Synthesis of zirconium carbide (ZrC) nanoparticles covered with graphitic “windows” by pulsed plasma in liquid
Liliang
Chen
a,
Chihiro
Iwamoto
b,
Emil
Omurzak
c,
Shintaro
Takebe
c,
Hiroki
Okudera
d,
Akira
Yoshiasa
e,
Saadat
Sulaimankulova
f and
Tsutomo
Mashimo
*g
aGraduate School of Science and Technology, Kumamoto University, Kumamoto 860-8555, Japan
bFaculty of Engineering, Kumamoto University, Kumamoto 860-8555, Japan
cPriority Organization for Innovation and Excellence, Kumamoto University, Kumamoto 860-8555, Japan
dFaculty of Natural System, Institute of Science and Engineering, Kanazawa University, Kanazawa 920-1192, Japan
eFaculty of Science, Kumamoto University, Kumamoto 860-8555, Japan
fInstitute of Chemistry and Chemical Technology, National Academy of Sciences, Chui pr. 267, Bishkek, 720071, Kyrgyzstan
gShock Wave and Condensed Matter Research Center, Kumamoto University, Kumamoto 860-8555, Japan. E-mail: mashimo@gpo.kumamoto-u.ac.jp; Fax: +81 96 342 3293; Tel: +81 96 342 3295
First published on 14th September 2011
Abstract
Cubic phase zirconium carbide (ZrC) nanoparticles encapsulated in graphitic carbon with an average size of about 10 nm were produced using pulsed plasma in liquid (PPL), in which zirconium metal electrodes provided the zirconium source and liquid ethanol the carbon. The quenching effect and near vacuum environment inherent in this pulsed plasma in liquid method resulted in the synthesis of nanoparticles with a uniform small size distribution and high purity. Lattice parameters of the obtained samples refined by the Rietveld method were larger than other reported data. Also, the emission lines of atoms Zr I, ions Zr II, atoms C I, ions C II and atoms H I were observed by an optical emission spectrum to gather information on the synthesis mechanism. The results of thermogravimetric and infrared ray emissivity of the nanoparticles showed that the graphitic carbon shells composed of a mere several layers with high transmissivity serve to act as “windows”, helping both to protect the ZrC nanoparticle inside from oxidation and to preserve its high infrared ray emissivity.
1. Introduction
Zirconium carbide (ZrC), except for cutting tools and ultra-high temperature applications1–3, is known as a main kind of far-infrared ceramics. Its selected thermal absorption property and high infrared ray emissivity can convert solar energy to heat effectively, thus endowing this material with potential for such applications as warmth-preserving functional textiles4 in a fine powder form. In addition, it also can be considered as a heat dissipation agent for computer cooling systems.However, the poor oxidation resistance of ZrC when produced as nanometer-sized particle restricts these above applications.5–8 Since 1993,9 graphitic carbon-coated nanoparticles10–14 have attracted a great deal of research globally, most theorizing that the carbon shell in these structures would be able to protect the inner core materials, thus improving anti-oxidation of ZrC nanoparticles in our case. Although some works on the synthesis of ZrC nanoparticles composed of such a structure by arc burning, mechanical alloying or the co-reduction-carburization method exist,15–17 these methods have failed to avoid thick carbon coatings, unwanted carbonaceous debris or size growth, all of which debilitate the light absorption and emissivity rate of the particles. In our method18 utilizing pulsed plasma in liquid, pulsed plasma appearing between two electrodes ablates and activates the metals which compose the rods, decomposing them and liquid molecules and enabling their interreaction. The minimal heat produced by the plasma with a short duration of about several microseconds as well as the quenching effect of the surrounding cool solution limit the obtained crystals' size, enabling the successful synthesis of very small size nanoparticles.
To minimize particle size and the thickness of carbon coating so as to optimize the balance between anti-oxidation and emissive properties, we perform the one-step synthesis method, pulsed plasma in liquid, for synthesis of graphitic carbon-encapsulated zirconium carbide nanoparticles in liquid ethanol.
2. Experimental
The experimental setup for pulsed plasma in liquid consisted of two main components: a low voltage alternating current (AC) electric power supply and a reactor including an anode and cathode, as shown in Fig. 1. In this study, pulses with single-pulse duration of about 3 μs at a frequency of 60 Hz and a near 20 A current were applied between two metallic rods of, 5 mm in diameter and 99.5% zirconium purity (Rare Metallic Co., Ltd.) submerged in liquid ethanol (99.5%, Kanto Kagaku Co., Ltd.). The cathode was set to vibrate at a frequency of 5000 vibrations per minute (vpm) within a 0–1 mm gap between tips during the experiment. This continuous vibration allowed maintenance of the pulsed plasma regardless of increasing rods' distance due to the consumption of the rods' zirconium. During the process, pulsed plasma was observed between the tips of two rods and the color of the liquid turned black gradually. After 1 h of reaction, the black nanoparticles were carefully separated by centrifugation from the liquid and then dried in air at 120 °C for 2 h.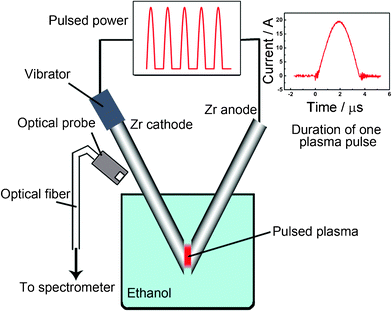 | ||
| Fig. 1 Schematics of the system to generate pulsed plasma in liquid ethanol (99.5%) by two metallic zirconium electrodes and a graph of one pulse duration. | ||
The morphology and microstructural characterization of prepared samples were observed by Field Emission Scanning Electron Microscopy (FESEM) (JEOL JSM-7600F) and High Resolution Transmission Electron Microscopy (HRTEM) (Philips Tecnai F20). The phase purity and structure of the nanocrystals were determined by powder X-ray diffraction (XRD) (Rigaku RINT 2000/PC) using Cu Kα radiation (40 kV, 200 mA) and a laser Raman microspectrometer (HORIBA Jobin Yvon HR 800) with a wavelength of 514.53 nm. Crystal parameters were calculated by Rietveld refinement. The hydrogen contents in our plasma sample and a commercial pure zirconium carbide sample (2 μm, 99%, Rare Metallic Co., Ltd.) for comparison were checked by the chemical analysis. The optical emission spectrum was obtained by an SEC2000-UV-VIS Spectrometer. Thermal analyses were performed on the SII Exstar 6000 TG/DTA 6300 model (Shimadzu Co., Japan) using a platinum crucible and the results compared with the above commercial zirconium carbide sample. Infrared ray emissivity of powders was tested in the wavelength range of 5–20 μm at 40 °C by a Fourier transform infrared spectrometer (FT-IR Spectrum GX, equipped with infrared radiation unit BFG-IR2 and FT-IR2 manufactured by PerkinElmer) and was compared with the above commercial ZrC sample and graphitic carbon powder with purity of 99.9995% (Wako Pure Chemical Industries, Ltd.).
3. Results and discussion
3.1 Characterization
FESEM examination was conducted to investigate the morphology characteristics of as-synthesized ZrC products by pulsed plasma in liquid ethanol. Fig. 2 displays a large quantity of several nm-sized uniform nanoparticles aggregated together.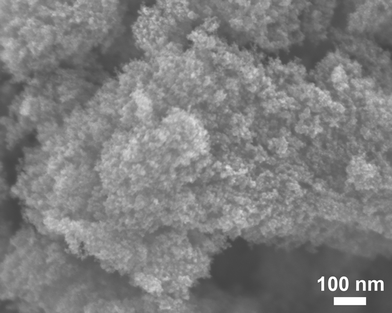 | ||
| Fig. 2 Field emission scanning electron microscopy (FESEM) image of ZrC nanoparticles grown in liquid ethanol (99.5%) by pulsed plasma. | ||
HRTEM observation of the ZrC samples revealed the nanoparticles to have an average size of about 10 nm and a distinct structure of shells and cores, as shown in Fig. 3. As indicated by the HRTEM image and schema on the right of Fig. 3, a single crystal ZrC with a cubic shape following the [110] direction was also found to be encapsulated in graphite layers. Comparing to the interplanar spacing of 0.335 nm in nature graphite with a fixed composition of stacking forms,19,20 and a little larger interlayer distance, about 0.34 nm, in multi-walled nanotubes because of ignoring stacking effects, the average layer separation of the shells in different shaped nanoparticles by our plasma method was the smallest (about 0.320 nm). The internal stress in the particles possibly due to the different coefficients of thermal expansion for ZrC core and graphite layers may help to explain the decrease of graphitic interlayer distances after cooling down from the hot plasma state during synthesis.
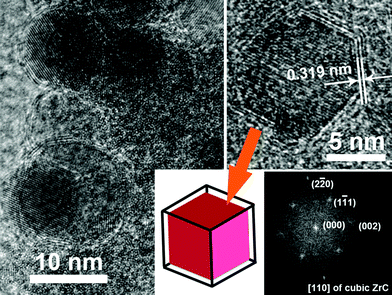 | ||
| Fig. 3 High-resolution TEM images of ZrC nanocrystals encapsulated in graphitic carbon by pulsed plasma in liquid ethanol (99.5%) with a schematic and a fast Fourier transform (FFT) image of one cubic single crystal of ZrC coated with carbon. | ||
To further confirm the composition and oxidation degree of obtained samples, energy-dispersive X-ray spectroscopy (EDX) was used during HRTEM observation; results are shown in Fig. 4. The negligible composition of the element oxygen expressed by EDX may be explained by the near-vacuum environment during synthesis by pulsed plasma in liquid as well as the fine protection of the carbon coating. In both the FESEM and HRTEM studies, no trace of unwanted carbonaceous debris such as nanotubes or flakes could be found. The carbon peak in the spectrum was due to the graphitic shells of the particles and the carbon membrane on the TEM grid, and Cu peak the TEM grid.
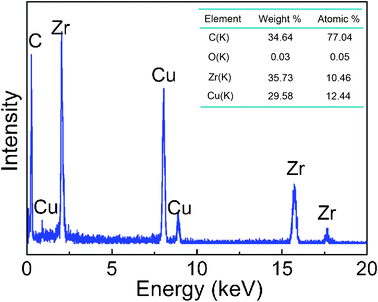 | ||
| Fig. 4 EDX pattern taken from the ZrC nanoparticle encapsulated in graphitic carbon synthesized by pulsed plasma in liquid ethanol (99.5%) in HRTEM image and the table of element content. | ||
The XRD pattern of prepared samples is shown in Fig. 5. All the intensive peaks correspond to face-center cubic (fcc) ZrC in agreement with the reference data in JCPDS (reference number 65-0973), indicating high purity of ZrC due to the anaerobic synthesis environment.
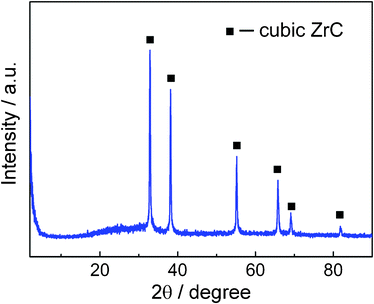 | ||
| Fig. 5 XRD powder pattern for the ZrC nanoparticle encapsulated in graphitic carbon by pulsed plasma in liquid ethanol (99.5%). | ||
Table 1 lists Rietveld refinement parameters of cubic structure zirconium carbide by pulsed plasma in liquid ethanol. The crystallographic structure of the synthesized ZrC sample is nicely refined using cubic Fm-3m space group (See Fig. 6) in the region of 20–90 degrees. Observed data are indicated by dots, and the calculated profile is indicated by a solid line. Short vertical bars below the pattern represent the positions of all possible Bragg reflections, and the line below the short vertical bars represents the difference between the observed and calculated patterns. Compared with other reported structure data21–24 listed in Table 2, the crystal parameter a of the prepared ZrC sample by pulsed plasma in liquid ethanol is the largest. As oxygen easily dissolves and substitutes in the ZrC lattice25 to form oxycarbide ZrOxCy24,26 with Zr–O bonding on the particle surface, and the Zr–O bonding force is stronger than Zr–C, one of the reasons of larger lattice parameter in the prepared sample may be the anaerobic environment during the plasma synthesis in liquid ethanol and the sufficient protection of graphitic carbon shells covering the ZrC nanoparticles. On the other hand, by elemental analysis, a commercial pure ZrC sample shows the hydrogen content is 0.30 wt%, whereas the hydrogen content in our product by plasma is larger, equal to 1.24 wt%. Hydrogen is known to be highly soluble in these materials,27 leading to a significant swelling, thus higher content of hydrogen in our sample helps to explain the high value of cell parameter. Additionally, the quenching effect by the surrounding cool liquid during plasma synthesis may also help to inhibit the crystal growth resulting in lattice expansion of nanocrystals.28
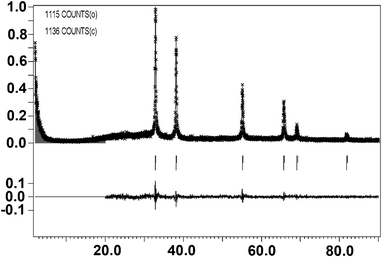 | ||
| Fig. 6 Rietveld refinement plot of cubic structure ZrC by pulsed plasma in liquid ethanol using cubic Fm-3m space group. Observed data are indicated by dots, and the calculated profile is indicated by a solid line. Short vertical bars below the pattern represent the positions of all possible Bragg reflections, and the line below the short vertical bars represents the difference between the observed and calculated patterns. | ||
| Sample | Zirconium carbide by pulsed plasma in liquid ethanol |
|---|---|
| Space group | Fm-3m |
| Lattices parameter a (Å) | 4.707(2) |
| Volume (Å3) | 104.3 |
| GOF | 1.14 |
| Rp | 11.70 |
| Rwp | 15.40 |
| Sample | Lattices parameter a (Å) | Reference |
|---|---|---|
| Prepared sample by pulsed plasma in liquid | 4.707(2) | — |
| ZrC by hot press and then polishing in air | 4.683 | [21] |
| ZrC0.87O0.08 | 4.690(1) | [22] |
| ZrC0.90O0.05 | 4.694(6) | [22] |
| ZrC0.92O0.03 | 4.695(9) | [22] |
| ZrC0.93O0.02 | 4.697(7) | [22] |
| ZrC with 20.03 wt% oxygen content | 4.680 | [23] |
| ZrC with 8.14 wt% oxygen conten | 4.690 | [23] |
| ZrC with 0.66 wt% oxygen content | 4.693 | [23] |
| ZrC with 3.3 wt% oxygen content | 4.691 | [24] |
| ZrC with 0.1 wt% oxygen content | 4.696 | [24] |
Raman spectra of the graphitic layer formed on the surface of ZrC nanoparticles show two broad peaks, at 1372.7 and 1597.5 cm−1 as shown in Fig. 7; conversely, the XRD pattern showed no diffraction peaks of graphite, as the diffraction diagram of a highly-defective material a few atomic layers thick should lead to very broad diffraction lines, hardly discriminated from an amorphous contribution in our sample. The peak ca 1597.5 cm−1 is conventionally referred to as the G mode, assigned to the C–C stretching vibration (E2g) of graphite, while the peak at 1372.7 cm−1 is conventionally referred to as the D mode of graphite, which is known to become active in small and defective crystallites of graphite.29,30 Thus, the D to G band ratio R (ID/IG) represents the concentration of defects in graphite,31 and the low value of R in our sample means the integrity of the graphitic shells is sufficiently high to protect the core material well from corrosion and oxidation.
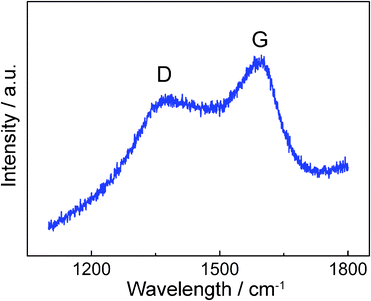 | ||
| Fig. 7 Micro-Raman spectrum of the ZrC nanoparticle encapsulated in graphitic carbon by pulsed plasma in liquid ethanol (99.5%). | ||
3.2 Optical emission spectrum and synthesis mechanism
To clarify the formation mechanism of carbon-encapsulated zirconium carbide nanoparticles by pulsed plasma in liquid, optical emission spectrum from the plasma discharge was collected using an optical probe placed adjacent to the beaker and transmitting via an optical fiber to a spectrometer, as shown in Fig. 8. The spectral lines were identified using reference data.32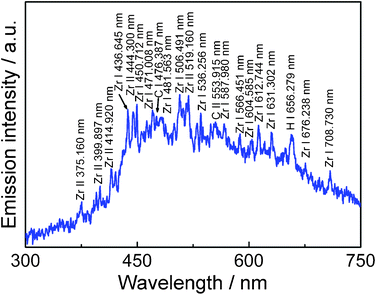 | ||
| Fig. 8 Optical emission spectrum from pulsed plasma in liquid ethanol (99.5%) with zirconium electrodes. | ||
The coexistence of atoms Zr I, ions Zr II, atoms C I, ions C II and atoms H I, apparent from the optical emission spectrum, and the structure consisting of a single shell with several carbon layers rather than multiple shells in one particle—as shown in HRTEM images—allows elucidation of the formation mechanism of the graphitic carbon coated ZrC particles as follows: It's assumed that, first, very hot plasma ablates the zirconium metal in the rod tips to form very active zirconium atoms Zr I; simultaneously, the liquid ethanol is decomposed by plasma to produce atoms C I and H I. In the plasma discharge zone, atoms Zr I and C I soon gain or lose electrons to transform into ions Zr II and C II, they then further interreact to produce ZrC clusters in a very short time. However, except the most part of hydrogen and oxygen come out from the liquid in the bubble form, some hydrogen, oxygen, and extra carbon atoms will dissolve into the ZrC clusters during this reaction. When ZrC clusters aggregate into crystallites and cool, the dissolved carbon atoms segregate out and form graphitic layers on the surface of the ZrC nanocrysals.
3.3 Anti-oxidation and infrared ray emissivity
To evaluate the anti-oxidation property of as-prepared ZrC nanoparticles by pulsed plasma in liquid, thermogravimetric analysis of carbide oxidation under air flow in comparison with the commercial microsize ZrC sample was carried out. The results as shown in Fig. 9 illustrate the high reactivity of small sized ZrC. The commercial sample starts to oxidize under air at about 270 °C, while our nano-scale particles obtained by plasma still keep in pure cubic ZrC at about 350 °C as shown in XRD pattern in Fig. 9; this is in vast opposition the 200 °C which has been to reported as the start of nanosized ZrC oxidation under air,22,33 and an even higher temperature than the above microsized commercial data. This anti-oxidation phenomenon is considered due to the protection of the graphitic carbon coating from oxygen, which can be confirmed by the mass loss below 400 °C in the TGA curve of prepared sample by plasma. With the temperature increasing, the coated carbon was first heated until it gasified, leading to a decreasing of total mass, and when the temperature over 400 °C, the core ZrC nanoparticles starts to be denuded and exposed to air and then quickly oxidize to zirconia.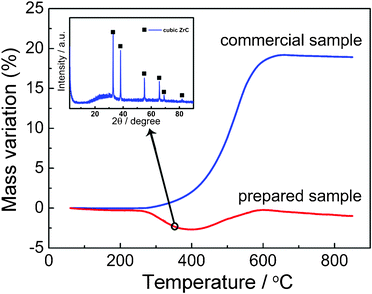 | ||
| Fig. 9 Thermogravimetric curve (under air) of the ZrC nanoparticle encapsulated in graphitic carbon by pulsed plasma in liquid ethanol (99.5%) compared with a commercial pure ZrC sample and XRD powder pattern for the prepared sample tested by TGA under air at 350 °C. | ||
Fig. 10 shows emissivity spectra of plasma-produced powders along with those of commercial particles and pure graphitic carbon powders as references at 40 °C; the figure reveals that the as-prepared powder sample has an emissivity of over 80% in the spectral range of 5–20 μm, much higher than commercial ones. This is due to the smaller size of ZrC nanoparticles produced by plasma and the good protection provided by its graphitic coating compared to the easy oxidation on the surface of nanosized ZrC particles.8 In addition, considering the reported high transmission property of carbon nanotube and graphene34,35 with a similar structure but different emission line shapes of pure carbon and ZrC especially in the region of wavelength between 5 to 10 μm in Fig. 10, the graphitic shells composed of a mere several layers on the surface of ZrC core particles may be considered to be analogous to a window which allow the light to pass through, preserving the high infrared ray emissivity of inside ZrC nanomaterials.
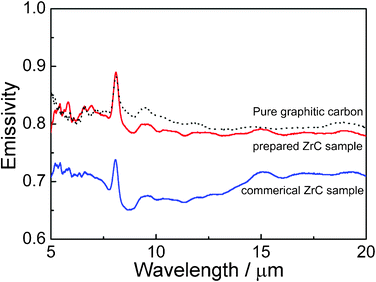 | ||
| Fig. 10 Spectral emissivity of ZrC nanoparticles encapsulated in graphitic carbon by pulsed plasma in liquid ethanol (99.5%) compared with the commercial pure ZrC sample and the pure graphitic carbon in the powder form at 40 °C. | ||
4. Conclusions
A pulsed plasma in liquid method for synthesis of graphitic “window” coated zirconium carbide nanoparticles in which pulsed plasma between two zirconium metallic rods in liquid ethanol allow preparation of pure small-sized ZrC particles of about 10 nm was presented. Larger lattice parameters of the nanoparticles are due to the anaerobic synthesis environment, dissolved hydrogen and quenching effect of surrounding liquid. Analysis of emission spectrum of the pulsed plasma suggests that only a few layers of graphitic carbon on the nanoparticles with core/shell structure were formed from the dissolved carbon in ZrC clusters during the synthesis process. These ZrC nanoparticles with graphitic “windows” exhibit good anti-oxidation properties and high infrared ray emissivity, enabling their potential for numerous thermal applications, such as warmth-preserving functional textiles and coating for cooling system. Furthermore, the method of decomposing organic solutions by the pulsed plasma in liquid is also considered to be able to cover “thin” graphitic shells on many kinds of nanomaterials, such as cobalt, SiC, etc., for the future applications in the anti-oxidation or photonic field.Acknowledgements
This work is supported by the China Scholarship Council, the Global Center of Excellence Program of Japan and Venture Business Laboratories of Kumamoto University, Japan.References
- X. M. He, L. Shu, H. B. Li and D. Weng, J. Mater. Res., 1999, 14, 615–618 CrossRef CAS.
- G. H. Reynolds, J. C. Janvier, J. L. Kaae and J. P. Morlevat, J. Nuc. Mat., 1976, 62, 9–16 CrossRef CAS.
- W. Sun, X. Xiong, B. Huang, G. Li, H. Zhang, Z. Chen and X. Zheng, Carbon, 2009, 47, 3368–3371 CrossRef CAS.
- Y. S. Nam, X. M. Cui, L. Jeong, J. Y. Lee and W. H. Park, Thin Solid Films, 2009, 517, 6531–6538 CrossRef CAS.
- S. Shimada, M. Yoshimatsu, M. Inagaki and S. Otani, Carbon, 1998, 36, 1125–1131 CrossRef CAS.
- H. Kitaoka, K. Ozawa, K. Edamoto and S. Otani, Solid State Commun., 2001, 118, 23–26 CrossRef CAS.
- S. Shimada, Solid State Ionics, 2001, 141–142, 99–104 CrossRef CAS.
- H. Kitaoka, K. Ozawa, K. Edamoto and S. Otani, Surf. Sci., 2002, 511, 359–365 CrossRef CAS.
- R. S. Ruoff, D. C. Lorentsm, B. Chan, R. Malhotra and S. Subramoney, Science, 1993, 259, 346–348 CAS.
- V. P. Dravid, J. J. Host, M. H. Teng, B. E. J. Hwang, D. L. Johnson, T. O. Mason and J. R. Weertman, Nature, 1995, 374, 602–602 CrossRef CAS.
- T. Hayashi, S. Hirono, M. Tomita and S. Umemura, Nature, 1996, 381, 772–774 CrossRef.
- R. Sergiienko, E. Shibata, A. Zentaro, D. Shindo, T. Nakamura and G. Qin, Acta Mater., 2007, 55, 3671–3680 CrossRef CAS.
- K. Suenaga, C. Colliex, N. Demoncy, A. Loiseau, H. Pascard and F. Willaime, Science, 1997, 278, 653–655 CrossRef CAS.
- W. Wu, Z. Zhu, Z. Liu, Y. Xie, J. Zhang and T. Hu, Carbon, 2003, 41, 317–321 CrossRef CAS.
- S. Bandow and Y. Saito, Jpn. J. Appl. Phys., 1993, 32, 1677–1680 CrossRef.
- J. Dong, W. Shen, X. Liu, X. Hu, B. Zhang, F. Kang, J. Gu, D. Li and N. Chen, Mater. Res. Bull., 2001, 36, 933–938 CrossRef CAS.
- C. Li, X. Yang, Z. Zhao and Y. Qian, Chem. Lett., 2002, 1088–1089 CrossRef CAS.
- L. Chen, T. Mashimo, E. Omurzak, H. Okudera, C. Iwamoto and A. Yoshiasa, J. Phys. Chem. C, 2011, 115, 9370–9375 CAS.
- A. H. R. Palser, Phys. Chem. Chem. Phys., 1999, 1, 4459–4464 RSC.
- S. M. Paek, E. Yoo and I. Honma, Nano Lett., 2009, 9, 72–75 CrossRef CAS.
- J. F. Alward, C. Y. Fong, M. El-Batanouny and F. Wooten, Phys. Rev. B: Solid State, 1975, 12, 1105–1117 CrossRef CAS.
- M. Dolle, D. Gosset, C. Bogicevic, F. Karolak, D. Simeone and G. Baldinozzi, J. Eur. Ceram. Soc., 2007, 27, 2061–2067 CrossRef CAS.
- Y. Yan, Z. Huang, X. Liu and D. Jiang, J. Sol-Gel Sci. Technol., 2007, 44, 81–85 CrossRef CAS.
- M. D. Sacks, C. Wang, Z. Yang and A. Jain, J. Mater. Sci., 2004, 39, 6057–6066 CrossRef CAS.
- S. K. Sarkar, A. D. Miller and J. I. Mueller, J. Am. Chem. Soc., 1972, 55, 628–630 CAS.
- A. Maitre and P. Lefort, Solid State Ionics, 1997, 104, 109–122 CrossRef CAS.
- I. E. Pavlov, Y. G. Zainulin, S. I. Alyamovskii and G. P. Shveikin, Sov. Powder Metall. Met. Ceram., 1975, 14, 1004–1006 CrossRef.
- L. Chen, P. Fleming, V. Morris, J. D. Holmes and M. A. Morris, J. Phys. Chem. C, 2010, 114, 12909–12919 CAS.
- M. Nakamizo, Tanso, 1977, 90, 105–113 CAS.
- G. Katagiri, Tanso, 1996, 175, 304–313 CAS.
- F. Tuinstra and J. L. Koening, J. Chem. Phys., 1970, 53, 1126–1130 CrossRef CAS.
- Y. Ralchenko, A. E. Kramida, J. Reader, NIST Atomic Spectra Database, Verson 4; National Institute of Standards and Technology: Gaithersburg, MD, 2010. Search PubMed.
- L. Combemale, Y. Leconte, X. Portier, N. Herlin-Boime and C. Reynaud, J. Alloys Compd., 2009, 483, 468–472 CrossRef CAS.
- G. Gruner, J. Mater. Chem., 2006, 16, 3533–3539 RSC.
- R. R. Nair, P. Blake, A. N. Grigorenko, K. S. Novoselov, T. J. Booth, T. Stauber, N. M. R. Peres and A. K. Geim, Science, 2008, 320, 1308–1308 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
