Electrochemical synthesis of large-area cobalt microparticle chain networks on Ni thin layer and their template applications†
Xiao-Hui
He
,
Gao-Ren
Li
*,
Zi-Long
Wang
,
Liang-Xin
Ding
and
Ye-Xiang
Tong
KLGHEI of Environment and Energy Chemistry/MOE Laboratory of Bioinorganic and Synthetic Chemistry/School of Chemistry and Chemical Engineer-ing/Institute of Optoelectronic and Functional Composite Materials, Sun Yat-sen University, Guangzhou, 510275, China. E-mail: ligaoren@mail.sysu.edu.cn; Fax: +86-20-84112245; Tel: +86-20-84110071
First published on 27th October 2011
Abstract
Large-area Co microparticle chain networks on Ni thin layer have been synthesized via a facile electrochemical route and tested as a promising magnetic material. They were also utilized as templates for the synthesis of noble metal microparticle chain networks with high electrocatalytic performances.
Materials with microstructures, such as rods, wires, tubes, and particle chains, often show novel electronic, magnetic, optical, or mechanical properties, holding great promise for a wide spectrum of applications.1–2 The synthesis and assembly of above microstructures into macroscopic architectures such as networks, arrays, or more complicated hierarchical structures are crucial for device applications. It is well known that the novel and robust networks tailored from 1D microstructures as building blocks are the foundations for constructing micro-devices. So far, although some networks have grown based on 1D microstructures,3–6 unfortunately, almost all of the previously mentioned synthesis methods only produce powders that must be suspended in binders to evaluate their performances. In addition, the reported small network structures make they difficult for actual applications for microdevices. A worth-while synthetic challenge, therefore, is to develop a facile and scalable approach to fabricate large-area network structures on conducting substrates that will make the super-structures have more application prospect for microdevices. Up to now, there are numerous approaches for creating microparticle assemblies, such as colloidal lithography,7 plasma-induced Ostwald ripening,8 and thermal chemistry method.9 Herein, we describe a convenient electrodeposition strategy may provide a solution. The distinct advantage of the reported electrodeposition route is the synthesis of large-area network structures that can be electrodeposited directly on a conducting substrate with excellent electrical contact without requiring post treatment.
Herein Co was used as an example to illustrate the construction of 2D microparticle chain networks by electrochemical route. As an important magnetic metal, various microstructured Co has been widely investigated because of their technological applications.10–12 However, up to now, assembling 1D Co microparticle chains into 2D micro-network structures still is a huge challenge in the modern material science. 1D microparticle chains are especially important because they can offer new opportunities to study their novel collective physicochemical properties that are evidently different from the detached microparticles,13 and they have shown many potential applications, such as plasmon waveguide,14 magnetic logic,15 and quantum cellular automata.16 The 2D microparticle chain networks will combine much more advantageous properties besides above applications, such as mechanical flexibility, optical transparency, high electrical conductivity, and so on. In this communication, we firstly reported the electrochemical synthesis of large-area 2D Co microparticle chain networks on conducting substrates with excellent electrical contact. In addition, large-area Pt and Pt–Pd alloy microparticle chain networks were also synthesized on conducting substrate by using Co microparticle chain networks as sacrificial templates, and this communication shows a novel route for the preparation of large-area noble metal microparticle chain networks.
The electrochemical deposition of Co was carried out in a simple three-electrode glass cell. A graphite rod was used as the counter electrode. A saturated calomel electrode (SCE) was used as the reference electrode that was connected to the cell with a double salt bridge system. The titanium sheet with uniform Ni thin layer of about 10 nm on the surface is used as working electrode. All the potential values determined in this study were the values versusSCE. Co microparticle chain networks were one-step electrodeposited in solution of 0.01 M·CoCl2 by galvanostatic electrolysis with current density of 1.0 mA cm−2 for 60 min at room temperature. Co microparticle chain networks were analyzed by X-ray energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), and scanning electron microscopy (SEM). Their magnetic properties were studied by magnetic property measurement system (MPMS XL-7). Pt and Pt–Pd alloy microparticle chain networks were prepared by immerging the prepared Co microparticle chain networks into 0.005 M·H2PtCl6 solution and 0.005 M·H2PtCl6 + 0.005 M·H2PdCl6 solution, respectively, for 120 min. The electrochemical properties of Pt microparticle chain networks were studied in solution of 0.5 M·H2SO4 + 1.0 M·CH3OH by cyclic voltammetry in a standard three-electrode cell at room temperature.
A SEM image of Co microparticle chain networks on Ni thin layer with low magnification is shown in Fig. 1(a), which shows large-area Co microparticle chain networks were successfully synthesized. The prepared Co microparticle chain networks are composed of many grids and extended to total surface of substrate. A typical rectangle-like grid in the networks with higher magnification is shown in Fig. 1(b) with long edges of about 55 μm and short edges of about 35 μm. It can be clearly observed that the edges of grids have hierarchical structure since they are consisted of Co microparticle chains. The sizes of these Co microparticles in the chains are about 1.0 μm. XRD pattern of Co microparticle chain networks is shown in Fig. S1(a) (ESI)†. The Co peaks in XRD pattern can be indexed to (100), (002), and (101) crystal faces of hexagonal-close-packed (hcp) Co phase (JCPDS 05-0727). No Co oxides, Co hydroxides, or other impurities are observed besides the peaks coming from the substrate, indicating the microparticle chain networks almost consist of pure Co. This result is consistent with EDS spectrum shown in Fig. S1(b) (ESI)†.
The evolution process of Co microparticle chain networks is investigated as shown in Fig. 2. Before electrodeposition of Co, the fresh Ni thin layer of about 10 nm is electrodeposited on titanium sheet that serves as substrate as shown in Fig. 2(a). For the electrodeposition of Co on Ni thin layer, it is unlike the most systems reported previously.17 Herein the nucleation process of Co exhibits a unique behavior of the deposits arranging systematically and regularly, and the deposited Co self-assembles into microparticle chains as shown in Fig. 2(b). With the deposition time increasing, Co microparticles grew bigger and more compact, and finally the hierarchical networks composed of Co microparticle chains were formed as shown in Fig. 2(c) and (d). Herein, the nucleation and growth direction of Co microparticles were controlled by grain boundaries. In addition, a control experiment was carried out, in which Co was directly deposited on the surface of titanium substrate. Without the freshly deposited Ni thin layer on titanium substrate, the flocculent Co is always obtained as shown Fig. S2†, and no Co microparticle chains are synthesized. Herein, the fresh Ni thin layer on titanium substrate is crucial for the formation of Co microparticle chain networks. By changing the deposition parameters, the different Co microparticle chain networks on Ni thin layers can be synthesized as shown in Fig. S3†.
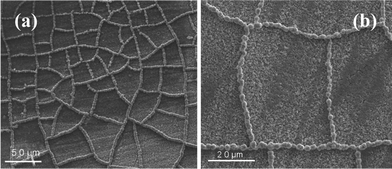 | ||
| Fig. 1 SEM images of Co microparticle chain networks with different magnifications. | ||
 | ||
| Fig. 2 The evolvement sequence of Co microparticle chain networks on Ni thin layer from (a)→(b)→(c)→(d). (a) 0 min; (b) 10 min; (c) 30 min; (d) 80 min. | ||
Magnetic hysteresis loops of Co microparticle chain networks on Ni thin film were measured at 300 K and 5 K, respectively, and they are shown in Fig. 3. The magnified curves among H = −2000 ∼+ 2000 Oe are shown in the inset. The ferromagnetic hysteresis loops are observed at 300 K and 5 K. Such magnetic irreversibility is also confirmed by zero-field cooling/field cooling (ZFC/FC) measurement (Fig. S5†). In the hysteresis loop measured at 300 K, the saturation magnetization (Ms) is about 48.2 emu/g. The remanent magnetization (Mr) is about 17.5 emu/g. The coercivity field (Hc) becomes as large as 102 Oe, which is a great enhancement compared with bulk Co value of 10 Oe18 and bulk Ni value of 0.7 Oe.19 The Hc of Co microparticle chain networks on Ni thin layer is also much higher than those of Co and Ni nanostructures prepared by solution route. For example, the Hc values of Co hollow mesospheres,19Co hollow nanospheres,20 and Ni nanoparticles21 at room temperature are only 40, 75, and 19.4 Oe, respectively, which are much lower than 102 Oe of Co microparticle chain networks. In addition, the Hc of Co microparticle chain networks on Ni thin layer is much higher than that of CoNi alloys (43.9∼53.2 Oe) at room temperature.22 When the measurement temperature was decreased to 5 K, the Hc of Co microparticle chain networks on Ni thin layer becomes larger, and it achieves about 329 Oe.
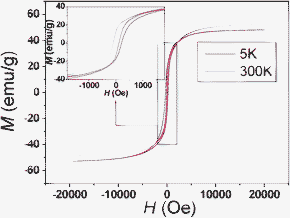 | ||
| Fig. 3 Magnetic hysteresis loops of Co microparticle chain networks on Ni thin layer at 5 and 300 K (the inset is the magnified hysteresis curves among −2000 ∼+ 2000 Oe). | ||
Recently, Pt as electrocatalyst has attracted much interest.23 Herein, by utilizing the synthesized Co microparticle chain networks on Ni thin layer as sacrificial templates, Pt microparticle chain networks on Pt thin layer were successfully synthesized when the Co microparticle chain networks on Ni thin layer were immersed into 0.005 M·H2PtCl6 solution for 120 min. Up to now, Pt microparticle chain networks on Pt thin layer are rarely reported. The typical SEM image of the obtained Pt sample is shown in Fig. 4(a). The thickness of Pt thin layer is about 10 nm, which is equal to the thickness of Ni thin layer. The sizes of Pt microparticles in the chains are about 2.5 μm. During the formation process, the following replacement reactions (1) and (2) happened:
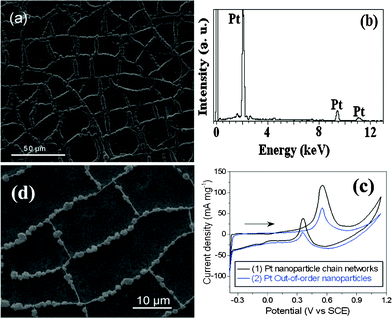 | ||
| Fig. 4 (a) SEM image of Pt microparticle chain networks on Pt thin layer. (b) EDS pattern of Pt microparticle chain networks on Pt thin layer; (c) CV curves of methanol oxidation on (1) Pt microparticle chain networks on Pt thin layer and (2) out-of-order Pt microparticles recorded in solution of 0.5 M·H2SO4 + 1.0 M·CH3OH at room temperature at 50 mV s−1; (d) SEM image of Pt–Pd alloy microparticle chain networks on Pt–Pd thin layer. | ||
2Co + PtCl62−→Pt + 2Co2++ 6Cl− (1)
2Ni + PtCl62−→Pt + 2Ni2++ 6Cl− (2)
The reaction (1) urged PtCl62− ions to form Pt atoms in the prim-ary sites of Co atoms with the disappearance of Co. Accordingly, in the end Co microparticle chain networks were entirely replaced by Pt microparticle chain networks with reaction time increasing. In addition, the Ni thin layer was also replaced by Pt thin layer via reaction (2). Finally, the Pt microparticle chain networks on Pt thin layer were successfully synthesized. The EDS pattern in Fig. 4(b) shows the obtained Pt sample is composed of pure Pt. Motivated by the unique microstuctures, the electrocatalytic activity of Pt microparticle chain networks on Pt thin layer was investigated. Curves (1) and (2) in Fig. 4(c) represent the cyclic voltammetry (CV) curves of Pt microparticle chain networks on Pt thin layer (Pt sample 1) and out-of-order Pt microparticles (Pt sample 2) with the same Pt loading in solution of 0.5 M·H2SO4 + 1.0 M·CH3OH at room temperature, respectively (the SEM image of Pt sample 2 is shown in Fig. S7†). The forward anodic peaks at 0.55 V in Fig. 4(c) correspond to the oxidations of methanol, and the peaks at 0.36 V in the reverse scan correspond to the oxidations of adsorbed CO or CO-like species. For Pt sample 1, the onset potential of methanol oxidation is 0.43 V, which is 40 mV more negative than that obtained on Pt sample 2. This result indicates a significant enhancement in the kinetics of methanol oxidation reaction for Pt sample 1. In addition, for Pt sample 1, the maximum peak current density of methanol oxidation in CV curve is higher than that of Pt sample 2. The above facts show Pt sample 1 exhibits much higher electrocatalytic activity for the oxidation of methanol compared with Pt sample 2. The unique microstructures of mutual connection networks that is much more favorable for the rapid electron transmission and the high utilization rate of Pt thin film are responsible for the high electrocatalytic performances of Pt sample 1.
To demonstrate the universality of the reported replacement reaction method for the synthesis of noble metal microparticle chain networks on noble metal thin layer, Pt-Pd alloy microparticle chain networks on Pt–Pd thin layer were also successfully prepared by immerging Co microparticle chain networks on Ni thin layer into 0.005 M·H2PtCl6 + 0.005 M·H2PdCl6 solution for 120 min at room temperature, and SEM image of the prepared sample is shown in Fig. 4(d). EDS pattern in Fig. S9† shows the obtained Pt–Pd sample is composed of Pt (52 at%) and Pd (48 at%).
In summary, large-area Co microparticle chain networks on Ni thin film have been synthesized by electrochemical deposition route at room temperature and ordinary atmospheric pressure without any surface capping agent. The electrochemical deposition route shows a facile and low-cost route. It has been found the prepared Co microparticle chain networks on Ni thin film showed much higher coercivity than those of bulk Co, bulk Ni, and Co and Ni nanostructures, holding the potential for microdevice applications. Co microparticle chain networks on Ni thin film have been successfully used as the sacrificial templates for the synthesis of large-area noble metal microparticle chain networks on noble metal thin layer, which showed the enhanced electrochemical properties as electrocatalysts.
This work was supported by NSFC (21073240, 51173212, 20873184, and 90923008) and the Fundamental Research Fund for the Central University (11lgzd14).
References
- (a) S. Guo, S. Dong and E. Wang, Chem. Commun., 2010, 46, 1869–1871 RSC; (b) M. Fischler, A. Sologubenko, J. Mayer, G. Clever, G. Burley, J. Gierlich, T. Carell and U. Simon, Chem. Commun., 2008, 169–170 RSC; (c) M.-J. Hu, Y. Lu, S. Zhang, S.-R. Guo, B. Lin, M. Zhang and S.-H. Yu, J. Am. Chem. Soc., 2008, 130, 11606–11607 CrossRef CAS; (d) Y.-J. Zhan and S.-H. Yu, J. Am. Chem. Soc., 2008, 130, 5650–5651 CrossRef CAS.
- (a) M. M. Bull, W. J. Chung, S. R. Anderson, S. Kim, I. Shim, H. Paik and J. Pyun, J. Mater. Chem., 2010, 20, 6023–6025 RSC; (b) P. Y. Keng, I. Shim, B. D. Korth, J. F. Douglas and J. Pyun, ACS Nano, 2007, 1, 279–292 CrossRef CAS; (c) B. D. Korth, P. Keng, I. Shim, S. E. Bowles, C. Tang, T. Kowalewski, K. W. Nebesny and J. Pyun, J. Am. Chem. Soc., 2006, 128, 6562 CrossRef CAS.
- S. S. Rahatekar, K. K. Koziol, S. R. Kline, E. K. Hobbie, J. W. Gilman and A. H. Windle, Adv. Mater., 2009, 21, 874 CrossRef CAS.
- J.-H. Huang, J. Kim, N. Agrawal, A. P. Sudarsan, J. E. Maxim, A. Jayaraman and V. M. Ugaz, Adv. Mater., 2009, 21, 3567 CrossRef CAS.
- (a) Z. Wu, M. G. Hahm, Y. J. Jung and L. Menon, J. Mater. Chem., 2009, 19, 463–467 RSC; (b) Y. Zhao, Y. Xie, J.-S. Jie, C.-Y. Wu and S. Yan, J. Mater. Chem., 2009, 19, 3378–3383 RSC; (c) J. N. Tiwari, F.-M. Pan, R. N. Tiwari and S. K. Nandi, Chem. Commun., 2008, 6516–6518 RSC.
- V. Maheshwari, J. Kane and R. F. Saraf, Adv. Mater., 2008, 20, 284 CrossRef CAS.
- (a) G. Duan, F. Lv, W. Cai, Y. Luo, Y. Li and G. Liu, Langmuir, 2010, 26, 6295–6302 CrossRef CAS; (b) M. Li, S. Johnson, H. Guo, E. Dujardin and S. Mann, Adv. Funct. Mater., 2011, 21, 851–859 CrossRef CAS.
- J. Tang, P. Photopoulos, A. Tserepi and D. Tsoukalas, Nanotechnology, 2011, 22, 235306 CrossRef CAS.
- X. N. Wang, J. Wang, M. J. Zhou, H. Wang, X. D. Xiao and Q. Li, J. Cryst. Growth, 2010, 312, 310–2314 CrossRef.
- (a) S. Liu, H. Gao, E. Ye, M. Low, S. H. Lim, S.-Y. Zhang, X. Lieu, S. Tripathy, W. Tremel and M.-Y. Han, Chem. Commun., 2010, 46, 4749–4751 RSC; (b) J. Liu and A. Wei, Chem. Commun., 2009, 4254–4256 RSC; (c) P. Zhou, Y. Li, P. Sun, J. Zhou and J. Bao, Chem. Commun., 2007, 1418–1420 RSC; (d) S.-H. Liu, H. Gao, E. Ye, M. Low, S. H. Lim, S.-Y. Zhang, X. Lieu, S. Tripathy, W. Tremel and M.-Y. Han, Chem. Commun., 2010, 46, 4749–4751 RSC; (e) Z. An, J. Zhang and S. Pan, CrystEngComm, 2010, 12, 500–506 RSC.
- (a) N. A. M. Barakat, B. Kim, S. J. Park, Y. Jo, M. H. Jung and H. Y. Kim, J. Mater. Chem., 2009, 19, 7371–7378 RSC; (b) F. Michalek, A. Lagunas, C. Jimeno and M. A. Pericàs, J. Mater. Chem., 2008, 18, 4692–4697 RSC; (c) J. Liu and A. Wei, Chem. Commun., 2009, 4254–4256 RSC; (d) T. Hang, A. Hu, M. Li and D. Mao, CrystEngComm, 2010, 12, 2799–2802 RSC; (e) J. Schällib-aum, F. H. D. Torre, W. R. Caseri and J. F. Löffler, Nanoscale, 2009, 1, 374–381 RSC.
- (a) X. Wang, H. Fu, A. Peng, T. Zhai, Y. Ma, F. Yuan and J. Yao, Adv. Mater., 2009, 27, 1636 CrossRef; (b) D. Li, R. S. Thompson, G. Bergmann and J. G. Lu, Adv. Mater., 2008, 20, 4575 CrossRef CAS; (c) I. Lisiecki, P.-A. Albouy and M.-P. Pileni, Adv. Mater., 2003, 15, 712 CrossRef CAS; (d) S.-H. Liu, H. Gao, E. Ye, M. Low, S. H. Lim, S.-Y. Zhang, X. Lieu, S. Tripathy, W. Tremel and M.-Y. Han, Chem. Commun., 2010, 46, 4749–4751 RSC; (e) H. Li and S. Liao, J. Mater. Chem., 2009, 19, 5207–5211 RSC.
- (a) S. Lin, M. Li, E. Dujardin, C. Girard and S. Mann, Adv. Mater., 2005, 17, 2553 CrossRef CAS; (b) M. H. Rashid, M. Raula and T. K. Mandal, J. Mater. Chem., 2011, 21, 4904–4917 RSC; (c) P.-G. Yin, L. Jiang, T.-T. You, W. Zhou, L. Li, L. Guo and S. Yang, Phys. Chem. Chem. Phys., 2010, 12, 10781 RSC; (d) A. Calleja, X. Granados, S. Ricart, J. Oró, J. Arbiol, N. Mestres, A. E. Carrillo, X. Palmer, F. Cano, J. A. Tornero, T. Puig and X. Obradors, CrystEngComm, 2011, 13 10.1039/C1CE05108C; (e) C. Hsieh and S. Hsieh, J. Mater. Chem., 2011, 21 10.1039/C1JM10136F.
- S. A. Maier, P. G. Kik, H. A. Atwater, S. Meltzer, E. Harel, B. E. Koel and A. A. Requicha, Nat. Mater., 2003, 2, 229 CrossRef CAS.
- K. Nakata, Y. Hu, O. Uzun, O. Bakr and F. Stellacci, Adv. Mater., 2008, 20, 4294 CrossRef CAS.
- R. P. Cowburn and M. E. Welland, Science, 2000, 287, 1466 CrossRef CAS.
- (a) D. Salyer and T. R. Sweet, Anal. Chem., 1958, 30, 1632–1635 CrossRef CAS; (b) V. D. Jović, B. M. Jović and M. G. Pavlović, Electrochim. Acta, 2006, 51, 5468 CrossRef; (c) W. Sun, X. Li, P. Qin and K. Jiao, J. Phys. Chem. C, 2009, 113, 11294 CrossRef CAS.
- S. Chikazumi, Physics of Magnetism, Wiley, New York 1964 Search PubMed.
- F. Liang, L. Guo, Q. Zhong, X. Wen, S. Yang, W. Zheng, C. Chen, N. Zhang and W. Chu, Appl. Phys. Lett., 2006, 89, 103105 CrossRef.
- H. Yoshikawa, K. Hayashida, Y. Kozuka, A. Horiguchi and K. Awaga, Appl. Phys. Lett., 2004, 85, 5287 CrossRef CAS.
- F. Davar, Z. Fereshteha and M. Salavati-Niasari, J. Alloys Compd., 2009, 476, 797 CrossRef CAS.
- H. C. Jang, S. H. Ju and Y. C. Kang, J. Alloys Compd., 2009, 478, 206–208 CrossRef CAS.
- (a) Q. Yuan, J. Zhuang and X. Wang, Chem. Commun., 2009, 6613–6615 RSC; (b) Y.-X. Xu, X. Zhao, X.-K. Jiang and Z.-T. Li, Chem. Commun., 2009, 4212–4214 RSC; (c) B. Lim, T. Yu and Y. Xia, Angew. Chem., Int. Ed., 2010, 49, 9819–9820 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental section and other experimental data. See DOI: 10.1039/c1ra00170a/ |
| This journal is © The Royal Society of Chemistry 2011 |
