Fe3+ (124 ppm), Co2+ (130 ppm), Ni2+ (128 ppm), Cu2+ (127 ppm), Zn2+ (131 ppm), Pb2+ (133 ppm) and Hg2+ (88 ppm)) by PSEA (the values are an average of three measurements) (a) and photographs showing the selective color change of PSEA by reaction with Hg2+; before adsorption, after treatment with 0.1 M mixture of Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+ and Hg2+, after treatment with each metal ions (b). | ||
Interestingly, the reaction of PSEA with Hg2+ resulted in vivid color change of particles from reddish orange to pale yellow (Fig. 5b). In contrast, after treatment of the other metal ions (Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+ and Pb2+), PSEA maintained the original color. To figure out the origin of color change and the formed mercury species on particles, we conducted the model reaction as shown in Fig. 6b. First, the red-orange compound (M2 in Fig. 3b) was formed by acid (HCl) treatment of 1,3-dimethylimidazoline-2-selenone.8d Second, when red-orange M2 was reacted with HgCl2, white-colored solids (Hg–SE) were immediately formed. Although the quality of X-ray diffraction data of the crystalline Hg–SE was not sufficient to be reported, single-crystal X-ray analysis showed the coordination of mercury ions to selenium in 2-selenone through the breaking of the weak Se–Se bond of M2. The similar Se–Se bond cleavage by Pt2+ was characterized by our group (Fig. 6b).8d Single-crystal X-ray structure of benzyl analogue of Hg–SE formed by same reaction is shown in Fig. 6c. Considering these observations, the color change and selective adsorption of PSEA are attributed to the coordination-induced cleavage of weak Se–Se bonding.
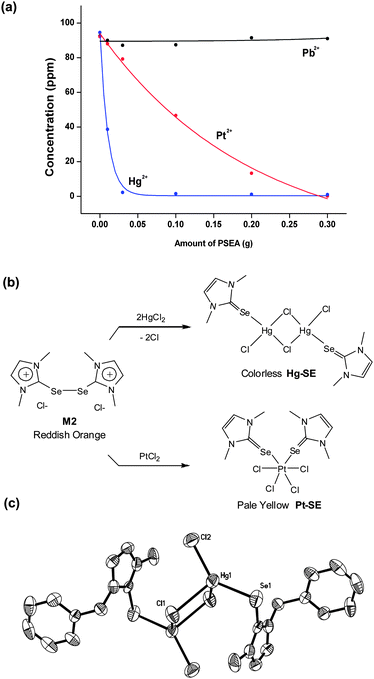 | ||
| Fig. 6 Comparison of adsorption ability for Hg2+(95 ppm) and Pt2+ (92 ppm) against Pb2+ (95 ppm) by PSEA (a) and the model reactions of Hg2+ and Pt2+ adsorption by PSEA (b) and X-ray structure of benzyl-analogue of Hg–SE (c). | ||
Surprisingly, PSEA showed nearly no interaction with Pb2+ ions (Fig. 5a). Recently, selective recovery of platinum ions from conventional metal ion mixtures has become an important issue due to the limited amount of platinum and the recent increased use in car devices.14 In particular, the selectivity for platinum ions against Pb2+ is important because lead is a common metal in a plethora of devices.15 Thus, we tested the adsorption selectivity toward Hg2+, Pt2+ and Pb2+ by changing the amount of PSEA.
As shown in Fig. 6a, the preference for adsorption was in the order of Hg2+ ≫ Pt2+ ≫ Pb2+, indicating that Hg2+ can be removed with a small amount of PSEA in advance, followed by the isolation of Pt2+ without significant adsorption of Pb2+. In addition, it is noteworthy that toxic Hg2+ is not a common material in car devices.
Conclusions
Submicron-sized polymer particles bearing imidazoline-2-selenone have been prepared by polymerization with MSE and DVB. The size and composition of the particles could be controlled by changing the reactant concentration. These particles have been applied as color-sensing adsorbents for halogens or mercury ions in aqueous solution after treatment with acid. This work effectively demonstrates the application of imidazoline selenone chemistry to material science.Acknowledgements
This work was supported by Hydrogen Energy R&D Center, a 21st century Frontier R&D Program. HYY thanks for grants NRF-2010-0029698 (Priority Research Centers Program) and R31-2008-10029 (WCU program).Notes and references
- Selected examples: (a) D. M. Browne and W. Thomas, Curr. Org. Chem., 2006, 10, 1893 CrossRef CAS; (b) M. Tiecco, L. Testaferri, C. Santi, C. Tomassini, R. Bonini, F. Marini, L. Bagnoli and A. Temperini, Org. Lett., 2004, 6, 4751 CrossRef CAS.
- (a) N. Esaki and H. Mihara, Biomed. Res. Trace Elem., 2008, 19, 308 CAS; (b) M. L. Copper, H.-O. Adami, H. Gronberg, F. Wiklund, F. R. Green and M. P. Rayman, Cancer Res., 2008, 68, 10171 CrossRef; (c) H. Araie, I. Suzuki and Y. Shiraiwa, J. Biol. Chem., 2008, 283, 35329 CrossRef CAS.
- Selected examples: (a) W. Ma, J. M. Luther, H. Zheng, Y. Wu and A. P. Alivisatos, Nano Lett., 2009, 9, 2072 Search PubMed; (b) G. Dukovic, M. G. Merkle, J. H. Nelson, S. M. Hughes and A. P. Alivisatos, Adv. Mater., 2008, 20, 4306 CrossRef CAS; (c) T. Mokari, S. E. Habas, M. Zhang and P. Yang, Angew. Chem., Int. Ed., 2008, 47, 5605 CrossRef CAS.
- T. Wirth, Angew. Chem., Int. Ed., 2000, 39, 3740 CrossRef CAS.
- (a) M. C. Aragoni, M. Arca, F. A. Devillanova, P. Grimaldi, F. Isaia, F. Lelj and V. Lippolis, Eur. J. Inorg. Chem., 2006, 2166 CrossRef CAS; (b) M. C. Aragoni, M. Arca, A. J. Blake, F. A. Devillanova, W.-W. du Mont, A. Garau, F. Isaia, V. Lippolis, G. Verani and C. Wilson, Angew. Chem., Int. Ed., 2001, 40, 4229 CrossRef CAS; (c) F. Bigoli, F. Demartin, P. Deplano, F. A. Devilanova, F. Isaia, V. Lippolis, M. L. Mercuri, M. A. Pellinghelli and E. F. Trogu, Inorg. Chem., 1996, 35, 3194 CrossRef CAS; (d) G. Roy, D. Das and G. Mugesh, Inorg. Chim. Acta, 2007, 360, 303 CrossRef CAS.
- S. Ahmad, A. A. Isab, A. R. Al-Arfaj and A. P. Arnold, Polyhedron, 2002, 21, 2099 CrossRef CAS.
- (a) J. Choi, J. Jin, I. G. Jung, J. M. Kim, H. J. Kim and S. U. Son, Chem. Commun., 2011, 47, 5241 RSC; (b) J. Choi, N. Kang, H. Y. Yang, H. J. Kim and S. U. Son, Chem. Mater., 2010, 22, 3586 CrossRef CAS; (c) S. U. Son and E. JangUS. Pat., 12–329058, 2008.
- (a) H. C. Cho, H. S. Lee, J. Chun, S. M. Lee, H. J. Kim and S. U. Son, Chem. Commun., 2011, 47, 917 RSC; (b) J. Choi, H. Y. Yang, H. J. Kim and S. U. Son, Angew. Chem., Int. Ed., 2010, 49, 7718 CrossRef CAS; (c) K. Jang, I. G. Jung, H.-J. Nam, D.-Y. Jung and S. U. Son, J. Am. Chem. Soc., 2009, 131, 12046 CrossRef CAS; (d) J. Choi, J. H. Ko, I. G. Jung, H. Y. Yang, K. C. Ko, J. Y. Lee, S. M. Lee, H. J. Kim, J. H. Nam, J. R. Ahn and S. U. Son, Chem. Mater., 2009, 21, 2571 CrossRef CAS; (e) J. Choi and S. U. Son, Kor. Pat., 10-2009-0003051, 2009; (f) K. H. Park, I. Ku, H. J. Kim and S. U. Son, Chem. Mater., 2008, 20, 1673 CrossRef CAS; (g) K. H. Park, K. H. Jang, Y. Cho, J. Chun, H. J. Kim, D. A. Sweigart and S. U. Son, Adv. Mater., 2007, 19, 2547 CrossRef CAS; (h) K. H. Park, K. Jang, S. U. Son and D. A. Sweigart, J. Am. Chem. Soc., 2006, 128, 8740 CrossRef CAS.
- W. Chen, Y. Zhang, L. Zhu, J. Lan, R. Xie and J. You, J. Am. Chem. Soc., 2007, 129, 13879 CrossRef CAS.
- (a) H. Wang, S. Zhang, M. Wang and X. Ge, Chem. Lett., 2008, 37, 1158 CrossRef CAS; (b) D. Zou, V. Derlich, K. Gandhi, M. Park, L. Sun, D. Kriz, Y. D. Lee, G. Kim, J. J. Aklonis and R. Salovey, J. Polym. Sci., Part A: Polym. Chem., 1990, 28, 1909 CrossRef CAS.
- We acknowledge that a referee suggested this explanation in the review process.
- The molar ratio of nitrogen to selenium can be regarded as 2.
- Recent example of the chalcogenide based-mercury adsorbent: J. Aguado, J. M. Arsuaga and A. Arencibia, Microporous Mesoporous Mater., 2008, 109, 513 CrossRef CAS.
- (a) N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and J. L. Wang, Science, 2007, 316, 732 CrossRef CAS; (b) C.-H. Kim, S. I. Woo and S. H. Jeon, Ind. Eng. Chem. Res., 2000, 39, 1185 CrossRef CAS; (c) S. Dai, M. C. Burleigh, Y. H. Ju, H. J. Gao, J. S. Lin, S. J. Pennycook, C. E. Barnes and Z. L. Xue, J. Am. Chem. Soc., 2000, 122, 992 CrossRef CAS; (d) J. Brown, L. Mercier and T. J. Pinnavaia, Chem. Commun., 1999, 69 RSC; (e) M. C. Burleigh, S. Dai, E. W. Hagaman and L. S. Lin, Chem. Mater., 2001, 13, 2537 CrossRef CAS; (f) X. Feng, G. E. Fryxell, L.-Q. Wang, A. Y. Kim, J. Liu and K. M. Kemner, Science, 1997, 276, 923 CrossRef CAS; (g) H. Narita, M. Tanaka and M. Kazuko, PCT Int. Appl., 2008, 23 Search PubMed; (h) D. Terekhov, O. Olurin, K. M. Khozan and N. Victor-Emmanuel, Can. Pat. Appl., 2008, 18 Search PubMed; (i) M. Bergeron and M. Richer-Lafleche, PCT Int. Appl., 2005, 45 Search PubMed.
- It is noteworthy that the wet-chemical separation method of the target noble metals from noble metal mixture containing platinum, palladium and gold has been well documented. See C. Ammen, Recovery and Refining of Precious Metals, Springer, 2nd ed., 1997 Search PubMed.
Footnote |
| † CCDC reference number 745623. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c1py00260k |
| This journal is © The Royal Society of Chemistry 2011 |
