CNT-network modified Ni nanostructured arrays for high performance non-enzymatic glucose sensors†
Jianhui
Zhu
a,
Jian
Jiang
a,
Jinping
Liu
a,
Ruimin
Ding
a,
Yuanyuan
Li
b,
Hao
Ding
a,
Yamin
Feng
a,
Guangming
Wei
a and
Xintang
Huang
*a
aInstitute of Nanoscience and Nanotechnology, Department of Physics, Central China Normal University, Wuhan, 430079, P. R. China. E-mail: jhzhu@phy.ccnu.edu.cn; xthuang@phy.ccnu.edu.cn; Fax: +86-027-67861185
bDepartment of Electronic Science and Technology, Huazhong University of Science and Technology, Wuhan, 430074, P.R. China
First published on 1st September 2011
Abstract
Ni nanostructured arrays (NAs) modified by entangled carbon nanotube (CNT) networks have been fabricated via a facile chemical vapor deposition (CVD) approach and applied as an electrode for non-enzymatic glucose sensors for the first time. Compared with previously reported Ni-contained biosensors, the electrode of CNT/Ni NAs possess higher performance for the detection of glucose, showing a low detection limit (1 μM, S/N = 3), a wide linear range (0.5–10 mM) and quite high sensitivity (∼1381 μA mM−1 cm−2). In addition, other excellent properties of the CNT/Ni NAs electrode, such as good reproducibility, long-term stability and anti-interferences, are demonstrated as well. The good analytical capability, low cost and facile preparation method make CNT/Ni NAs promising for amperometric non-enzymatic glucose detection.
Introduction
The development of novel glucose sensors with high sensitivity, reliability, fast response and good selectivity has gained special focus for decades, driven by their practical use in the control and treatment of diabetes.1 As a category of glucose sensors, though enzymes-based biosensors can well meet the requirements of good selectivity and high sensitivity, the drawbacks arising from their thermal/chemical instabilities of immobilized enzymes and the complex fabrication procedures have hindered their further progress.2,3 In parallel, varieties of enzymes-free glucose sensors have recently triggered great interest and been widely exploited on the basis of direct electrochemical oxidation of glucose.4 Generally, the electroactivity of electrode materials plays a prominent role on glucose detection because it exerts a significant influence on the sensitivity of the sensors. Early research mainly focused on the noble metal-based (like Pt, Au) amperometric glucose sensors.5,6Pt-based bimetallic (like Pt/Ru, Pt/Bi and Pt/Pb) amperometric sensors are of particular interest for glucose detection owing to their high electrocatalytic ability, good sensitivity and selectivity to the electro-oxidation of glucose.7–9 Nevertheless, many unfavorable factors (e.g., high cost, toxicity, etc.) of heavy metals severely handicap their future commercial applications.10 As a consequence, in pursuit of improvement in glucose detection, more efforts need to be devoted to searching for electrode materials with low-cost and superior electrocatalytic behaviors.It is noteworthy that enzymeless electro-oxidation of glucose can also be achieved with some transition metal-based catalysts.11 Among catalysts, Ni-contained materials are one of the most attractive candidates.12 The electrocatalytic effect of Ni-based sensors generally relies on the Ni2+/Ni3+ redox couple that results from the Ni(OH)2/NiOOH system forming on the working electrode surface in alkaline electrolyte media. For the determination of glucose, the Ni-based sensors are normally prepared by depositing Ni nanoparticles on electrodes, such as carbon ceramic electrodes, diamond and metallic substrates.13–15 One severe issue with these modified electrodes is the gradual degradation of the catalytic activity for extended periods of time under applied potential.3 Hence, to address this issue and advance the glucose sensors, the preparation of novel Ni-based electrodes with good operational stability and durability are still highly desirable.
Carbon-based nanomaterials are quite satisfactory for biosensor applications on account of its low cost, good conductivity, easily functionalized properties, large surface areas and excellent corrosion resistance in various electrolytes.16–19Multi-walled carbon nanotubes (MWCNTs) are of potential interest for electrochemical applications.1,18–20As reported, metal/CNT composites with enhanced mechanical and electrical characteristics can exhibit improved and unique functionality.21 Encouraged by the high electrocatalytic capability of pure metallic nanomaterials and the good stability of MWCNTs, in this communication, we demonstrate a novel type of ultra-sensitive and stable enzymeless glucose sensors on the basis of CNT/Ni hybrid materials. To the best of our knowledge, it’s the first time that the CNT/Ni composite nanostructured arrays (NAs) have been fabricated via a facile CVD approach and adopted directly as non-enzymatic biosensors for glucose detection. The merits of low cost, facile preparation and good analytical capabilities make CNT/Ni NAs promising for the development of effective glucose sensors.
Experimental
Synthesis
D-(+)-Glucose, D-fructose, uric acid (UA) and ascorbic acid (AA) were purchased from Sinopharm Chemical Reagent Co., Ltd. All reagents were of analytical grade and used without further purification. In a typical synthesis, Ni2(OH)2CO3 nanowall arrays were selected as precursors and prepared on conductive substrates beforehand via a hydrothermal method according to our previous work.22 CNT/Ni hybrid NAs were fabricated through a chemical vapor deposition (CVD) process carried out in a horizontal, quartz tube-furnace system. Ni2(OH)2CO3 precursors on a stainless alloy (area: 2 × 4 cm2) were placed in the center of the quartz tube furnace. Hereafter, ethylene glycol (2 mL) serving as a carbon source was loaded in an alumina boat and placed at the upstream zone of the quartz tube (the distance from alumina boat to the quartz-tube center: ∼20 cm). Prior to heating, the quartz-tube reactor was sealed and flushed with nitrogen gas (rate: 150 sccm) for 30 min. Then, the furnace was heated to 650 °C at a heating rate of 10 °C min−1 under a nitrogen flow (100 sccm), kept at 650 °C for 15 min, followed by a natural cool-down process. The final product is black in color and was collected and kept in the dry electric oven. For a comparative study, we also fabricated NiO-based electrodes and pure CNT electrodes. The preparation procedure for NiO was quite similar to that of CNT/Ni composite NAs. The only difference was that without the introduction of a carbon source, Ni2(OH)2CO3 precursors were directly subjected to a calcination treatment at 500 °C for 2 h under a nitrogen flow. The electrode of CNTs was prepared by a traditional method. In detail, 0.2 g MWCNTs (purchased from Chinese academy of science, Chengdu), 0.13 g Polyvinylidene Fluoride (PVDF) and ∼0.5 g N, N-dimethylacethylamide were mixed and ground to uniform size. Hereafter, the mixture was pasted evenly onto the stainless alloy substrate. After drying in an electric oven at 60 °C for 1 h, the CNT electrode was taken out, tailored (area: 3 × 3 mm2) and directly used as the working electrode.Characterization and electrochemical measurements
Final products were characterized using powder X-ray diffraction (XRD, Bruker D-8 Avance, Cu Kα radiation; λ = 1.5418 Å), Raman spectroscopy (Witech CRM200, 532 nm), scanning electron microscopy (SEM, JSM-6700F, 5KV) and transmission electron microscopy (TEM and HRTEM, JEM-2010FEF; 200 KV). Electrochemical impedance spectroscopy (EIS), cyclic voltammetry and amperometry experiments were all performed on the CHI760D electrochemical workstation utilizing a three electrode system that was composed of platinum auxiliary, saturated calomel electrode reference (SCE) and a CNT/Ni NAs (3 × 3 mm2) working electrode. All the electrochemical experiments were performed in 0.5 M NaOH solution in air at room temperature. The current–time curves were recorded in a stirred cell for successive additions of glucose solution at an operating potential of 0.55 V. It was also worth noting that the CNT/Ni nanocomposite alloy was directly subjected to the electrochemical measurements, saving the carbon black/polymer binders and tedious post-treatments.23 Comparative studies were also performed by using NiO NAs and CNTs respectively as the working electrode under the same conditions.Results and discussion
Fig. 1A shows the XRD pattern of CNT/Ni NAs on stainless alloy substrates. Except for diffraction peaks arising from the alloy substrate, all the other peaks appearing at 2θ of 44.5° and 51.8° can be well indexed to metallic Ni (JCPDS card No. 04-0805), corresponding to the facets of (111) and (200), respectively. A Raman spectra of the CNT/Ni NAs has been made in the range of 900–2000 cm−1 and displayed in Fig. 1B. It can be seen that two broad characteristic peaks centered at 1339 cm−1 and 1590 cm−1, respectively are attributable to the D- and G- vibration modes, giving evidence to the existence of carbon materials.24 Representative SEM images of CNT/Ni hybrid NAs are shown in Fig. 2A and B. As can be seen, after the CVD process, CNT/Ni NAs are still able to maintain the interconnected nanowall array morphology, which is identical to that of Ni2(OH)2CO3 precursors (See Fig. S1, ESI†).22 The top-open network structure of the electrode effectively generates a large quantity of void spaces, greatly facilitating the ions or glucose molecules in the electrolyte diffusing into the active material film. Apart from that, this architecture also increases the contact area between the solid electrode and the electrolyte, offering more reaction sites for the electrochemical catalysis and thus boosting the electrode performance. To get clearer observations of the hybrid nanostructures, one selected place in Fig. 2A is magnified and shown in Fig. 2B. From this high-resolution SEM image, it is obvious that a dense entanglement of curly nanofibres are uniformly growing on a single nanowall.25 Typical observations of TEM (Fig. 2C) and HRTEM (Fig. 2D) are made and used to characterize the nanofibres. From the TEM image, it is observed that the nanofibre with a mean diameter of 20 nm has got a tubular structure. The HRTEM image further reveals that the as-grown nanofibre exhibits a multi-walled nature; the lattice fringe of tube wall is calculated to be 0.34 nm, which is in agreement with the (006) face of hexagonal carbon. All the characterization results provide good evidence of the presence of MWCNTs on the nanowalls.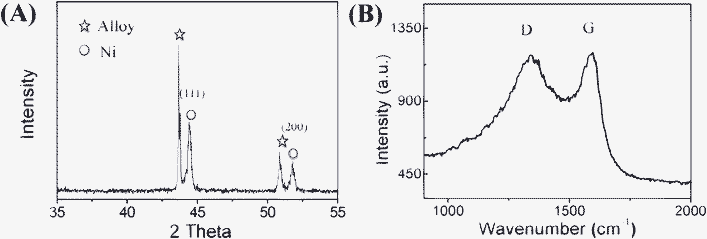 | ||
| Fig. 1 (A) XRD pattern and (B) Raman spectrum of the CNT/Ni NAs built on a stainless alloy substrate. | ||
The electrocatalytic behavior of both the CNT/Ni hybrid and NiO NAs electrodes towards the oxidation of glucose was initially investigated by cyclic voltammetry in a potential range 0.1 V to 0.7 V. The cyclic voltmmograms (CVs) of the CNT/Ni NAs electrode in 0.5 M NaOH in the absence and presence of glucose at a scan rate of 50 mV s−1 are presented in Fig. 3A. It can be clearly seen that in alkaline electrolyte medium, the CNT/Ni NAs electrode exhibited a pair of well-defined redox peaks (curve c), which correspond to the Ni(II)/Ni(III) redox couple. By contrast, for the case of NiO NAs, only a poor voltammetric response was detected, as demonstrated in the inset of Fig. 3A. To account for the apparent enhanced electrochemical activity of the CNT/Ni NAs electrode, we believe that the high electrochemical behavior of nanosized Ni and the possible synergistic effect from carbon nanotubes are the two main factors. The peak currents increased gradually during the successive scans in the NaOH solution; until a steady current state is reached, a certain amount of glucose solution was injected into the electrolytic cell and its response was recorded in Fig. 3A. Upon the addition of 250 μM glucose to 50 mL NaOH (the concentration of glucose being 5 mM), notable enhancement of the oxidative peak current, as well as an anodic shift in the oxidative peak potential, could be observed while the reduction peak current decreased slightly without any potential shift (Fig. 3A, curve b). With the increase of glucose concentration, the enhancement of the oxidation peak is more evident (Fig. 3A, curve a). This can be ascribed to the electro-oxidation of glucose into glucolactone, which according to the following reactions: Ni(III) + glucose → Ni(II) + glucolactone, is an irreversible process.12 The catalytic conversion of the Ni(III) to Ni(II), which is prior to the electrochemical reduction, can enrich the Ni(II) ions existing in the electrolyte solution and as a result boosts the anodic reactions.12Electrochemical impedance spectroscopy (EIS) spectra were conducted under open-circuit conditions in the frequency range of 50–1×106 Hz to identify the Faraday impedance (Zf) of different samples, as shown in Fig. 3B. In principle, Zf comprises Warburg impedance (Zw) reflected by a line with an angle close to 45 degrees in the low frequency region, and serially connected resistance (R) (charge-transfer/surface-film resistance) reflected by the diameter of the semicircle in the high frequency range. After curve fitting, it is clear that the CNT/Ni NAs electrodes have a lower R than NiO nanowall electrodes, indicative of a good conducting capability, which is considered to be beneficial to the quick electron transfer from the reactive sites to current collector, effectively enhancing the detecting sensitivity and shortening the response time. The influence of scanning rates on the cyclic voltammogram response of the CNT/Ni nanohybrid electrode was also evaluated and the results are displayed in Fig. 3C. It can be seen that the CNT/Ni electrode redox peak current rises with an increase of the scanning rates, and both the anodic and cathodic peak currents show linear response to the scan rates ranging from 10 to 100 mV s−1 (Fig. 3D), indicating a surface controlled process.1,11
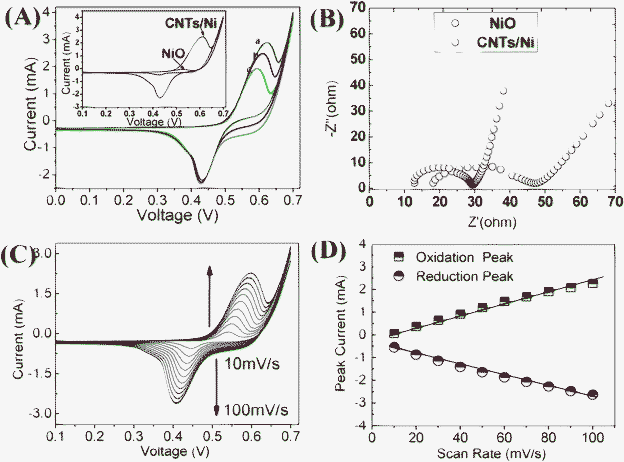 | ||
| Fig. 3 (A) CV plots of the CNT/Ni NAs electrode in 0.5 M NaOH (a) in the presence of 10 mM glucose, (b) in the presence of 5 mM glucose and (c) in the absence of glucose. The inset shows the CV plots of different electrodes (CNT/Ni; NiO). (B) Electrochemical impedance spectroscopy measurements in 0.5 M NaOH using CNT/Ni and NiO electrodes. (C) CVs of CNT/Ni electrode in 0.5 M NaOH at different scan rates. (D) The plots of peak currents versus scan rates. | ||
The glucose sensing properties of the CNT/Ni hybrid and NiO NAs have been investigated. Amperometric responses of the two electrodes were measured at an applied potential of 0.55 V in a continuously stirred 0.5 M NaOH solution. With the glucose solution added into electrolyte, the response is recorded shown in Fig. 4A. For the CNT/Ni NAs electrode, upon the successive introduction of 0.5 mM glucose, fast responses (95% of the steady-state current achieved within 5 s) can be observed. Meanwhile, the detection limit was measured to be 1 μM at a signal-to-noise ratio of 3 (Fig. 4B), demonstrating a rapid and sensitive response at the glucose concentration variation. This result is superior to that achieved by previous work.12,26 In comparison, for the case of the NiO NAs electrode, when the same amount of glucose was introduced, only a small current variation can be achieved within 10 s. The calibration plots of both electrodes are depicted in Fig. 4C. The CNT/Ni NAs electrode gives a linear dependence (R = 0.995) in the glucose concentration range 0.5–10 mM, with a sensitivity up to ∼1381 μA mM−1 cm−2, which is much higher than that of NiO NAs electrode (∼268 μA mM−1 cm−2). In addition, to ensure the Ni in the electrode plays a dominant role in the glucose detection, we compared the sensing properties of CNT/Ni hybrid NAs to the pure CNT electrode (See Fig. S2, ESI†).
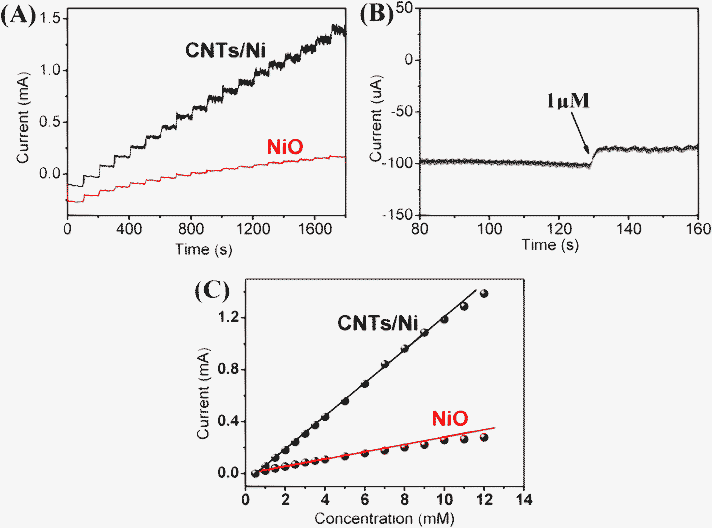 | ||
| Fig. 4 (A) Chronoamperometric current responses of CNT/Ni and NiO electrodes for successive additions of 0.5 mM glucose to a stirred 0.5 M NaOH solution at 0.55 V. (B) The amperometric response to 1 μM glucose for the CNT/Ni electrode. (C) Calibration curves of glucose concentration on the CNT/Ni electrode and NiO electrode. | ||
Obviously, the CNT/Ni hybrid electrode exhibits prominent electrocatalytic performance on glucose sensing in terms of the sensitivity and detecting limit. By referring to the literature,27,28 we consider that the outstanding electrocatalytic activity of the CNT/Ni NAs electrode can be attributed to the following reasons. On the one hand, nanoscale metallic Ni has better electrochemical properties compared with NiO.28 Secondly, it is considered that CNTs could have a collaboration on the electrocatalytic oxidation of glucose. The thermal decomposition of the Ni2(OH)2CO3 precursors and the consumption of the Ni catalysts in the CVD process would form a loosely packed CNT/Ni hybrid structure. This is, to some extent, beneficial for the ionic transportation in the electrode and the increase of reaction area; nevertheless, the yielded boundaries between Ni nanoparticles are detrimental to quick electron transfer. Conductive CNTs on the nanowalls can make up for this problem. The CNTs are highly entangled with each other, offering electron-transfer pathways and forming a solid network structure on the Ni NAs. As a result, Ni nanoparticles are well connected and fastened by the CNTs, which would further enhance the electroconductivity and electrocatalytic properties of the sensing electrode.
The selectivity of the glucose biosensors is highly important for practical use in the control and treatment of diabetes. The common interfering species coexisting with glucose in blood system include uric acid (UA) and ascorbic acid (AA), and the normal physiological level of glucose is about 3–8 mM, which is much higher than the concentrations of interfering species like UA (0.1 mM) and AA (0.1 mM).26 Considering fructose is isomeric with glucose, we performed the interference test towards fructose (FO) as well. Fig. 5 (A) shows the amperometric response of CNT/Ni NAs electrode towards the addition of 1 mM glucose and 0.1 mM FO, AA and UA in a stirred 0.5 M NaOH at an applied potential of 0.55 V.3 Compared to the sharp current response of glucose (2.76 mA), the interfering species showed negligible current variation, demonstrating sufficient selectivity to be applied for the analysis of human blood. The reproducibility of the sensor was investigated by comparing the response currents of ten CNT/Ni electrodes prepared under the same conditions. The relative standard deviation (RSD) of the response current is 3.82% for 2.0 mM glucose. The stability of the glucose biosensor has been investigated through the amperometric response to 0.5 mM glucose at 0.55 V in NaOH at intervals over half a month, and the CNT/Ni electrode was stored at 60 °C in an electric oven when not in use. The data collected from the repeated experiments carried out everyday for 15 consecutive days (See Fig. 5 (B)) indicate that the used electrode maintains at least 90% of the initial sensitivity in the continuous tests. These results suggest the sensors based on CNT/Ni have good reproducibility and stability.
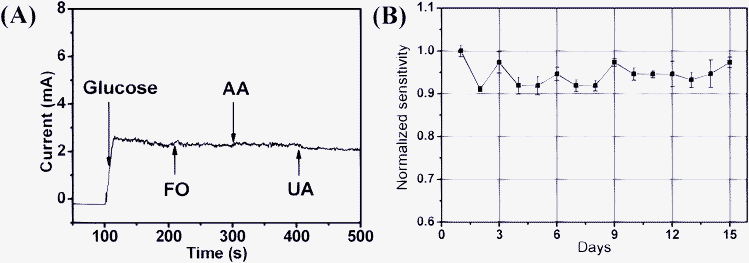 | ||
| Fig. 5 (A) Amperometric responses of the CNT/Ni electrode for the injection of 1 mM glucose, 0.1 mM fructose, AA and UA to stirred 0.5 M NaOH at 0.55V. (B) Normalized sensitivity of the prepared electrode to glucose tested every one day by amperometric measurements for 15 consecutive days. | ||
Conclusion
In summary, CNT/Ni hybrid NAs were successfully synthesized by a facile CVD process and used as a novel electrode for non-enzymatic glucose sensing. The CNT/Ni nanocomposite electrode exhibits higher electrochemical performance for glucose oxidation than traditionally used NiO NAs electrodes, including a faster response time (less than 5s), a lower detection limit of 1 μM and a linear dependence in the glucose concentration ranging from 0.5–10 mM with a high sensitivity of ∼1381 μA mM−1 cm−2. The introduction of entangled CNTs paved on the Ni NAs are believed to stabilize the CNT/Ni NAs electrode even in the presence of common interfering species. Ease of fabrication, fast response, high sensitivity as well as long-term stability makes the sensor proposed in this study ideal for the pharmaceutical and clinical detection of glucose.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 50872039), Natural Science Foundation of Hubei Province (No. 2010CDB04005) and China Postdoctoral Science Foundation (20090460996).References
- J. Chen, W. D. Zhang and J. S. Ye, Electrochem. Commun., 2008, 10, 1268 CrossRef CAS.
- C. X. Guo, X. T. Zheng, Z. S. Lu, X. W. Lou and C. M. Li, Adv. Mater., 2010, 22, 5164 CrossRef CAS.
- C. X. Guo and C. M. Li, Phys. Chem. Chem. Phys., 2010, 12, 12153 RSC.
- J. P. Liu, C. X. Guo, C. M. Li, Y. Y. Li, Q. B. Chi, X. T. Huang, L. Liao and T. Yu, Electrochem. Commun., 2009, 11, 202 CrossRef CAS.
- C. X. Guo, Z. M. Sheng, Y. Q. Shen, Z. L. Dong and C. M. Li, ACS Appl. Mater. Interfaces, 2010, 2, 2481 CAS.
- Y. Z. Xian, Y. Hu, F. Liu, Y. Xian, H. T. Wang and L. T. Jin, Biosens. Bioelectron., 2006, 21, 1996 CrossRef CAS.
- F. Xiao, F. Q. Zhao, D. P. Mei, Z. R. Mo and B. Z. Zeng, Biosens. Bioelectron., 2009, 24, 3481 CrossRef CAS.
- G. Wittstock, A. Strübing, R. Szargan and G. Werner, J. Electroanal. Chem., 1998, 444, 61 CrossRef CAS.
- J. P. Wang, D. F. Thomas and A. C. Chen, Anal. Chem., 2008, 80, 997 CrossRef CAS.
- P. H. Hindle, S. Nigro, M. Asmussen and A. C. Chen, Electrochem. Commun., 2008, 10, 1438 CrossRef.
- Z. H. Zhu, L. N. Qu, Q. J. Niu, Y. Zeng, W. Sun and X. T. Huang, Biosens. Bioelectron., 2011, 26, 2119 CrossRef CAS.
- X. Li, J. P. Liu, J. Jiang, R. M. Ding, Y. Y. Hu, A. Z. Hu and X. T. Huang, Sens. Actuators, B, 2010, 147, 241 CrossRef.
- K. E. Toghill, L. Xiao, M. A. Phillips and R. G. Compton, Sens. Actuators, B, 2010, 147, 642 CrossRef.
- T. Watanabe and Y. Einaga, Biosens. Bioelectron., 2009, 24, 2684 CrossRef CAS.
- A. Salimi and M. Roushani, Electrochem. Commun., 2005, 7, 879 CrossRef CAS.
- J. P. Liu, Y. Y. Li, J. Jiang and X. T. Huang, Dalton Trans., 2010, 39, 8693 RSC.
- Y.-S. Chen and J.-H. Huang, Biosens. Bioelectron., 2010, 26, 207 CrossRef CAS.
- S. K. Smart, A. I. Cassady, G. Q. Lu and D. J. Martin, Carbon, 2006, 44, 1034 CrossRef CAS.
- A. Huczko, H. Lange, M. Bystrzejewski and P. Baranowski, Fullerenes, Nanotubes, Carbon Nanostruct., 2005, 13, 141 CrossRef CAS.
- D. Ragupathy, A. I. Gopalan and K. P. Lee, Electrochem. Commun., 2009, 11, 397 CrossRef CAS.
- X. H. Kang, Z. B. Mai, X. Y. Zou, P. X. Cai and J. Y. Mo, Anal. Biochem., 2007, 363, 143 CrossRef CAS.
- J. H. Zhu, J. Jiang, J. P. Liu, R. M. Ding, H. Ding, Y. M. Feng, G. M. Wei and X. T. Huang, J. Solid State Chem., 2011, 184, 578 CrossRef CAS.
- J. Jiang, Y. Y. Li, J. P. Liu and X. T. Huang, Nanoscale, 2011, 3, 45 RSC.
- J. Chen, J. Z. Wang, A. I. Minett, Y. Liu, C. Lynam, H. K. Liu and G. G. Wallace, Energy Environ. Sci., 2009, 2, 393 CAS.
- J. Jiang, J. P. Liu, X. T. Huang, Y. Y. Li, R. M. Ding, X. X. Ji, Y. Y. Hu, Q. B. Chi and Z. H. Zhu, Cryst. Growth Des., 2010, 10, 70 CAS.
- H. H. Ai, X. T. Huang, Z. H. Zhu, J. P. Liu, Q. B. Chi, Y. Y. Li, Z. K. Li and X. X. Ji, Biosens. Bioelectron., 2008, 24, 1048 CrossRef CAS.
- M. S. Wu, C. Y. Huang and K. H. Lin, Electrochem. Solid-State Lett., 2009, 12, A129 CrossRef CAS.
- Q. Jiang, L. Jiang, H. Hou, J. Qi, S. Wang and G. Sun, J. Phys. Chem. C, 2010, 114, 19714 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1ra00280e |
| This journal is © The Royal Society of Chemistry 2011 |

