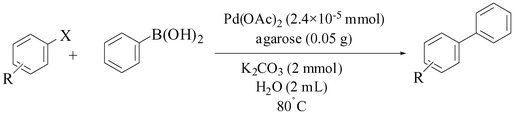
Agarose hydrogel as an effective bioorganic ligand and support for the stabilization of palladium nanoparticles. Application as a recyclable catalyst for Suzuki–Miyaura reaction in aqueous media†
Habib
Firouzabadi
*,
Nasser
Iranpoor
*,
Mohammad
Gholinejad
and
Faezeh
Kazemi
Department of Chemistry, College of Sciences, Shiraz University, Shiraz, 71454, Iran. Fax: 98 711 2286008; Tel: 98 711 2284822E-mail: firouzabadi@chem.susc.ac.ir; iranpoor@chem.susc.ac.ir
First published on 5th September 2011
Abstract
In this article, the usefulness of an agarose hydrogel as an effective bioorganic ligand and support for the entrapment and stabilization of palladium nanoparticles is described. We have applied this catalyst under low loading of the supported palladium nanoparticles for the Suzuki–Miyaura coupling reaction of different aryl iodides, bromides and chlorides with phenylboronic acid in the absence of phosphorous ligands or any organic co-solvents or phase transfer additives. The catalyst was efficiently recycled for six runs, retaining its activity within the limit of the experimental error. ICP analysis of the catalyst shows Pd content of the agarose hydrogel is a low amount. The analysis of the reaction mixture by the ICP technique also showed the leaching of the catalyst was negligible. The work-up of the mixture can also be handled under highly green conditions in water. However, this catalytic system can be easily applied for scaled-up protocols.
Introduction
In recent years, intensive consideration has been paid to reduce issues that are responsible for pollution in our environment. For this concern, chemical industries are under stress from different sources. Along this line, they have adopted green chemistry practices, such as waste prevention, using recyclable catalysts, with low levels of metal leaching, and the use of less toxic solvents and reagents. Therefore the replacement of expensive, flammable and toxic organic solvents with water as a green solvent, which is cheap, plentiful, nontoxic and nonflammable has been under consideration.1–7Over the past few decades, rigorous attention has been paid to the use of nanoparticles of materials, and compared with their corresponding bulk compounds, for different aims.8 Trapping of nanoparticles in gels are reported in the literature. Recently, preparation of gold nanoparticles in agarose,9 fabrication of agar-conjugated Fe3O4 magnetic nanoparticles,10 using cellulose as supporting medium of Ag, Au and Pt nanoparticles11 and crystal nucleation in agarose gels are reported.12 Alongside this effort, we have also reported a Pd nanoparticle supported ionic liquid-modified xerogel.13 Very recently, palladium nanoparticle supported gelatin as a bioorganic reductant, ligand and support for ligand- and amine-free Sonogashira–Hagihara reactions has been presented by us.14
Agarose oligomers are composed of repeated residues of 1,3-linked β-D-galactopyranose and 1,4-linked 3,6-anhydro-α-L-galactopyranose. Agarose is freely soluble in hot water. The molecular weight of this compound is about 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, which forms a gel network to produce double helices stabilized by water molecules bound inside the double helical cavities.15Agarose contains a vast number of free hydroxyl groups and has the capacity to form a gel network (Fig. 1).
000, which forms a gel network to produce double helices stabilized by water molecules bound inside the double helical cavities.15Agarose contains a vast number of free hydroxyl groups and has the capacity to form a gel network (Fig. 1).
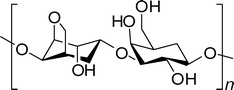 | ||
| Fig. 1 Repeated residues of 1,3-linked β-D-galactopyranose and 1,4-linked 3,6-anhydro-α-L-galactopyranose in agarose oligomer molecules. | ||
Carbon–carbon bond formation is one of the most important tasks in organic synthesis. One of the frequently utilized palladium-catalyzed C–C couplings is the Suzuki–Miyaura reaction, which affords substituted biaryls from aryl halides and phenylboronic acid.16–19 The discovery of this reaction has put enormous impact upon the preparation of essential basic biaryl valuable compounds for chemical industries and academia. Since the unearthing of the reaction, a large number of biaryl compounds are obtainable in bulk, which are used in pharmaceutics, agrochemicals, cosmetics, conducting polymers, liquid crystalline materials and many others.
In this article, for the first time, we have paid attention to the use of agarose hydrogel as the support and ligand for the successful entrapment and stabilization of palladium nanoparticles. The agarose hydrogel supported Pd nanoparticles have been used as the catalyst for Suzuki–Miyaura reactions of different aryl iodides, bromides and more importantly, chlorides with phenylboronic acid in water. The reactions occurred under phosphorous-free conditions, low loading of palladium nanoparticles and in the absence of any organic co-solvents and phase transfer additives.
Experimental
General:
Palladium acetate (containing 48.4% Pd) was purchased from Alfa Aesar. Agarose, phenylboronic acid and K2CO3 were purchased from Merck and aryl halides were purchased from Merck, Fluka and Acros. NMR spectra were recorded on a Bruker Avance DPX-250 Spectrometer (1H NMR 250 MHz and 13C NMR 62.9 MHz) in CDCl3 with tetramethylsilane (TMS) as the internal standard. UV-vis spectra were recorded on a Perkin Elmer, Lambda 25 UV-vis Spectrometer. Scanning electron microscopy (SEM) images were obtained by XL-30 FEG SEM, Philips, at 20 KV. Transmission electron microscopy (TEM) images were obtained by TEM, Philips CM10. X-Ray diffraction spectra were recorded by XRD, D8, Advance, Bruker, axs. Palladium content of the agarose hydogel was measured by a ICP analyzer; Varian, Vista- pro and atomic absorption analysis.Gram-scale preparation of palladium nanoparticles supported on the agarose hydrogel:
Agarose (1 g) was dissolved in water (100 mL) at 80 °C. To this solution, an acidic solution of Pd(OAc)2 (0.022 g, 1 mM) was added and diluted with water (100 mL). To the resulting solution, a solution of citric acid (20 mL, 4 mM) was added dropwise upon which a gray–brown color developed. After refluxing for 1 h and cooling the mixture to room temperature, a gray–brown gel mass of the hydrogel was formed (Fig. 2A). Drying of the resulting agarose hydrogel mass was performed under a flow of the air over night and dried in vacuum for 24 h, resulting in a stable black powder, which has been used as the catalyst (Fig. 2B).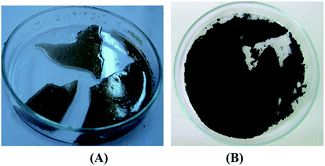 | ||
| Fig. 2 (A) Gray–brown agarose hydorogel supported Pd nanoparticles. (B) Black powder of the dried agarose hydrogel supported Pd nanoparticles after drying. | ||
General procedure for Suzuki–Miyaura reaction catalyzed by palladium nanoparticles supported on the agarose hydrogel:
Aryl halide (1 mmol) and phenylboronic acid (0.18 g, 1.5 mmol) were added to a flask containing agarose hydrogel supported Pd nanoparticles (0.05 g, which contains 55 × 10−5 g, 2.4 × 10−5 mmol of Pd as determined by ICP analysis) and K2CO3 (0.27 g, 2 mmol) in water (2 mL). The resulting gel-like mixture was stirred at 80 °C in the air for the appropriate reaction time (Table 2). After completion of the reaction (monitored by TLC or GC), the reaction mixture was cooled to room temperature, resulting in a dark gel-like mass of the hydrogel in the reaction vessel. The gel-like mass was washed with diethyl ether (5 × 2 mL) and the ethereal solution was evaporated to give the pure product. Further purification, if it was necessary, was performed on a silica gel column eluted with n-hexane/Et(OAc)2 to give the pure biphenyl product in high to excellent yields (Table 2).| Entry | Base | Time (h) | Isolated Yield (%) |
|---|---|---|---|
| 1 | none | 12 | 0 |
| 2 | K2CO3 | 0.6 | 90 |
| 3 | K3PO4 | 1 | 89 |
| 4 | Cs2CO3 | 1.5 | 85 |
| 5 | Et3N | 1.5 | 75 |
| 6 | DBU | 1 | 75 |
| Entry | Ar–X | Time (h) | Product | Isolated yield (%)a |
|---|---|---|---|---|
| a The reactions of aryl iodides and bromides were conducted at 80 °C, and aryl chlorides were performed at 100 °C b In parenthesis, time and the isolated yield of the large-scale reaction using 15 mmol of 4-iodoanisole and 23 mmol of phenylboronic acid is presented c The reactions conducted in a sealed tube at 100 °C because of the high vapor pressure of the substrates. | ||||
| 1 |
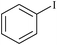
|
0.3 | 1a | 92 |
| 2 |
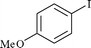
|
0.6 (0.6) | 1b | 90 (88)b |
| 3 |
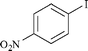
|
0.3 | 1c | 93 |
| 4 |
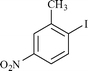
|
1 | 1d | 89 |
| 5 |
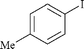
|
0.5 | 1e | 89 |
| 6 |
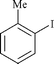
|
6 | 1f | 83 |
| 7 |
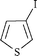
|
1 | 1g | 89 |
| 8 |
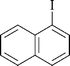
|
5 | 1h | 92 |
| 9 |
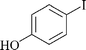
|
1 | 1i | 85 |
| 10 |
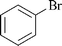
|
0.5 | 1a | 90 |
| 11 |
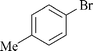
|
0.6 | 1e | 90 |
| 12 |
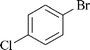
|
1 | 1j | 84 |
| 13 |
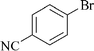
|
3 | 1k | 89 |
| 14 |
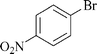
|
1 | 1c | 87 |
| 15 |
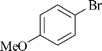
|
5 | 1b | 80 |
| 16 |
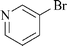
|
1 | 1l | 90 |
| 17 |
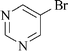
|
1 | 1m | 85 |
| 18 |
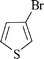
|
2 | 1g | 84 |
| 19 |
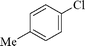
|
24 | 1e | 78c |
| 20 |
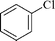
|
24 | 1a | 80c |
| 21 |
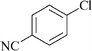
|
24 | 1a | 80 |
| 22 |
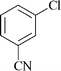
|
24 | 1n | 52 |
| 23 |
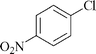
|
24 | 1c | 70 |
| 24 |
|
24 | 1o | 62 |
A typical reaction presenting a highly green protocol for the reaction of 4-iodoanisole with phenylboronic acid using Pd nanoparticles supported on the agarose hydrogel in water:
To a flask (5 mL) containing the agarose hydrogel Pd- supported nano-catalyst (0.05 g, which contains 55 × 10−5 g, 2.4 × 10−5 mmol of Pd species as determined by ICP analysis) in water (2 mL) were added 4-iodoanisole (0.23 g, 1 mmol), phenylboronic acid (0.18 g, 1.5 mmol) and K2CO3 (0.27 g, 2 mmol) at 80 °C and stirred for 40 min. After completion of the reaction (TLC), the resulting hot reaction mixture was filtered quickly through a thick cellulose filter paper under diminished pressure. The filter cake was washed with a portion of hot water (3 mL) and the combined hot aqueous filtrates were allowed to cool down to room temperature upon which 4-methoxybiphenyl was isolated as white crystals in 78–80% yield, m.p 87–89 °C, lit.87–88;191H NMR (250 MHz, CDCl3) δ (ppm): 7.51–7.57 (m, 4H), 7.41 (t, 1H, J = 7.2), 7.28 (t, 1H, J = 7.2), 6.98 (d, 2H, J = 7.5), 3.85 (s, 3H); 13C NMR (62.9 MHz, CDCl3) δ (ppm): 159.2, 140.9, 133.8, 128.8, 128.4, 128.2, 127.1, 114.3, 55.3.Water of the filtrate was distilled off yielding a solid crude material, identified as the waste of the reaction.
Recycling of the catalyst:
4-Iodoanisole (0.23 g, 1 mmol), phenylboronic acid (0.18 g, 1.5 mmol) and K2CO3 (0.27 g, 2 mmol) were added to a flask (5 mL) containing the agarose hydrogel Pd- supported nano-catalyst (0.05 g) in water (2 mL) at 80 °C. After completion of the reaction (GC), the reaction mixture was cooled to room temperature to give a dark gelatinous mass of the hydrogel. The resulting gel-like compound was washed with diethyl ether. The ethereal phase was separated and the resulting dark gray gelatinous mass was used for another batch of the reaction. This recycling was repeated for six runs without a noticeable drop in the catalytic activity of the catalyst (Table 3).| Run | Time (min) | GC Conversion (%) |
|---|---|---|
| 1 | 25 | 100 |
| 2 | 25 | 100 |
| 3 | 27 | 100 |
| 4 | 30 | 100 |
| 5 | 33 | 100 |
| 6 | 32 | 100 |
Results and Discussion
For the preparation of the catalyst, the agarose was dissolved in hot water followed by the addition of a solution of Pd(OAc)2 in water. In order to convert Pd(II) to Pd(0) species, a solution of citric acid in water was added dropwise. On cooling the mixture to room temperature, a gray–brown hydrogel appeared in the reaction vessel (Fig. 2A). Drying of the gelatinous compound in the air and under vacuum at room temperature produced a black powder (Fig. 2B).The initial stage of our investigation was focused on the characterization of the resulting solid. First of all, we approved the oxidation state of Pd on the surface of agarose by UV-vis spectra, which confirmed the conversion of Pd(II) to Pd(0) by the disappearance of the peak at 420 nm (Fig. 3).14 This was further confirmed by the appearance of peaks at (111), (200), (220) and (311) crystallographic planes related to the formation of Pd(0) by XRD analysis.6b (see ESI†)
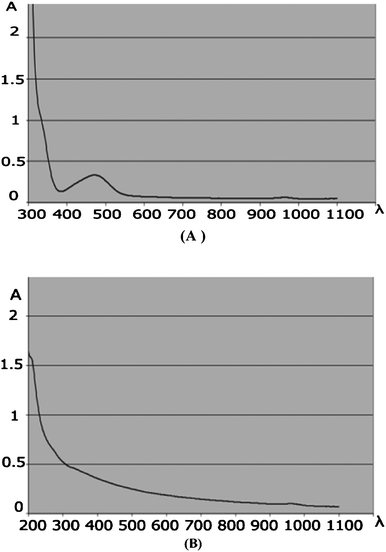 | ||
| Fig. 3 (A) UV-vis spectra of Pd(0) supported on agarose hydrogen generated by citric acid reduction and (B) Pd(II) in the presence of agarose hydrogel at room temperature before reduction. | ||
The TEM image of the hydrogel (Fig. 4), indicates that the average size of the palladium particles entrapped in the agarose hydrogel are in the range of 10–30 nm. The SEM image (Fig. 5) of the hydrogel showed the regular dispersion of the palladium nano-particles on the surface of the agarose hydrogel.
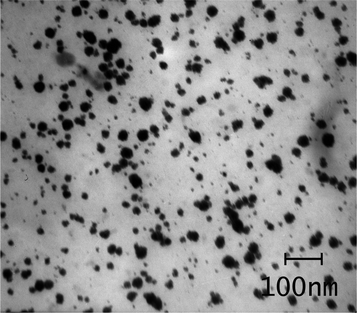 | ||
| Fig. 4 TEM image of the agarose supported Pd nanoparticles, which shows the size distribution of the particles to be around 10–30 nm. | ||
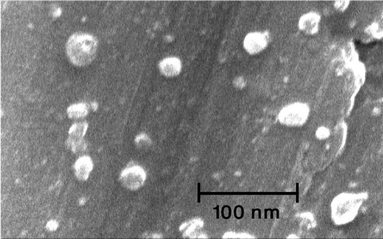 | ||
| Fig. 5 SEM image of the agarose hydrogel supported Pd nanoparticles, which shows the regular dispersion of the palladium nanoparticles on the surface of the agarose hydrogel. | ||
In order to show the applicability of this new bioorganic supported Pd(0) nanoparticles as a catalyst for carbon–carbon bond formation, we studied the Suzuki–Miyaura reaction of structurally different aryl halides in the presence of the hydrogel in water. We chose the reaction of 4-iodoanisole (1 mmol) with phenylboronic acid (1.5 mmol) using K2CO3 (1.5 mmol) as the base by means of 0.05 g of the hydrogel carrying Pd nanoparticles in water at 80 °C. The amount of Pd species supported on agarose (0.05 g) was determined by ICP analysis to be 55 × 10−5 g, 2.4 × 10−5 mmol of Pd. The reaction proceeded smoothly and after 30–40 min, the desired biphenyl product was obtained in a 90% isolated yield. Also, in order to check the effect of different bases upon the reaction rate, we studied the above mentioned model reaction in the presence of different bases. The results showed that K2CO3 was the most suitable base for the reaction as summarized in Table 1.
Then, we applied these conditions for the coupling of structurally different aryl chlorides, bromides and iodides with phenylboronic acid in the presence of the catalyst. However, the reaction of aryl iodides and aryl bromides were performed in 0.3–6 h with excellent isolated yields (80–93%). The results of this study are shown in Table 2. The reaction of aryl chlorides at 100 °C with the same stoichiometric amounts of the reagents used for the aryl iodides .The reactions proceeded with slower rates than mentioned for aryl iodides and bromides to give the desired products in 24 h with good isolated yields (52–80%). Moreover, we have also shown that this nano-catalyst can be applied the scaled-up reactions. For this aim, 4-iodoanisole (3.5 g, 15 mmol) was reacted with phenylboronic acid (4.2 g, 23 mmol) under similar optimized reaction conditions. The reaction proceeded well to produce the desired biphenyl compound in an 88% isolated yield (Table 2, entry 2).
We have also shown that the reaction could be performed under highly green conditions. For this aim, the reaction of 4-iodoanisole with phenylboronic acid at 80 °C was used as a model reaction. After completion of the reaction, the resulting hot mixture was filtered through a thick cellulose filter paper. The filter paper took up all the agarose hydrogel Pd supported mass by the formation of strong hydrogen bonds between agarose hydrogel catalyst and the cellulose material. The hot filtrate, upon cooling to room temperature, released the organic product as a white crystalline compound in highly pure state in a 78–80% yield, mp 87–89 °C, literature values 87–88 °C.20 The aqueous filtrate was distilled under diminished pressure to separate water from the wastes of the reaction. After the completion of the distillation, a solid crude waste mixture was obtained, which can be discarded safely.
Since palladium salts are expensive and some of them are toxic, their recycling is very important from an economical and environmental point-of-view. For this purpose, recycling of the agarose hydrogel supported Pd nanocatalyst for the reaction of 4-iodoanisole with phenylboronic acid at 80 °C was studied. After the first run, the resulting mixture was cooled down to room temperature to give the dark mass of the Pd nanocatalyst hydrogel. The resulting gel was first washed with diethyl ether and was reused for the second run of the reaction. This process was repeated for six rotations with success with the preservation of the catalytic activity within the limit of experimental errors (Table 3). ICP analysis of the ethereal solution after the first run, showed the amount of leaching of Pd into the ethereal solution was <2%. In order to get more information about the alteration of the morphology of the catalyst during the reaction, the hydrogel mass was separated and dried after the first run under vacuum. The SEM image of the resulting solid (Fig. 6) showed the regularity of dispersion of Pd-nanoparticles has been preserved and was more or less the same as the SEM image of the catalyst before the reaction (Fig. 5). We believe, the lack of high aggregation of the catalytic particles during the reaction is responsible for the successful recycling of the catalyst for six runs without an appreciable decrease in its catalytic activity (Table 3).
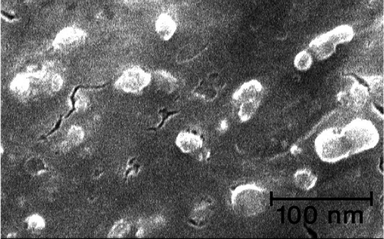 | ||
| Fig. 6 The SEM image of the agarose hydrogel supported Pd nano-catalyst after the first run of the reaction of 4-iodoanisole with phenylboronic acid, which shows the conservation of a regular dispersion of the particles on the surface of the agarose hydrogel. | ||
However, we have also shown that the presence of the agarose hydrogel is important and plays a crucial role in the formation and stabilization of the nanoparticles by retardation of the agglomeration process. Conglomeration of the catalyst particles plays an important function upon the yields and the rates of the reactions. In order to show these effects, a solution of citric acid in water (0.1 M, 10 mL) was added, dropwise with stirring, to the solution of Pd(OAc)2 in hot water (100 mL) in the absence of the agarose. The SEM image of the powder obtained by drying of the above mentioned mixture, revealed extensive agglomeration of the particles in the absence of the agarose hydrogel (Fig. 7).
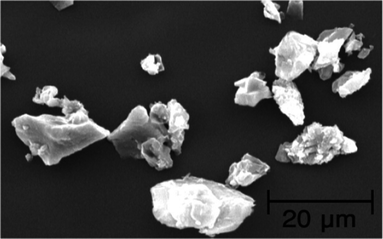 | ||
| Fig. 7 The SEM image of the Pd(0) catalyst generated from the reduction of Pd(OAc)2 by the addition of citric acid, which shows extensive agglomeration of Pd particles in the absence of the agarose hydrogel. | ||
However, in Table 4, we have compared the results of the catalytic activity of agarose hydrogel supported Pd nanoparticles with the catalysts generated in the absence of the agarose hydrogel and a mixture of agarose hydrogel with the Pd(0) generated from Pd(OAc)2. The results clearly show the distinct difference using the agarose hydrogel supported Pd nanoparticles for the reaction (Table 4).
| Entry | Catalyst | Time (min) | GC (%) |
|---|---|---|---|
| 1 | Agarose hydrogel supported Pd nanoparticles containing Pd(0) catalyst generated from 55 × 10−5 g, 2.4 × 10−5 mmol of Pd(OAc)2 | 30 | 100 |
| 2 | A mixture of agarose(0.05 g) hydrogel and 55 × 10−5 g , 2.4 × 10−5 mmol of Pd(0) generated from Pd(OAc)2 by the addition of citric acid | 30 | 65 |
| 3 | 55 × 10−5 g, 2.4 × 10−5 mmol of Pd(0) generated from Pd(OAc)2 by the addition of citric acid in the absence of the agarose hydrogel | 30 | 35 |
However, in order to show the general effect of the particle size upon the reaction of aryl halides (I, Br) using a mixture of bulk Pd(OAc)2 and the agarose hydrogel were also studied. A comparison of the results in Table 5 with the results tabulated in Table 1, shows that the time of the reactions has been elongated, which clearly supports the positive effect of using Pd nanoparticles in these reaction.
| Entry | Ar–X | Time (h) | Product | Isolated yield (%) |
|---|---|---|---|---|
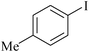
| 1 |
|
1 | 1e | 86 |
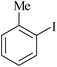
| 2 |
|
9 | 1f | 84 |
| 3 |
|
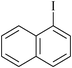
10 | 1h | 82 |
| 4 |
|
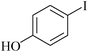
3 | 1i | 83 |
| 5 |
|
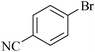
11 | 1k | 90 |
| 6 |
|
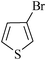
18 | 1g | 45 |
At the end, in order to show the merit and the reactivity of palladium nanoparticles supported on the agarose hydrogel as the catalyst, a comparison with some other reported homogeneous and heterogenenous palladium catalysts is presented in Table 6. As the presented results show, the time of the reactions are much faster than the other catalysts presented in the table and also the yields are higher.
| Entry | Ar–X | Catalyst | Time (h) | Isolated yield (%) |
|---|---|---|---|---|
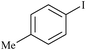
| 1 |
|
PVC–EDA–SA–Pd0 | 1 | 5421 |
| 2 |
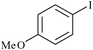
|
PVC–EDA–SA–Pd0 | 0.6 | 7621 |
| 3 |
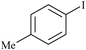
|
Agarose hydrogel supported Pd nanoparticles | 0.5 | 89 |
| 4 |
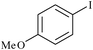
|
Agarose hydrogel supported Pd nanoparticles | 0.6 | 90 |
| 5 |
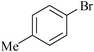
|
Organostannoxane-supported palladium nanoparticles | 8 | 9922 |
| 6 |
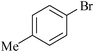
|
SS–Pd | 6 | 8223 |
| 7 |
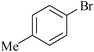
|
Agarose hydrogel supported Pd nanoparticles | 0.6 | 90 |
| 8 |
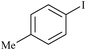
|
SS-Pd | 6 | 9023 |
| 9 |
|
MCM-41–SH–Pd(0 | 6 | 9624 |
| 10 |
|
Agarose hydrogel supported Pd nanoparticles | 0.5 | 89 |
| 11 |
|
Agarose hydrogel supported Pd nanoparticles | 0.3 | 93 |
Conclusively, in this study, we have introduced the preparation of palladium nanoparticles supported on the agarose hydrogel as a degradable bioorganic ligand and support for the stabilization of palladium nanoparticles. The agarose hydrogel entrapped palladium nanoparticles showed excellent catalytic activities for Suzuki–Miyaura reactions of various aryl iodides, bromides and chlorides under phosphine-free conditions in the absence of any organic co-solvent or phase transfer agents in water as a green media.
The hydrogel catalyst was simply separated from the aqueous reaction mixture and was recycled for six runs without appreciable loss of its catalytic activity. The effective recycling of the catalyst can be considered as a clue for tight entrapment and stabilization of Pd nanoparticles in water by the agarose hydrogel. ICP analysis of the first recycled catalyst also supports this axiom by showing low concentration of the leached Pd species into the reaction mixture. In the presence of this catalyst, scaling the reaction was easily achieved for the model reaction. In addition, using the naturally occurring agarose is a great advantage of the method. Agarose is not toxic, cheap, is degradable in nature and is soluble in hot water, which makes its use as a powerful ligand very attractive and practical applications and for the reactions conducted in water for transition metal catalysis. However, we have also shown that the isolation of the final product for a model reaction can also be performed in water without using any organic solvents.
Acknowledgements
We gratefully acknowledge the support of this work by the Shiraz University Research Council and TWAS Chapter of Iran based at ISMO. We also highly appreciate the valuable help of Professor K. Izadpanah Jahromi for the TEM images.References
- P. Anastas and M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686–694 CrossRef CAS
.
- R. A. Sheldon, Chemtech, 1994, 24, 38–47 CAS
.
- C. Jimenez-Gonzalez, A. D. Curzons, D. J. C. Constable and V. Cunningham, Int. J. Life Cycle Assess., 2004, 9, 114–121 CrossRef
.
-
(a)
P. T. Anastas, J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press: Oxford, 1998 Search PubMed
; (b) P. Tundo, P. T. Anastas, D. S. Black, J. Breen, T. Collins, S. Memoli, J. Miamoto, M. Poliakoff and W. Tumas, Pure Appl. Chem., 2000, 72, 1207–1228 CrossRef CAS
.
-
(a)
P. A. Grieco, Organic Synthesis in Water, Blackie Academic and Professional: London, 1998 Search PubMed
; (b) C. Li, T. H. Chan,. Organic Reactions in Aqueous Media, John Wiley & Sons: New York, 1997 Search PubMed
; (c) B. Cornils, W. A. Hermann, Aqueous–Phase Organometallic Chemistry: Concepts and Applications,Wiley-Interscience: Weiheim, 1998 Search PubMed
.
-
(a) H. Firouzabadi, N. Iranpoor and M. Abbasi, Adv. Synth. Catal., 2009, 351, 755–766 CrossRef CAS
; (b) H. Firouzabadi, N. Iranpoor and M. Gholinejad, Tetrahedron, 2009, 65, 7079–7084 CrossRef CAS
; (c) H. Firouzabadi, N. Iranpoor and A. Garzan, Adv. Synth. Catal., 2005, 347, 1925–1928 CrossRef CAS
; (d) H. Firouzabadi, N. Iranpoor, A. A. Jafari and E. Riazymontazer, Adv. Synth. Catal., 2006, 348, 434–438 CrossRef CAS
; (e) H. Firouzabadi, N. Iranpoor and F. Nowrouzi, Chem. Commun., 2005, 789–791 RSC
; (f) H. Firouzabadi, N. Iranpoor and M. gholinejad, J. Organomet. Chem., 2010, 695, 2093–2097 Search PubMed
; (g) H. Firouzabadi, N. Iranpoor and M. Abbasi, Tetrahedron, 2009, 65, 5293–5301 Search PubMed
; (h) H. Firouzabadi, N. Iranpoor and M. Gholinejad, J. Mol. Catal. A: Chem., 2010, 321, 110–116 CrossRef CAS
; (i) H. Firouzabadi, N. Iranpoor and M. Gholinejad, Adv. Synth. Catal., 2010, 352, 119–124 CrossRef CAS
; (j) H. Firouzabadi, N. Iranpoor and M. Abbasi, Bull. Chem. Soc. Jpn., 2010, 83, 698–702 Search PubMed
; (k) H. Firouzabadi, N. Iranpoor, A. Ghaderi, M. Ghavami and S. J. Hoseini, Bull. Chem. Soc. Jpn., 2011, 84, 100–109 Search PubMed
.
-
(a) C. A. Fleckenstein and H. Plenio, Green Chem., 2007, 9, 1287–1291 RSC
; (b) C. A. Fleckenstein and H. Plenio, J. Org. Chem., 2008, 73, 3236–3244 CrossRef CAS
; (c) B. Karimi, D. Elhamifar, J. H. Clark and A. J. Hunt, Chem. Eur. J, 2010, 16, 8047–8053 CAS
; (d) T. Nishikata and B. H. Lipshutz, J. Am. Chem. Soc., 2009, 131, 12103–12105 CrossRef CAS
; (e) A. Krasovskiy, C. Duplais and B. H. Lipshutz, Org. Lett., 2010, 12, 4742–4744 CrossRef CAS
; (f) C. Duplais, A. Krasovskiy, A. Wattenberg and B. H. Lipshutz, Chem. Commun., 2010, 46, 562–564 RSC
; (g) B. H. Lipshutz, T. B. Petersen and A. R. Abela, Org. Lett., 2008, 10, 1333–1336 CrossRef CAS
.
-
(a) A. Roucoux, J. Schulz and H. Patin, Chem. Rev., 2002, 102, 3757 CrossRef CAS
; (b) M. Moreno-Mañas and R. Pleixats, Acc. Chem. Res., 2003, 36, 638 CrossRef CAS
; (c) G. Schmid, Nanoparticles , Wiley-VCH, Weinheim, 2004 Search PubMed
; (d) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS
; (e) D. Astruc , Nanoparticles and Catalysis, Wiley-VCH, Weinheim, 2008 Search PubMed
.
- H. Faoucher, P. Nativo, K. Black, J. B. Claridge, M. Gass, S. Romani, A. L. Bleloch and M. Brust, Chem. Commun., 2009, 6661–6663 RSC
.
- S. Hsieh, B. Y. Huang, S. L. Hsieh, C. C. Wu, C. H. Wu, P. Y. Lin, Y. S. Huang and C. W. Chang, Nanotechnology, 2010, 21, 445601–44607 Search PubMed
.
-
(a) J. Cai, S. Kimura, M. Wada, S. Kuga and L. Zhang, Chem. Sus. Chem., 2008, 1, 149–154 CrossRef CAS
; (b) J. Cai, S. Kimura, M. Wada and S. Kuga, Biomacromolecules, 2009, 10, 87–94 CrossRef CAS
.
- C. Duffus, P. J. Camp and A. J. Alexander, J. Am. Chem. Soc., 2009, 131, 11676–11677 CrossRef CAS
.
- A. Safavi, N. Maleki, N. Iranpoor, H. Firouzabadi, A. R. Banazadeh, R. Azadi and F. Sedaghati, Chem. Commun., 2008, 14, 6155–6157 Search PubMed
.
- H. Firouzabadi, N. Iranpoor and A. Ghaderi, Org. Biomol. Chem., 2011, 9, 865–871 RSC
.
- C. Araki, Bull. Chem. Soc. Jpn., 1956, 29, 543–544 Search PubMed
.
-
(a) A. Suzuki, J. Organomet. Chem., 1999, 576, 147–168 CrossRef CAS
; (b) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS
; (c) F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047–3101 CrossRef CAS
; (d) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS
; (e) E. Giralt and P. Lloyd-Williams, Chem. Soc. Rev., 2001, 30, 145–157 RSC
; (f) J. Hassan, M. Sevignon, C. Gozzi, E. Schultz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1469 CrossRef CAS
; (g) H. Firouzabadi, N. Iranpoor and M. Gholinejad, J. Organomet. Chem., 2010, 695, 2093–2097 Search PubMed
.
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS
.
- S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633–9695 CrossRef CAS
.
- D. A. Alonso and C. Najera, Chem. Soc. Rev., 2010, 39, 2891–2902 RSC
.
- W.-J. Zhou, K.-H. Wang, J.-X. Wang and Z.-R. Gao, Tetrahedron, 2010, 66, 7633–7641 Search PubMed
.
- J. Liu, Y.-Q. Li and W.-J. Zheng, Monatsh. Chem., 2009, 140, 1425–1429 CrossRef CAS
.
- V. Chandrasekhar, R. S. Narayanan and P. Thilagar, Organometallics, 2009, 28, 5883–5888 CrossRef CAS
.
- P. Das, D. Sharma, A. K. Shil and A. Kumari, Tetrahedron Lett., 2011, 52, 1176–1178 Search PubMed
.
- Q. Xu, W. Hao and M. Cai, Catal. Lett., 2007, 118, 98–102 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c1ra00480h |
| This journal is © The Royal Society of Chemistry 2011 |