Dextran-silane coating chemistry for SiO2-based suspension array system
Rong
Cao†
abc,
Zhuoxuan
Lu†
a,
Demin
Duan
ac,
Zhuan
Zhao
ac,
Xingli
Du
a and
Jiong
Li
*a
aSuzhou Institute of Nano-tech and Nano-bionics, Chinese Academy of Sciences, Suzhou, 215123, P. R. China. E-mail: jli2006@sinano.ac.cn; Fax: (+86)512-62603079; Tel: (+86)512-62872644
bTechnical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China
cGraduate University of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 13th September 2011
Abstract
Suspension arrays provide an efficient way to identify and quantify biopolymers (usually proteins or nucleic acid sequences) in high throughput. Among various suspension arrays, self-encoded silica particle arrays, which benefit from higher sample throughput and better material stability, are attracting increasing attention. Popular methods of attaching biomolecules to silica particle surfaces include adsorption and covalent binding viaamine-silane or aldehyde-silane chemistry. One drawback of these immobilization strategies is the relatively low binding efficiency. Herein, we present a method for increasing the binding capacity of biomolecules on silica particles by employing three-dimensional (3D) derivatized natural macromolecule (dextran) matrixes. And we demonstrate the preparation of protein arrays to substantiate the applicability of our approach. Derivatized dextran-coated particles can be easily incorporated in biopolymers and provide a versatile support for bioanalysis. This new approach with dextran-silane chemistry for biomolecule binding allows signals from immobilization and interaction to have higher signal-to-noise ratios than traditional two-dimensional (2D) methods. We believe that the proposed method could provide a perspective on the improvement of self-encoded silica particle arrays.
Introduction
Recent advances in chemical biology and bioengineering have led to the development of biosensors and microarrays.1–3 In particular, microarrays provide an efficient way for multiplexed biomolecule analysis. These technologies have substantially influenced many areas, such as genetic analysis, combinatorial chemistry and clinical diagnosis.4,5 Presently, there are two broad classes of microarrays: planar arrays and suspension (particle-based) arrays. In comparison to planar arrays, suspension arrays, on account of faster reaction kinetics, higher sample throughput and better quality control, have been widely used in genomics and proteomics research.6,7As promising tools for high-throughput screening, suspension arrays generally have two different encoding options: spectral and graphical.8 Spectral encoding, usually performed with fluorescent labeling, has achieved commercial success (e.g., Luminex's xMAP Technology).9–11 However, these fluorescence-encoded bead arrays suffer from several disadvantages, including the limited encoding available, higher costs for manufacture and detection, plus the potential spectral crosstalk of fluorescent signals between encoding and analyte detection.8,12 Recently, graphical encoding, featuring identifiable patterning of optical elements on the particle, is beginning to offer the potential to circumvent these limitations posed by spectral encoding. Some typical cases include cylindrical silica particles (http://www.illumina.com/), dot-coded polymer particles12 and shape-coded silica nanotubes.13 Among them, in virtue of the ease of micropatterning by photolithographic techniques, low intrinsic fluorescence and a variety of surface modification strategies, silica particles (SiO2) make themselves highly suitable to fabricate graphically encoded suspension arrays.14–16
Nowadays, a variety of silane chemistries have been widely used to facilitate the immobilization of biomolecules for microarrays, such as amino, epoxy or aldehyde silane.15,17 However, because of these two-dimensional (2D) matrixes, the immobilization capacity is limited, which results in relatively low array sensitivity.18 To overcome this problem of planar arrays, several approaches for fabricating three-dimensional (3D) matrixes have been used to achieve higher immobilization capacity, like polyacrylamide19 and agarose coating on glass surfaces.20 In view of this, the 3D polymer derivatization of silica particles should also provide an efficient way to reinforce the binding capacity and signal strength of silica particle-based arrays.
In this paper, we report a method with dextran-silane chemistry that enhances the capacity of binding biomolecules onto the silica particle surface. Generally, silica particles derivatized with aldehyde dextran (AD) and carboxymethyl dextran (CMD) are used for bioassay applications. As specific examples, we chose spheral silica beads as the model system to demonstrate the conjunctive use of pure silica and derivatized natural macromolecule (dextran). Then, we described the construction of protein microarrays. Under optimized experimental conditions, CMD-coated beads had excellent biomolecular binding capacities, which could offer great flexibility in routine genomic and proteomic applications. We hope the polymer coating technology can be applied to a diverse range of biological macromolecule assays based on silica particles.
Experimental section
Materials
Silica beads (4.54 μm, ∼1 × 1010 beads g−1) were obtained from Bangs Laboratories, Inc. 3-(2-Aminoethylamino)propyl-dimethoxymethylsilane, 3-glycidoxypropyltrimethoxysilane, glutaraldehyde and bromoacetic acid were obtained from Sigma-Aldrich. DextranT70, T500 and T2000 (weight-averaged molecular weight = 70, 500 and 2000 kDa) were purchased from Pharmacia. 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS) and phosphate-buffered saline (PBS, pH 7.4) were purchased from Sangon Biological Engineering Technology & Services Co., Ltd. DyLight™ 488-conjugated goat anti-rabbit IgG (DL488 anti-rabbit IgG) was purchased from Jackson ImmunoResearch Labs. Other proteins were obtained from ZhongShan Golden Bridge Biotechnology Co., Ltd. All chemicals and solvents were of analytical grade and ultrapure water (18 MΩ cm) was used throughput the work.Instrumentation
Reactions with silica beads were performed in centrifuge tubes at room temperature with continuous mixing by the Vortex-Genie 2 mixer. Beads were collected by centrifugation at 9000 rpm. All fluorescence measurements were performed with a Nikon Eclipse Ti inverted research microscope after experiments. More specifically, a drop (1 μL) of sample beads in corresponding buffer was transferred to a hydrophobic glass slide. Before the droplet was dried out, fluorescence of settled beads was collected with the FITC filter (using a 40× objective, if not specified). Measurements of all the optical parameters (e.g., exposure time and gain value) were kept constant in related measurements. Bead fluorescence intensities were calculated based on 40 random beads with the ImageJ software.Chemical modifications of silica beads
Silica beads were washed with ethanol prior to derivatization with the silane reagents.(a) Amino silanization: Silica beads (∼106) were suspended in 100 μL of 2 vol% 3-(2-aminoethylamino)propyl-dimethoxymethylsilane in 95% ethanol for 0.5 h, similar to the process used for glass surfaces.21 Following silanization, amino-silanized beads were rinsed thoroughly with ethanol followed by ddH2O.
(b) Epoxy silanization: Silica beads (∼106) were suspended in 100 μL of 100% 3-glycidoxypropyltrimethoxysilane solution for 3 h, similar to the process used for glass surfaces.22 Subsequently, epoxy-silanized beads were rinsed thoroughly with ethanol followed by ddH2O.
(c) Aldehyde modification: Amino-silanized beads (∼106) were dispersed in 100 μL of 2.5 vol% glutaraldehyde/PBS solution. Then beads were shaken for 2 h and washed thoroughly with PBS.
Derivatized-dextran coating on silanized beads
(a) AD coating on amino beads: typically, amino beads (∼106) were mixed with 1 mL oxidized AD solution (0.13 g sodium periodate was added to the solution of 0.1 g dextranT2000 in 10 mL 0.1 M pH 5.0 acetate buffer, freshly prepared, 10 min prior to use) in the dark for 4 h, and then rinsed by 0.1 M pH 5.0 acetate buffer. As the same, silica beads and epoxy beads, which reacted with oxidized AD solution as described above, were used as the control.(b) CMD coating on epoxy beads: Typically, CMD was prepared by dissolving 1 g dextranT500 in 10 mL of 2 M NaOH containing 1.39 g bromoacetic acid. After stirred for 24 h, the pH of the reaction mixture was adjusted by 2 M H2SO4. Then epoxy beads (∼106) were mixed with 1 mL reaction mixture for 24 h, and rinsed by ddH2O. As the same, silica beads and amino beads, which reacted with prepared CMD solution as described above, were used as the control.
Following the procedures as mentioned above, a variety of reaction parameters were examined to identify optimal conditions for derivatized-dextran coating.
Immobilization of proteins on derivatized dextran-coated beads
Fluorescence-labeled protein (DL488 anti-rabbit IgG) was directly immobilized onto surfaces of different beads for signal detection, including AD-coated beads, CMD-coated beads and aldehyde-derivatized beads.(a) AD-coated beads (∼106) were incubated with 100 μL of 7.5 μg mL−1DL488 anti-rabbit IgG in 0.1 M pH 5.0 acetate buffer for 4 h in the dark, and then washed by 0.1 M pH 5.0 acetate buffer with 0.1% Tween 20.
(b) CMD-coated beads were activated with the mixed solution (20 mg mL−1 NHS and 40 mg mL−1 EDC in 0.1 M pH 5.0 MES buffer, containing 0.15 M NaCl) for 0.5 h. Then activated beads (∼106) were washed and resuspended in 10 mM pH 5.0 acetate buffer to react with 100 μL of 7.5 μg mL−1DL488 anti-rabbit IgG for 4 h in the dark. Finally, the beads were washed with PBST (pH 7.4, containing 0.1% Tween 20).
(c) Aldehyde-derivatized beads (∼106) were incubated with 100 μL of 7.5 μg mL−1DL488 anti-rabbit IgG in 0.1 M pH 5.0 acetate buffer for 4 h in the dark, and then washed by 0.1 M pH 5.0 acetate buffer with 0.1% Tween 20.
Fabrication and processing of protein microarrays
(a) Antibody-antigen interaction and recognition: 50 μg mL−1 rabbit IgG were added to activated CMDT500-coated beads with continuous mixing for 4 h, as described above. After washing, the beads were resuspended in excess quenching solution (50 mM Tris, pH 7.4) for 0.5 h, followed by 1% (w/v) BSA in PBS for 0.5 h. Then beads were incubated with 0.1–50 ng mL−1DL488 anti-rabbit IgG (each 104 beads for 50 μL DL488 anti-rabbit IgG solution) for 2 h in the dark, rinsed, and imaged to determine fluorescent intensity. Control samples (i.e., aldehyde-derivatized beads) were obtained through the same experimental procedures.(b) Sandwich immunoassay: As a typical experiment for protein detection, a three-component sandwich assay is illustrated in Fig. 1. We immobilized goat anti-rabbit IgG (50 μg mL−1) on CMDT500-coated beads. Then, after quenching as mentioned above, the beads were mixed with 2 μg mL−1 rabbit IgG, mouse IgG and goat IgG for 2 h, respectively. After washed by PBST buffer, the beads were incubated with 7.5 μg mL−1DL488 anti-rabbit IgG for 2 h in the dark, rinsed, and imaged to determine fluorescent intensity.
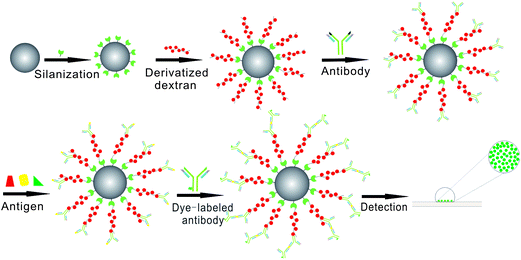 | ||
| Fig. 1 Schematic diagram of protein sandwich microarrays based on our dextran-silica system. Detailed schematic of how derivatized-dextran coated silica beads can be used to provide the detection of specific antigen using a sandwich immunoassay approach. Our model protein arrays (with goat anti-rabbit IgG) were treated with different antigen (rabbit IgG, mouse IgG and goat IgG), and then incubated with DL488 anti-rabbit IgG. Images were obtained with a Nikon Eclipse Ti inverted research microscope. | ||
Results and discussion
Design of 3D matrixes on silica particles
Considering that the objective of microarray technology is the study of biomolecular interactions, the provision of optimal binding conditions is a crucial feature of microarray support materials. As a popular macromolecule, dextran with excellent biocompatibility, available molecular weights and possible functionalization,23–26 has been widely used in planar-array fabrication to provide the high-sensitivity and high-selectivity attributes for biomolecule detection (e.g., commercially available BIAcore Sensor Chip CM5).27,28As a new class of dextran applications here, the most prevalent derivatized dextrans have been applied to yield 3D matrixes on silica particles via covalent immobilization, as shown schematically in Fig. 2. AD and CMD have obviously different molecular structures, which bring on different coating chemistries. Covalent coating of AD could be accomplished via reactions between amino groups of the beads and aldehyde groups of oxidized dextran (Fig. 2A), while epoxy groups on the surface provide binding sites for hydroxyl group-containing CMD (Fig. 2B).
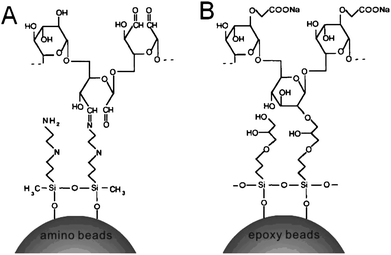 | ||
| Fig. 2 Schematic representation of derivatized-dextran coating on silanized silica beads. (A) AD coating: coupling of AD to amine-functionalized beads via a Schiff's reaction. (B) CMD coating: coupling of CMD to epoxy-functionalized beads via the epoxide ring-opening reaction. | ||
First, DL488 anti-rabbit IgG was directly immobilized on the surface of silica beads derivatized with AD and CMD, respectively. This treatment was to test the capacity of biomolecule immobilization. We further performed two types of experiments using derivatized dextran-coated beads as models for protein microarrays.
Optimization of AD coating on amino beads
Periodate oxidation specifically cleaves the vicinal glycols in dextran to form their open-chain dialdehyde derivatives. Each cis-diol group consumes one molecule of periodate. And it is well known that the degradation rate of oxidized dextran becomes higher as pH increases.29,30 Considering the alkaline degradation of oxidized dextran, 0.1 M pH 5.0 acetate buffer was chosen as reaction medium. We investigated the performance of AD coating by controlling the extent of oxidation and varying the concentration of AD. Hydroxyl groups in dextran were selectively oxidized to aldehyde groups by sodium periodate with different molar ratios of sodium periodate to monosaccharide units (Ms Mm−1), ranging from 1/4 to 2. Fig. 3A shows the influence of Ms Mm−1 on protein immobilization. The high molar ratio (≥ 3/4) resulted in significantly increased protein loading. The most obvious explanation was that sufficient oxidation provided more aldehyde groups along the oxidized chains to react specifically with primary amines of protein. On the other hand, the effect of AD concentration on protein immobilization was not as striking as that of the degree of oxidation. The immobilized amount of protein exhibited a low dependence on the AD concentration (Fig. 3B). To get the maximum binding capacity, 2% (w/v) AD (Ms Mm−1 = 1) was chosen for the following experiments.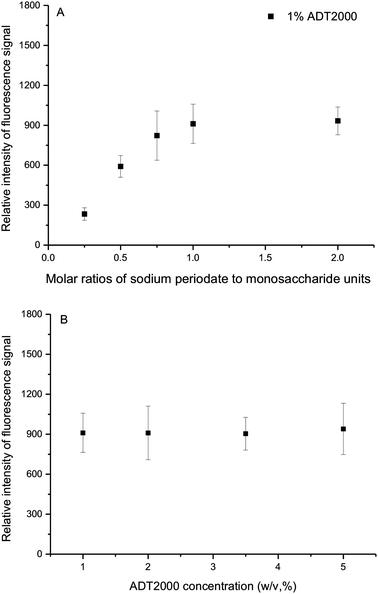 | ||
| Fig. 3 Detection of immobilized DL488-anti rabbit IgG on AD-coated beads. (A) Effect of variation of molar ratios of sodium periodate to monosaccharide units (Ms Mm−1), while keeping the concentration of ADT2000 at 1% (w/v). (B) Effect of variation of ADT2000 concentration, while keeping Ms Mm−1 = 1. | ||
Optimization of CMD coating on epoxy beads
The synthesis of CMD in aqueous solution using bromoacetic acid and strong alkaline conditions allows of highly substituted CMD with good reproducibility and high yield. The formation of carboxylic groups along the backbone of dextran endows the polymer with negative charges, which permit a rapid electrostatic adsorption of positively charged biopolymers. Especially for protein arrays, it can accelerate covalent immobilization of positively charged protein on negatively charged CMD matrix. This would minimize expensive protein usage.Here, we put emphasis on the dependence of CMD coating on the pH of the reaction medium and CMD concentration. As shown in Fig. 4A, the performance of CMD coating was sensitive to pH conditions. Since silanized particles are unstable at high pH and dextran is susceptible to hydrolysis at low pH, slightly alkaline conditions (pH 9.2) would be chosen to assist CMD coating in this study. With CMD solution concentration above 10%, the immobilized amount of protein could reach a plateau (Fig. 4B). And in the case of CMD-coated beads without EDC/NHS activation, no significant fluorescence signal was detected to indicate the success of the coating procedure. To get the maximum binding capacity, 10% (w/v) CMD (pH 9.2) was chosen for the following experiments.
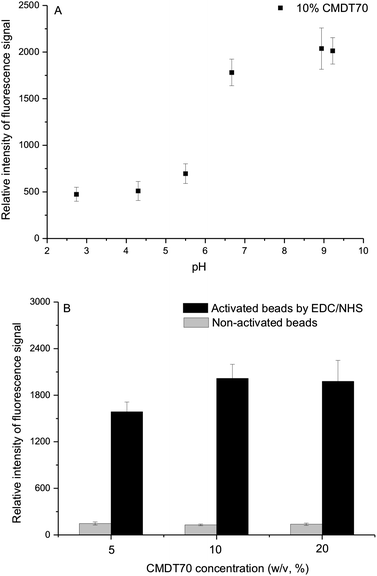 | ||
| Fig. 4 Detection of immobilized DL488-anti rabbit IgG on CMD-coated beads. (A) Effect of variation of pH. Detailed reaction conditions: 1 g dextranT70 in 10 mL of 2 M NaOH containing 1.39 g bromoacetic acid were stirred for 24 h, then pH adjusted by 2 M H2SO4. (B) Effect of variation of CMDT70 concentration. Detailed reaction conditions: 0.5 g, 1 g, and 2 g dextranT70, respectively, in 10 mL of 2 M NaOH containing 1.39 g bromoacetic acid were stirred for 24 h, then adjusted to pH 9.2. | ||
Derivatized-dextran modification on different silanized beads
The proportion of protein immobilized on silica beads could vary with different chemistries (Fig. 5). With AD coating on amino beads, it seemed more efficient for protein immobilization. The other types of silica particles displayed different behaviors, depending on the failure of covalent coating of AD (Fig. 5A). Surprisingly, the results with CMD coating on silanized beads were exactly opposite. The results indicated the success of the CMD coating in all cases of silica beads (Fig. 5B). We believed that these results were due to physical adsorption of CMD, which was in good agreement with the results of glass slides by Elender et al.31 It is important to note that there is a drastic reduction of molecular weight in the dextran oxidation process. Namely, oxidation of dextran produces a decrease in the stiffness of the polymer with a chain scission as a simultaneous reaction, which may lead to the failure of AD physical adsorption on silica particles.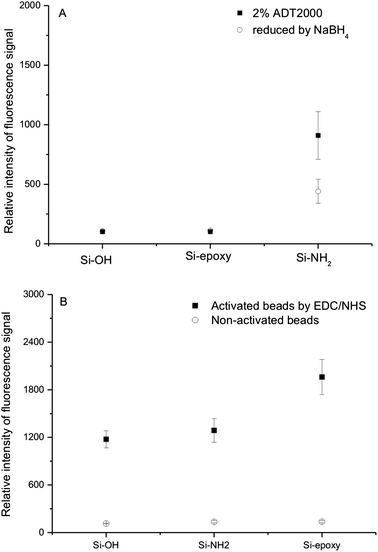 | ||
| Fig. 5 Detection of immobilized DL488-anti rabbit IgG on different treated silica beads. (A) AD coating on different beads. Detailed reaction conditions: the concentration of ADT2000 at 2% (w/v), Ms Mm−1 = 1. AD-coated beads were treated with 1% NaBH4 in PBS buffer before protein immobilization (circles). Beads were not involved with NaBH4 treatment (squares). (B) CMD coating on different beads. Detailed reaction conditions: 2 g dextranT70 in 10 mL of 2 M NaOH containing 1.39 g bromoacetic acid were stirred for 24 h, then adjusted to pH 9.2. Beads were not activated with EDC/NHS (circles). Beads were activated with EDC/NHS (squares). | ||
Comparison of immobilization methods
With the optimized protocol, we compared the performance of our derivatized dextran-coated beads with the widely used 2D beads (i.e., aldehyde-derivatized beads). On the basis of these observations from Fig. 6, the coating derived from CMD would be recognized as a more efficient method to increase immobilization of protein for silica particles. We also evaluated the effect of CMD with different molecular weights. It was apparent that with increasing molecular weight of CMD, there was an increase in polymer layer thickness, which in turn affected protein immobilization. In the case of CMDT2000, the fluorescence signal was as three times as that of aldehyde-derivatized beads (Fig. 6B). Even pure silica beads coated with high molecular weight CMD had a strong fluorescence signal. Namely, the physical adsorption of high molecular weight CMD was resistant to the incubation and stringent rinsing steps, which seemed very promising and practical for simplifying our bead-coating processing. And as shown in Fig. 7, CMD-coated beads exhibited high monodispersity due to their hydrophilic surfaces, which was important for suspension arrays.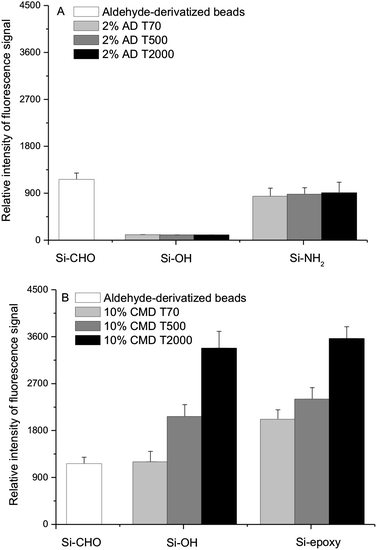 | ||
| Fig. 6 Effect of derivatized dextran with different molecular weights on protein immobilization. (A) AD coating. Detailed reaction conditions: the concentration of ADT70, T500 and T2000 at 2% (w/v), Ms Mm−1 = 1. (B) CMD coating. Detailed reaction conditions: 1 g dextranT70, T500 and T2000 in 10 mL of 2 M NaOH containing 1.39 g bromoacetic acid was stirred for 24 h, then adjusted to pH 9.2. | ||
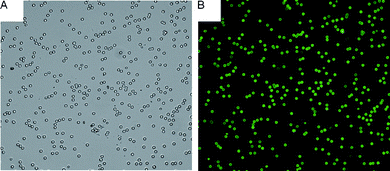 | ||
| Fig. 7 Image of immobilized DL488-anti rabbit IgG on 10% CMDT500-coated beads. (A) Optical image (using a 20× objective). (B) Fluorescence image (using a 20× objective). | ||
Assessment of protein microarray performance
To examine the performance of protein microarrays based on our CMD coating, we selected two miniaturized types of experiments using IgG and anti-IgG as models. As an example for a miniaturized sandwich immune fluorescence assay, we immobilized goat anti-rabbit IgG on CMDT500-coated beads. And a small sample of rabbit IgG, mouse IgG and goat IgG, respectively, was incubated with beads. Because of the specific binding between IgG and its corresponding antibody, anti-rabbit IgG-modified beads would interact with corresponding rabbit IgG. Then a strong fluorescence signal could be observed after incubation with DL488 anti-rabbit IgG, which indicated the formation of antibody/antigen/antibody sandwich. As anticipated, the results revealed that only rabbit IgG bound selectively to the beads (Fig. 8A). In the case of goat IgG and mouse IgG, the fluorescence signal was almost the same as background signal of the glass slide.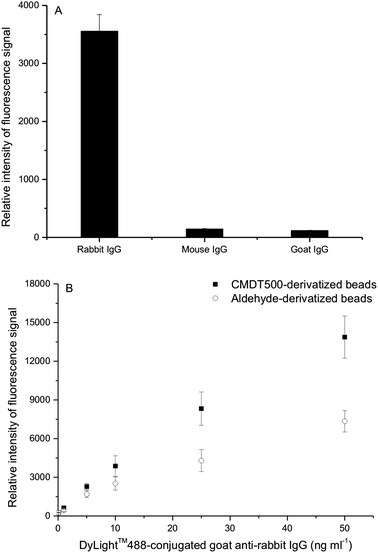 | ||
| Fig. 8 Protein microarrays. (A) Specific and non-specific binding discrimination. (B) Fluorescence signal as a function of DL488 anti-rabbit IgG concentration. CMDT500 coating conditions: 1 g dextranT500 in 10 mL of 2 M NaOH containing 1.39 g bromoacetic acid were stirred for 24 h, then adjusted to pH 9.2. | ||
In the other instance, rabbit IgG was immobilized and then incubated with DL488 anti-rabbit IgG. The fluorescence response of rabbit IgG arrays based on CMDT500 and aldehyde-derivatized beads as a function of DL488 anti-rabbit IgG is shown in Fig. 8B. Measurements were obtained with a dilution series of labeled antibody concentrations. The enhanced performance of our array based on CMDT500 beads could be attributed to the higher loading density of the capture antigen in our CMD-coated beads than aldehyde-derivatized beads. Significant fluorescence signal amplification by our beads allowed arrays by CMD coating to achieve a lower limit-of-detection. Besides, the normalized result clearly showed that the fluorescence signal intensity was linear in a broad concentration range (Fig. 8B). In the light of excellent experimental results obtained even by a normal fluorescence microscope, we believe that the CMD-coated beads could be a robust platform for suspension arrays.
Conclusions
Silica particles are a topic of considerable interest in biomolecule analysis platforms, due to their low intrinsic fluorescence, facile chemical modification and ease of micropatterns. The broad and interdisciplinary interest arises not only from SiO2-based suspension arrays but also from chip-based microanalytical systems (e.g., a capillary with 103 μm arrayed silica beads).32 These recently developed technologies usually rely on tens-of-micrometre particles. It is important to realize that larger silica particles are more easily spun down, less likely to stick firmly together, and more easily resuspended. In view of this, we chose silica beads (<5 μm) as the model and even the small size of derivatized-dextran coated beads presented good performance for protein arrays. Therefore, our silica particle arrays with dextran-silane chemistry would be extraordinarily suited for biomolecule analysis platform requiring varied particle size. That is, small-size monodispersity highlights the suitability of our substrate for the applications.Experience from planar arrays has shown that the formation of 3D matrixes on the surface, by introducing polysaccharides, improves the capacity of probe immobilization and retains biomolecules' activity. As an enlargement of these approaches, we present a method involved with the conjunctive use of silica particles and polysaccharides for particle arrays. For AD coating, the approach is similar to the planar array. However, unlike the procedure of classic CMD application for surface plasmon resonance (SPR) biochips described by Löfås,33 we have preformed the carboxymethylation of dextran prior to use, because silica particles are unstable under strong alkaline conditions which is necessary for the synthesis of CMD.
There is a common view that AD and CMD need to be exhaustively dialyzed before use. In our protocol, no effort is put into the dialysis of AD and CMD prior to use. However, without the dialysis, CMD coating has a very high capacity for protein immobilization. We have tried AD and CMD coating with the dialysis, but this does not give a better result (data not shown). Free carboxylic acid groups on the surface endow the bead with good water solubility, while aldehyde groups at the surface seem more hydrophobic. Thus, CMD beads with good hydrophilic performance reduce the likelihood for hydrophobic interactions as a potential cause for aggregation like AD beads. Besides, the homogeneous water environment provided by the 3D matrixes could help to keep proteins in their active states.
In this paper, we have only described the design and fabrication of derivatized dextran-coated silica beads for protein detection in particle-based arrays, which could display some advantages over prevalent 2D coupling chemistry. Although what we have demonstrated here is an interesting finding, future efforts will still focus on further optimization of experimental conditions for serial applications, like DNA arrays. As previously suggested, in combination with an appropriate SiO2-based bioanalytical platform, such CMD-coated particles can be used in a wide range of applications.
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (No.60701018) and Suzhou Municipal Science and Technology Project (ZXG0711).References
- H. A. Clark, M. Hoyer, M. A. Philbert and R. Kopelman, Anal. Chem., 1999, 71, 4831 CrossRef CAS.
- D. J. Harrison, K. Fluri, K. Seiler, Z. Fan, C. S. Effenhauser and A. Manz, Science, 1993, 261, 895 CrossRef CAS.
- M. Han, X. Gao, J. Z. Su and S. Nie, Nat. Biotechnol., 2001, 19, 631 CrossRef CAS.
- S. P. A. Fodor, R. P. Rava, X. C. Huang, A. C. Pease, C. P. Holmes and C. L. Adams, Nature, 1993, 364, 555 CrossRef CAS.
- D. Stoll, J. Bachmann, M. F. Templin and T. O. Joos, Drug Discovery Today: Targets, 2004, 3, 24 CrossRef CAS.
- H. Zhou, S. Roy, H. Schulman and M. J. Natan, Trends Biotechnol., 2001, 19, 34 CrossRef.
- J. P. Nolan and L. A. Sklar, Trends Biotechnol., 2002, 20, 9 CrossRef CAS.
- C. N. LaFratta and D. R. Walt, Chem. Rev., 2008, 108, 614 CrossRef CAS.
- D. A. A. Vignali, J. Immunol. Methods, 2000, 243, 243 CrossRef CAS.
- K. L. Kellar and M. A. Iannone, Exp. Hematol., 2002, 30, 1227 CrossRef CAS.
- S. A. Dunbar, Clin. Chim. Acta, 2006, 363, 71 CrossRef CAS.
- D. C. Pregibon, M. Toner and P. S. Doyle, Science, 2007, 315, 1393 CrossRef CAS.
- B. He, S. J. Son and S. B. Lee, Anal. Chem., 2007, 79, 5257 CrossRef CAS.
- S. K. Chiu, M. Hsu, W. C. Ku, C. Y. Tu, Y. T. Tseng, W. K. Lau, R. Y. Yan, J. T. Ma and C. M. Tzeng, Biochem. J., 2003, 374, 625 CrossRef CAS.
- G. Steinberg, K. Stromsborg, L. Thomas, D. Barker and C. Zhao, Biopolymers, 2004, 73, 597 CrossRef CAS.
- H. Niino, Y. Kawaguchi, T. Sato, A. Narazaki and R. Kurosaki, Appl. Surf. Sci., 2007, 253, 8287 CrossRef CAS.
- M. K. Walsh, X. Wang and B. C. Weimer, J. Biochem. Biophys. Methods, 2001, 47, 221 CrossRef CAS.
- V. Afanassiev, V. Hanemann and S. Wölfl, Nucleic Acids Res., 2000, 28, e66 CrossRef CAS.
- D. Proudnikov, E. Timofeev and A. Mirzabekov, Anal. Biochem., 1998, 259, 34 CrossRef CAS.
- C. A. Koch, P. C. H. Li and R. S. Utkhede, Anal. Biochem., 2005, 342, 93 CrossRef CAS.
- N. Zammatteo, L. Jeanmart, S. Hamels, S. Courtois, P. Louette, L. Hevesi and J. Remacle, Anal. Biochem., 2000, 280, 143 CrossRef CAS.
- W. Kusnezow, Y. V. Syagailo, S. Rüffer, K. Klenin, W. Sebald, J. D. Hoheisel, C. Gauer and I. Goychuk, Proteomics, 2006, 6, 794 CrossRef CAS.
- Y. S. Lin, E. Renbutsu, M. Morimoto, Y. Okamura, T. Tsuka, H. Saimoto, Y. Okamoto and S. Minami, J. Nanosci. Nanotechnol., 2009, 9, 2558 CrossRef CAS.
- H. Mouaziz, R. Veyret, A. Theretz, F. Ginot and A. Elaissari, J. Biomed. Nanotechnol., 2009, 5, 172 Search PubMed.
- Y. S. Lin, Y. Okamoto and S. Minami, J. Biomed. Nanotechnol., 2010, 6, 247 Search PubMed.
- K. B. Park, Y. I. Jeong, K. C. Choi, S. G. Kim and H. K. Kim, J. Nanosci. Nanotechnol., 2011, 11, 4240 CrossRef CAS.
- D. Usov and G. B. Sukhorukov, Langmuir, 2010, 26, 12575 CrossRef CAS.
- B. Johnsson, S. Löfås and G. Lindquist, Anal. Biochem., 1991, 198, 268 CrossRef CAS.
- J. Devakumar and V. Mookambeswaran, Bioconjugate Chem., 2007, 18, 477 CrossRef CAS.
- E. V. Novikova, E. V. Tishchenko, A. A. Iozep and B. V. Passet, Russ. J. Appl. Chem., 2002, 75, 985 CrossRef CAS.
- G. Elender, M. Kühner and E. Sackmann, Biosens. Bioelectron., 1996, 11, 565 CrossRef CAS.
- Y. Kohara, H. Noda, K. Okano and H. Kambara, Nucleic Acids Res., 2002, 30, e87 CrossRef.
- S. Löfås, Pure Appl. Chem., 1995, 67, 829 CrossRef CAS.
Footnote |
| † Joint first authors, contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2011 |
