Brønsted acidic ionic liquid: a simple, efficient and recyclable catalyst for regioselective alkylation of phenols and anti-Markovnikov addition of thiols to alkenes
Ziyauddin S.
Qureshi
,
Krishna M.
Deshmukh
,
Kishor P.
Dhake
and
Bhalchandra M.
Bhanage
*
Department of Chemistry, Institute of Chemical Technology, N. Parekh Marg Matunga, Mumbai, 400 019, India. E-mail: bhalchandra_bhanage@yahoo.com; bm.bhanage@ictmumbai.edu.in; Fax: +91-22-3361-1020
First published on 19th September 2011
Abstract
A highly efficient protocol for the alkylation of phenols and anti-Markovnikov addition of thiols with various alkenes has been developed using a Brønsted acidic ionic liquid, N-methyl-2-pyrrolidone hydrogen sulfate [NMP]+HSO4−, under solvent free conditions. The developed methodology offers appreciable advantages with respect to excellent yield, short reaction time and high regioselectivity. The C–C and C–S bond formation has been achieved under metal free conditions.
Introduction
Alkylated phenols and their derivatives are industrially important compounds as they find wide applications for the synthesis of resins,1 durable surface coatings,2 varnishes,3 printing inks,4antioxidants,5 flame retardants,6 UV absorbers,7fungicide and insecticide formulations,8,9 petroleum additives10 and fragrances.11 Classically, alkylation of phenols was carried out by Friedel–Crafts alkylation using Lewis acid type catalysts.12Alkylation of phenol with alkene is also an alternative, which is usually catalyzed by several different catalysts including Brønsted acids,13 Lewis acids14 and zeolites.15 Recently, rhenium complex,16chloroindate(III) ionic liquid17 and iron containing mesoporous aluminosilicate18catalysts were reported for the alkylation of phenols with alkenes. However drastic reaction conditions, low selectivity, by-product formation, higher temperature, longer reaction time and use of organic solvents are the major drawbacks of these reported methods.On the other hand, thioethers and thioesters play a very important role in number of biological and chemical processes19 and are useful building blocks for the synthesis of various organosulfur compounds.20 The addition of thiols across double bonds may proceed via an electrophilic pathway involving ionic processes or a free radical pathway.21 Electrophilic addition is catalyzed by either a protic22 or Lewis acid23 to provide the branched Markovnikov product. Very few catalysts, such as H-Rh-zeolite,24silica nanoparticles,25CeCl3,26InI27 and reaction in aqueous media,28 are reported for the addition of thiol to an alkene in an anti-Markovnikov fashion.29 These procedures are associated with several disadvantages such as unsatisfactory yield, longer reaction time and a lack of a detailed systematic study.
Over the past few decades, ionic liquids (ILs) have attracted considerable attention as an environmentally benign alternative for organic synthesis procedures, where it play an important role as novel solvents, catalysts and reagents.30 Features that make the ionic liquids attractive include a lack of vapor pressure, non-volatility, non-flammability and thermal stability over a wide temperature range. In continuation of our interest in applications of ionic liquids for several organic transformations,31 herein we report a facile and highly efficient protocol for alkylation of phenols and anti-Markovnikov addition of thiols with alkenes under solvent free conditions (Scheme 1).
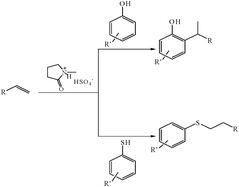 | ||
| Scheme 1 Brønsted acidic ionic liquid catalyzed alkylation of phenol and anti-Markovnikov addition of thiols to alkenes | ||
Results and discussion
Initially, the alkylation of phenol with styrene in the presence of p-toluene sulfonic acid was selected as a model reaction and the influence of various reaction parameters such as catalyst screening, solvent, catalyst loading, mole ratio of substrates, reaction time and temperature was studied (Table 1).| Entry | Catalyst | Solvent | Time (h) | Temp. (°C) | Yield (%)b |
|---|---|---|---|---|---|
a Reaction conditions: styrene (0.5 mmol), phenol (2.0 mmol), solvent (3 mL), catalyst (10 mol%).
b
GC yield.
c
Catalyst (100 mg).
d
Catalyst (5 mol%).
e
Catalyst (20 mol%).
f
Styrene: Phenol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
g
Styrene:Phenol (2.0 1).
g
Styrene:Phenol (2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5).
h
n.r states no reaction. 0.5).
h
n.r states no reaction.
|
|||||
| 1 | p-TSA | CH3CN | 3 | 80 | 23 |
| 2 | Cu(OTf)2 | CH3CN | 3 | 80 | n.r h |
| 3c | Montmorillonite k-10 | CH3CN | 3 | 80 | 65 |
| 4 | NaHSO4 | CH3CN | 3 | 80 | 66 |
| 5 | KHSO4 | CH3CN | 3 | 80 | 64 |
| 6 | [NMP]+HSO4− | CH3CN | 3 | 80 | 69 |
| 7 | [HMIM]+HSO4− | CH3CN | 3 | 80 | 58 |
| 8 | -SO3H functionalized IL | CH3CN | 3 | 80 | 15 |
| 9 | [NMP]+HSO4− | 1,4-Dioxane | 3 | 80 | 32 |
| 10 | [NMP]+HSO4− | Cyclohexane | 3 | 80 | 70 |
| 11 | [NMP]+HSO4− | THF | 3 | 80 | 33 |
| 12 | [NMP]+HSO4− | Toluene | 3 | 80 | 47 |
| 13 | [NMP]+HSO4− | — | 3 | 80 | 73 |
| 14d | [NMP]+HSO4− | — | 3 | 80 | 70 |
| 15e | [NMP]+HSO4− | — | 3 | 80 | 75 |
| 16f | [NMP]+HSO4− | — | 3 | 80 | 47 |
| 17g | [NMP]+HSO4− | — | 3 | 80 | 10 |
| 18 | [NMP]+HSO4− | — | 1 | 80 | 61 |
| 19 | [NMP]+HSO4− | — | 2 | 80 | 73 |
| 20 | [NMP]+HSO4− | — | 2 | 100 | 68 |
| 21 | [NMP]+HSO4− | — | 2 | 60 | 59 |
| 22 | [NMP]+HSO4− | — | 2 | rt | 35 |
| 23 | None | — | 4 | 80 | n.r h |
Various solid acid catalysts and ionic liquids such as p- toluene sulfonic acid, Cu(OTf)2, montmorillonite K-10, NaHSO4, KHSO4, [NMP]+HSO4−, [HMIM]+HSO4−, and –SO3H functionalized ionic liquid were screened (Table 1, entries 1–8), where [NMP]+HSO4− was found to be best catalyst providing 69% yield of the desired ortho-alkylated product (Table 1, entry 6) under screening conditions. We observed that reaction was more favorable under solvent free conditions rather than in conventional organic solvents; an oligomer of styrene was observed as a major side product (Table 1, entries 6, 9–13). Considering this, further studies were carried out in neat conditions.
In an effort to determine the optimum concentration of the catalyst, various amounts of catalyst concentrations were studied ranging from 5 to 20 mol% (Table 1, entries 13–15). The optimum results were observed with 10 mol% of catalyst (73% yield) demonstrating that further increase in the catalyst concentration has no significant effect on the yield of ortho-alkylated phenol (Table 1, entry 13). A substrate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for styrene:phenol was found to be best for this transformation (Table 1, entries 13, 16–17). The other reaction parameters like reaction time and temperature were also studied and were observed to work smoothly at 80 °C within an appreciable time period of 2 h (Table 1, entries 18–22). Also, it is noteworthy to mention that the reaction was carried out in the absence of catalyst under the optimized reaction conditions, however no product formation was observed thus emphasizing that the [NMP]+HSO4− was solely responsible for catalyzing the reaction with higher yields (Table 1, entry 23). Hence, the optimized reaction conditions for regioselective alkylation of phenol with styrene are: catalyst [NMP]+HSO4− (10 mol%), solvent (neat), at 80 °C for 2 h.
4 for styrene:phenol was found to be best for this transformation (Table 1, entries 13, 16–17). The other reaction parameters like reaction time and temperature were also studied and were observed to work smoothly at 80 °C within an appreciable time period of 2 h (Table 1, entries 18–22). Also, it is noteworthy to mention that the reaction was carried out in the absence of catalyst under the optimized reaction conditions, however no product formation was observed thus emphasizing that the [NMP]+HSO4− was solely responsible for catalyzing the reaction with higher yields (Table 1, entry 23). Hence, the optimized reaction conditions for regioselective alkylation of phenol with styrene are: catalyst [NMP]+HSO4− (10 mol%), solvent (neat), at 80 °C for 2 h.
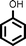
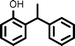
Once the best reaction conditions were established, several substituted phenols possessing various electron-withdrawing and electron-donating groups, including styrene derivatives were investigated for alkylation reaction and were found to furnish good to excellent yields of the desired products (Table 2, entries 1–15).
| Entry | Phenol | Alkene | Product | Yield (%)b[Ref.] |
|---|---|---|---|---|
| a Reaction conditions: alkene (0.5 mmol), phenol (2.0 mmol), [NMP]+HSO4− (10 mol%), temperature (80 °C), time (2 h). b GC yield. c Recyclability study for three consecutive cycles. d In parenthesis yield obtained in a large-scale experiment α-methyl styrene (5 mmol) and p-Cresol (20 mmol). | ||||
| 1 |
|
|
|
7318 |
| 2 |
|
|
|
8918 |
| 3 |
|
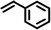
|
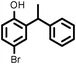
|
8218 |
| 4 |
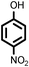
|
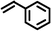
|
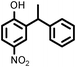
|
6918 |
| 5 |
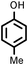
|
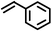
|
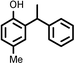
|
9218 |
| 6 |
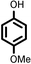
|
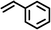
|
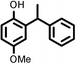
|
9518 |
| 7 |
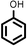
|
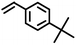
|
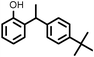
|
8733 |
| 8 |
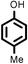
|
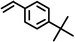
|
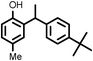
|
89(89, 87 83)c33 |
| 9 |
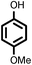
|
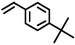
|
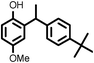
|
9033 |
| 10 |
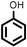
|
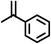
|
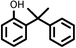
|
8133 |
| 11 |
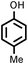
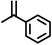
|
|
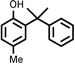
|
85 (91)d33 |
| 12 |
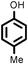
|
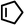
|
|
9333 |
| 13 |
|

|
|
9533 |
| 14 |
|
|
|
8516 |
| 15 |
|
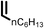
|
|
5516 |
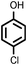
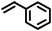
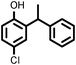
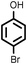
Reaction of phenol with styrene provided the ortho-alkylated phenol in 73% yield and para-alkylated phenol in 27% yield (Table 2, entry 1). It was observed that, phenols bearing electron-withdrawing groups such as Cl, Br, and NO2 gave moderate to good yields of the respective ortho-alkylated products (Table 2, entries 2–4) while electron-donating groups such as Me and OMe on phenol gave excellent results (Table 2, entries 5–6). Further, we explored the scope of the [NMP]+HSO4− ionic liquid for alkylation of phenols by varying styrene derivatives. Reaction of hindered 4-tert-butyl styrene with phenol provided a mixture of ortho and para-alkylated phenol 87% and 13% respectively (Table 1, entry 7). Moreover, the 4-tert-butyl styrene smoothly undergoes alkylation with different phenol derivatives providing 87–90% yield of the expected products (Table 2, entry 8–9). In addition, it was observed that α-methyl styrene under acidic conditions reacted with phenol and provided the preferred ortho-alkylated product in 81% and 19% of para-alkylated phenol (Table 2, entry 10) while the phenol derivative also reacted well with α-methyl styrene providing 85% yield of the respective ortho-alkylated product (Table 2, entry 11).
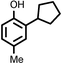
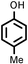
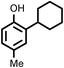
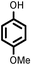
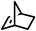
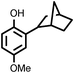
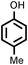
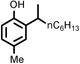
Several aliphatic cyclic and acyclic alkenes for the present reaction were also investigated. Aliphatic cyclic alkenes such as cyclopentene, cyclohexene and norbornene are selectively incorporated to the ortho position of the phenol under the present reaction conditions (Table 2, entries 12–14). The reaction between p-cresol and 1-octene provided the ortho-alkylated phenol derivative in a moderate yield 55% (Table 2, entry 15).
Encouraged with the successful application of the developed catalytic system for ortho-alkylation of phenols with various alkenes, we focused our attention on the ortho-alkylation of thiophenols with alkenes under similar reaction conditions. However, we observed that instead of the ortho-alkylated thiol product, the thiol prefers anti-Markovnikov linear addition with alkenes to give the corresponding product. Considering the latest results obtained, we then explored the developed methodology for the anti-Markovnikov addition of thiols to different alkenes (Table 3, entries 1–18).
| Entry | Thiol | Alkene | Product | Time | Yield (%)b[Ref.] |
|---|---|---|---|---|---|
| a Reaction conditions: alkene (0.5 mmol), thiol (2.0 mmol), [NMP]+HSO4− (10 mol%), temperature (80 °C). b Isolated yield. | |||||
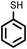
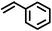
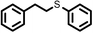
| 1 |
|
|
|
2 | 7828 |
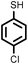
| 2 |
|
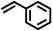
|
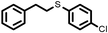
|
2 | 9328 |
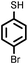
| 3 |
|
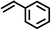
|
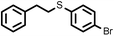
|
3 | 8428 |
| 4 |
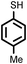
|
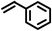
|
|
2 | 9228 |
| 5 |
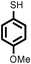
|
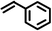
|
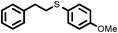
|
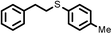
2 | 9528 |
| 6 |
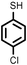
|
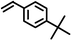
|
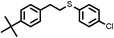
|
2 | 7928 |
| 7 |
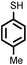
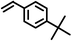
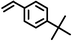
|
|
|
2 | 8828 |
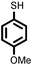
| 8 |
|
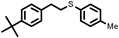
|
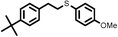
|
2 | 9133 |
| 9 |
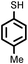
|
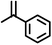
|
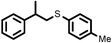
|
2 | 7728 |
| 10 |
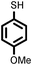
|
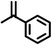
|
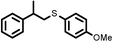
|
2 | 8028 |
| 11 |
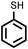
|
|
|
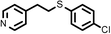
3 | 9133 |
| 12 |
|
|
|
3 | 8933 |
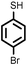
| 13 |
|
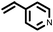
|
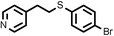
|
3 | 8333 |
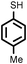
| 14 |
|
|
|
2 | 9433 |
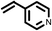
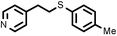
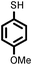
| 15 |
|
|
|
2 | 9633 |
| 16 |
|
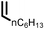
|

|
2 | 8325 |
| 17 |
|
|
|
2 | 9525 |
| 18 |
|
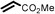
|

|
2 | 9625 |
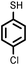
Firstly, to explore the general applicability of the reaction, several substituted thiophenols bearing electron-withdrawing and electron-donating groups were reacted with styrene under the present reaction conditions. We noticed that all the additions were regioselective and anti-Markovnikov in nature and no Markovnikov adducts were isolated in any case studied, hence highlighting the unique nature of catalyst towards different substituted thiol substrates (Table 3, entries 1–5). Furthermore, the reaction of styrene derivatives such as 4-tert-butyl styrene and α-methyl styrene with different substituted thiophenols were also examined and was found to afford the respective thioethers in 77–91% yield (Table 3, entries 6–10).
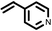
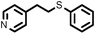
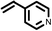
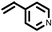
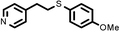
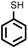
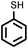
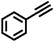
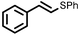
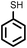
To best of our knowledge, a general method for the synthesis of linear anti-Markovnikov addition product from 4-vinyl pyridine and thiophenol derivatives has not yet been reported. Considering which, we attempt to study the applicability of our developed catalytic system for the anti- Markovnikov addition of 4-vinyl pyridine with different substituted thiophenol including both electron-donating and electron-withdrawing groups. In favor, we observed good to excellent yields of desired products under the present reaction conditions (Table 3, entries 11–15). Alkenes such as 1-octene were found to react with thiophenol to provide the corresponding linear thioether in high yield (Table 3, entries 16). The addition of thiophenol with phenyl acetylene was highly selective in nature and achieved a 95% yield of thioether within 2 h (Table 3, entry 17). Moreover, [NMP]+HSO4− ionic liquid effectively catalyzed the addition of thiophenol with methyl acrylate in a Michael addition to furnish the respective addition product in admirable yield (Table 3, entry 18).
Conclusions
In conclusion, we developed a simple, inexpensive methodology comprising Brønsted acidic ionic liquid N-methyl-2-pyrrolidone hydrogen sulfate, [NMP]+HSO4−, as an efficient and recyclable catalyst for the regioselective alkylation of phenols and the anti-Markovnikov addition of thiols to alkenes. This metal-free method exhibited remarkable catalytic activity and higher yields of desired products within a short reaction time under solvent free conditions. The developed protocol thus presents a general process for the preparation of valuable biologically active species and industrial chemicals.Experimental section
All chemicals and reagents were procured from commercial suppliers and used without further purification. The products were characterized using 1H NMR, 13C NMR spectra (Varian Mercury 300 NMR Spectrometer) and IR (Perkin-Elmer FT-IR) spectroscopic techniques. The progress of the reaction was monitored using GC analysis (Perkin-Elmer, Clarus 400) (BP-10 GC column, 30 m × 0.32 mm ID, film thickness 0.25 mm). Products were confirmed by GC-MS (Shimadzu GC-MS QP 2010). All the compounds are already well known in the literature.33Typical procedure for the synthesis of ionic liquid
In this study, several ILs used were synthesized according to the procedures reported in the literature.32Synthesis of N-methyl-2-pyrrolidone hydrogen sulfate [NMP]+HSO4− ionic liquid: 1-methyl-2-pyrodidone (0.2 mol) was taken into a 250 mL three necked flask with a magnetic stirrer. Then equimolar concentrated sulphuric acid (98 wt%) was added drop wise slowly into the flask at 80 °C for 12 h. The mixture was then extensively washed with ether (10 × 3) to remove non-ionic residues and dried under vacuum by a rotary evaporator to obtain the viscous clear liquid.
A typical experimental procedure for the alkylation of phenol with alkene
To 10 mL of sealed tube, alkene (0.5 mmol), phenol (2.0 mmol) and [NMP]+HSO4− (10 mol%) were added and the tube was properly sealed. The reaction mixture was heated at 80 °C for a desired time and was cooled to room temperature on completion of the reaction. Alkene conversion as well as product formation was monitored by gas chromatography. In order to separate the ionic liquid, a small amount of water (2 × 5 mL) was added to the reaction mixture and the alkylated product was extracted in ethyl acetate (3 × 5 mL). The residue obtained was purified with column chromatography (silica gel, 60–120 mesh; PE-EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05) to afford the desired ortho-alkylated phenol product. The structures of the obtained products were confirmed by GC-MS, 1H NMR, 13C NMR, and IR spectroscopic techniques. The purity of the compounds was determined by GC-MS analysis.
05) to afford the desired ortho-alkylated phenol product. The structures of the obtained products were confirmed by GC-MS, 1H NMR, 13C NMR, and IR spectroscopic techniques. The purity of the compounds was determined by GC-MS analysis.
A typical experimental procedure for the anti-Markovnikov addition of thiol with alkene
To a 10 mL sealed tube, alkene (0.5 mmol), thiol (2.0 mmol) and [NMP]+HSO4− (10 mol%) were added and properly sealed. The reaction mixture was heated at 80 °C for desired time and after completion of the reaction it was cooled to room temperature. Alkene conversion and product formation was monitored using gas chromatography. To separate the ionic liquid, a small amount of water (2 × 5 mL) was added to the reaction mixture and the addition product was extracted with ethyl acetate (3 × 5 mL). The residue obtained was purified by column chromatography (silica gel, 60–120 mesh; PE-EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05) to afford the desired thioethers product. The structures of the products were confirmed by GC-MS, 1H NMR, 13C NMR, and IR spectroscopic techniques. The purity of compounds was determined by GC-MS analysis.
05) to afford the desired thioethers product. The structures of the products were confirmed by GC-MS, 1H NMR, 13C NMR, and IR spectroscopic techniques. The purity of compounds was determined by GC-MS analysis.
Typical procedure for recycling the [NMP]+HSO4− ionic liquid catalyst for the alkylation of p-cresol with 4-tert-butyl styrene
After completion of reaction, the reaction mixture was cooled to r.t., and was then extracted with di-isopropyl ether (5 × 10 mL). Catalyst was then dried under reduced pressure for 1 h. The dried catalyst was then used for catalyst recyclability experiment. It was observed that the recovered catalyst could be reused for three consecutive cycles for the regioselective alkylation of p-cresol with 4-tert-butyl styrene with a slight decrease in yield of desired product.Characterization of selected compounds
1H NMR (300 MHz, CDCl3): δ = 1.25 (s, 3 H), 1.57 (d, J = 7.33 Hz, 3 H), 3.74 (s, 3 H), 4.27 (q, J = 7.08 Hz, 1 H), 4.35 (s, 1 H), 6.65 (d, J = 2.57 Hz, 1 H), 6.67 (s, 1 H), 6.81 (d, J = 2.57 Hz, 1 H), 7.15 (d, J = 8.43 Hz, 2 H), 7.27 (d, J = 8.43 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 21 (CH3), 29.1 (CH), 31.3 (3CH3), 38.2 (C), 55.6 (OCH3), 111.4 (CH), 114.3 (CH), 116.5 (CH), 125.5 (2CH), 127.1 (2CH), 133.7 (C), 141.9 (C), 147.4 (C), 149.1 (C), 153.6 (C); IR (KBr) ν = 3411, 2963, 1506, 1462, 1429, 1363, 1269, 1203, 1034, 836, 801, 713, 578 cm−1; MS (EI, 70 ev): m/z (%) = 284 (57), 269 (48), 228 (16), 213 (15), 198 (4), 165 (4), 150 (100), 135 (21), 120 (23), 91 (12), 77 (7), 57(21).
1H NMR (300 MHz, CDCl3): δ = 2.89 (t, J = 7.7 Hz, 2 H), 3.14 (t, J = 7.7 Hz, 2 H), 7.17–7.25 (m, 5 H), 7.29 (d, J = 7.3 Hz, 2 H), 7.40 (d, J = 8.4 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 35.1 (SCH2), 35.4 (CH2), 119.7 (C), 126.5 (CH), 128.5 (4CH), 130.6 (2CH), 131.9 (2CH), 135.7 (C), 139.9 (C); IR (KBr) ν = 3026, 2923, 2857, 1603, 1496, 1473, 1453, 1386, 1092, 1069, 1007, 807, 730, 697, 480 cm−1; MS (EI, 70 ev): m/z (%) = 295 (40), 293 (39), 203 (23), 201 (23), 157 (4), 122 (64), 106 (100), 77 (18), 51 (22).
1H NMR (300 MHz, CDCl3): δ = 1.31 (s, 9 H), 2.89 (t, J = 7.9 Hz, 2 H), 3.14 (t, J = 7.9 Hz, 2 H), 7.13 (d, J = 8.4 Hz, 2 H), 7.25–7.28 (m, 4 H), 7.32 (d, J = 8.4 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 31.4 (3 CH3), 34.5 (C), 35.1 (SCH2), 35.4 (CH2), 125.5 (2CH), 128.2 (2CH), 129.1 (2CH), 130.5 (2CH), 132 (C), 135.1 (C), 137 (C), 149.5 (C); IR (KBr) ν = 2961, 1514, 1476, 1390, 1363, 1268, 1095, 1011, 812, 563, 487 cm−1; MS (EI, 70 ev): m/z (%) = 304 (42), 289 (7), 161 (59), 147 (60), 132 (16), 131 (16), 117 (28), 105 (18), 91 (17), 77 (8), 57 (100), 45 (25), 41 (13).
1H NMR (300 MHz, CDCl3): δ = 1.32 (s, 9 H), 2.33 (s, 3 H), 2.89 (t, J = 7.9 Hz, 2 H), 3.13 (t, J = 7.9 Hz, 2 H), 7.13–7.15 (m, 4 H), 7.23–7.34 (m, 4 H); 13C NMR (75.43 MHz, CDCl3): δ = 21.1 (CH3), 31.4 (3 CH3), 34.4 (C), 35.3 (SCH2), 35.7 (CH2), 125.4 (2CH), 128.2 (2CH), 129.8 (2CH), 129.9 (2CH), 132.7 (C), 136 (C), 137.3 (C), 149.2 (C); IR (KBr) ν = 2962, 1492, 1363, 1268, 1092, 1017, 805, 562, 493 cm−1; MS (EI, 70 ev): m/z (%) = 284 (63), 161 (46), 137 (100), 117 (19), 105 (12), 91 (27), 77 (9), 57 (86), 45 (29).
1H NMR (300 MHz, CDCl3): δ = 1.29 (s, 9 H), 2.83 (t, J = 7.9 Hz, 2 H), 3.06 (t, J = 7.9 Hz, 2 H), 3.78 (s, 3 H), 6.85 (d, J = 8.8 Hz, 2 H), 7.10 (d, J = 8 Hz, 2 H), 7.30 (d, J = 8 Hz, 2 H), 7.36 (d, J = 8.8 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 31.4 (3 CH3), 34.4 (C), 35.4 (SCH2), 37.1 (CH2), 55.3 (OCH3), 114.5 (2CH), 125.4 (2CH), 126.4 (2CH), 128.2 (2CH), 133.1 (C), 137.3 (C), 149.1 (C), 158.8 (C); IR (KBr) ν = 2960, 1592, 1493, 1465, 1363, 1284, 1245, 1179, 1106, 1033, 824, 562 cm−1; MS (EI, 70 ev): m/z (%) = 300 (62), 161 (40), 153 (76), 138 (18), 131 (14), 117 (15), 109 (20), 91 (13), 77 (9), 57 (100), 45 (16).
1H NMR (300 MHz, CDCl3): δ = 2.92 (t, J = 7.7 Hz, 2 H), 3.17 (t, J = 7.8 Hz, 2 H), 7.13 (d, J = 6.2 Hz, 1 H), 7.22–7.38 (m, 6 H), 8.51 (d, J = 5.8 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 33.9 (SCH2), 34.7 (CH2), 123.9 (2CH), 126.4 (CH), 128.9 (2CH), 129.6 (2CH), 135.4 (C), 149 (2CH), 149.5 (C); IR (KBr) ν = 3060, 2928, 1601, 1478, 1437, 1415, 795, 740, 688, 482 cm−1; MS (EI, 70 ev): m/z (%) = 215 (69), 123 (100), 106 (97), 77 (30), 65 (13), 51 (22), 45 (52).
1H NMR (300 MHz, CDCl3): δ = 2.90 (t, J = 7.7 Hz, 2 H), 3.15 (t, J = 7.5 Hz, 2 H), 7.12 (d, J = 6.2 Hz, 2 H), 7.26–7.28 (m, 4 H), 8.52 (d, J = 5.7 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 34.2 (SCH2), 34.6 (CH2), 123.9 (2CH), 129.1 (2CH), 131 (2CH), 132.4 (C), 134 (C), 148.8 (2CH), 149.5 (C); IR (KBr) ν = 2928, 2851, 1601, 1476, 1412, 1388, 1218, 1095, 1011, 807, 488 cm−1; MS (EI, 70 ev): m/z (%) = 249 (48), 159 (8), 157 (50), 108 (11), 106 (100), 77 (19), 45 (48).
1H NMR (300 MHz, CDCl3): δ = 2.90 (t, J = 7.5 Hz, 2 H), 3.15 (t, J = 7.5 Hz, 2 H), 7.11 (d, J = 5.9 Hz, 2 H), 7.21 (d, J = 8.8 Hz, 2 H), 7.42 (d, J = 8.4 Hz, 2 H), 8.51 (d, J = 5.5 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 33.9 (SCH2), 34.5 (CH2), 120.2 (C), 123.8 (2CH), 131 (2CH), 132 (2CH), 134.7 (C), 148.8 (2CH), 149.5 (C); IR (KBr) ν = 3021, 2927, 1601, 1558, 1473, 1415, 1218, 1092, 1057, 1007, 804, 570, 481 cm−1; MS (EI, 70 ev): m/z (%) = 295 (25), 293 (25), 203 (17), 201 (17), 122 (60), 106 (100), 77 (15), 45 (12).
1H NMR (300 MHz, CDCl3): δ = 2.32 (s, 3 H), 2.87 (t, J = 7.5 Hz, 2 H), 3.11 (t, J = 7.5 Hz, 2 H), 7.08–7.13 (m, 4 H), 7.27 (d, J = 8 Hz, , 2 H), 8.49 (d, J = 5.8 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 21 (CH3), 34.7 (SCH2), 34.8 (CH2), 123.9 (2CH), 129.8 (2CH), 130.6 (2CH), 131.6 (C), 136.7 (C), 149.1 (2CH), 149.6 (C); IR (KBr) ν= 3027, 2923, 1601, 1559, 1493, 1414, 1281, 1219, 1091, 966, 802, 571, 497 cm−1; MS (EI, 70 ev): m/z (%) = 229 (82), 137 (100), 106 (65), 91 (40), 77 (25), 65 (15), 45 (58).
1H NMR (300 MHz, CDCl3): δ = 2.86 (t, J = 7.5 Hz, 2 H), 3.07 (t, J = 7.5 Hz, 2 H), 3.8 (s, 3 H), 6.87 (d, J = 8.8 Hz, 2 H), 7.10 (d, J = 5.9 Hz, 2 H), 7.37 (d, J = 8.8 Hz, , 2 H), 8.49 (d, J = 5.9 Hz, 2 H); 13C NMR (75.43 MHz, CDCl3): δ = 34.9 (SCH2), 36 (CH2), 55.3 (OCH3), 114.6 (2CH), 123.9 (2CH), 125.4 (2CH), 133.7 (C), 149.2 (2CH), 149.5 (C), 159.2 (C); IR (KBr) ν = 2927, 2840, 1600, 1494, 1462, 1415, 1284, 1246, 1180, 1030, 828, 798 cm−1; MS (EI, 70 ev): m/z (%) = 245 (100), 153 (83), 138 (31), 125 (13), 109 (39), 107 (41), 106 (69), 96 (10), 77 (24), 45 (27).
Acknowledgements
The financial assistance from Indira Gandhi Centre for Atomic Research (IGCAR), Kalpakkam, India is kindly acknowledged.References
- D. Juhue and J.-M. Sage, US Pat., 7488784, 2009 Search PubMed.
- G. R. John, U. S. Pat., 4613384, 1986 Search PubMed.
- C. H. Thomas, U. S. Pat., 4500689, 1985 Search PubMed.
- M. L. Davi and F. Gnudi, Water Res., 1999, 33, 3213 CrossRef CAS.
- A. Takahashi, T. Toyoda, C. Kondo, A. Tamura, S. Yamaguchi and S. Asai, Japan Pat., 07098885, 1978 Search PubMed.
- B. J. Stucker, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Chapter on Flame Retardants; published online 2000 Search PubMed.
- (a) D. H. Lorenz and B. A. Gruber, US Pat., 1981, 4260768 Search PubMed; (b) R. Liebler, S. Besecke and M. Munzer, US Pat., 1986, 4576870 Search PubMed.
- J. R. Roberts, G. C. Volgas and P. T. Delashmit, US Pat. Appl., 0262061A1, 2008 Search PubMed.
- W. Wolff, R. Faber and K. Ruclack, US Pat., 3849556, 1974 Search PubMed.
- (a) L. A. Potolovskii, V. N. Vasileva, N. M. Kukui, M. V. Nazarova and T. I. Kirsanova, Chem. Technol. Fuels Oils, 1972, 8, 920 CrossRef; (b) K. J. L. Paciorek, R. H. Kratzer, J. Kaufman, T. I. Ito and J. H. Nakahara, US Pat., 4192757, 1980 Search PubMed.
- (a) K.-G. Fahlbusch, F.-J. Hammerschmidt, J. Panten, W. Pickenhagen, D. Schatkowski, K. Bauer, D. Garbe and H. SurburgUllmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Flavours and fragrances, 2003, vol. 14, pp. 73–200 Search PubMed.
- (a) G. A. Olah In Friedal-Crafts Chemistry; Wiley: New York, 1973 Search PubMed; (b) G. A. Olah, B. M. Trost, I. Fleming and Eds, Oergamon Press: Oxford, 1991; vol. 3, p 293.; (c) M. Bandini and A. U. Melloni, Angew. Chem., Int. Ed., 2004, 43, 550 CrossRef CAS.
- (a) H. Fiege, H.-W. Voges, T. Hamamoto, S. Umemura, T. Iwata, H. Miki, Y. Fujita, H.-J. Buysch, D. Garbe and W. Paulus, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, phenol derivatives; published online 2000 Search PubMed; (b) B. Das, M. Krishnaiah, K. Laxminarayana, K. Damodar and D. N. Kumar, Chem. Lett., 2008, 38, 42 CrossRef.
- A. Lange andH. P. Rath, US Pat.20030187171A1, 2003; Search PubMedThompson-Hayward Chemical Company, GB Pat. 1142233, 1969.
- A. Vinu, B. M. Devassy, S. B. Halligudi, W. Bohlmann and M. Hartmann, Appl. Catal., A, 2005, 281, 207 CrossRef CAS.
- Y. Kuninobu, T. Matsuki and K. Takai, J. Am. Chem. Soc., 2009, 131, 9914 CrossRef CAS.
- H. Q. Nimal Gunaratne, J. L. Tobias and K. R. Seddon, New J. Chem., 2010, 34, 1821 RSC.
- S. Halder and S. Koner, J. Org. Chem., 2010, 75, 6005 CrossRef.
- R. J. Cremlyn, An Introduction to Organo-Sulfur Chemistry, Wiley & Sons, New York, 1996 Search PubMed.
- (a) M. E. Peach, Thiols as Nucleophiles, in The Chemistry of the Thiol Group, ed. S. Patai, John Wiley & Sons, London, 1979, 721 Search PubMed; (b) S. Oae, Organic Sulfur Chemistry, CRC Press, Boca Raton, 1991. Search PubMed.
- D. P. Curran, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, New York, Vol. 4, 1991, p. 715 Search PubMed.
- (a) T. Posner, Ber. Dtsch. Chem. Ges., 1905, 38, 646 CrossRef; (b) C. G. Screttas and M. Micha-Screttas, J. Org. Chem., 1979, 44, 713 CrossRef CAS; (c) F. Wolf and H. Z. Finke, Z. Chem., 1972, 12, 180 CrossRef CAS.
- (a) T. Mukaiyama, T. Izawa, K. Saigo and H. Takai, Chem. Lett., 1973, 355 CrossRef CAS; (b) M. Belley and R. Zamboni, J. Org. Chem., 1989, 54, 1230 CrossRef CAS; (c) S. Kanagasabapathy, A. Sudalai and B. C. Benicewicz, Tetrahedron Lett., 2001, 42, 3791 CrossRef CAS.
- P. Kumar, R. K. Pandey and V. R. Hedge, Synlett, 1999, 1921 CrossRef CAS.
- S. Banerjee, J. Das, R. P. Alvareza and S. Santra, New J. Chem., 2010, 34, 302 RSC.
- C. C. Silveira, S. R. Mendes and F. M. Libero, Synlett, 2010, 790 CrossRef CAS.
- B. C. Ranu and T. Mandal, Tetrahedron Lett., 2006, 47, 6911 CrossRef CAS.
- (a) B. C. Ranu and T. Mandal, Synlett, 2007, 925 CrossRef CAS; (b) B. Movassagh and M. Navidi, Arkivoc, 2008,(xv), 47 CAS.
- K. Griesbaun, Angew. Chem., Int. Ed. Engl., 1970, 9, 273 CrossRef.
- (a) P. Wasserscheid and T. WeltonIonic Liquids in Synthesis, 2nd ed., Wiley-VCH, 2002 CrossRef; (b) R. D. Rogers and K. R. Seddon, Ionic Liquids as Green Solvents: Progress and Prospects, ACS Symposium Series 856, American Chemical Society, Oxford University Press, Oxford, 2003. Search PubMed; (c) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS; (d) P. Wasserscheid and W. Keim, Angew. Chem. Int. Ed., 2000, 39, 3772 CrossRef CAS; (e) R. Sheldon, Chem. Commun., 2001, 2399 RSC.
- (a) S. R. Jagtap and B. M. Bhanage, J. Chem. Res., 2007, 370 CrossRef CAS; (b) A. G. Panda, S. R. Jagtap, N. S. Nandurkar and B. M. Bhanage, Ind. Eng. Chem. Res., 2008, 47, 969 CrossRef CAS; (c) Z. S. Qureshi, K. M. Deshmukh, M. D. Bhor and B. M. Bhanage, Catal. Commun., 2009, 10, 833 CrossRef CAS; (d) K. P. Dhake, Z. S. Qureshi, R. S. Singhal and B. M. Bhanage, Tetrahedron Lett., 2009, 50, 2811 CrossRef CAS; (e) K. M. Deshmukh, Z. S. Qureshi, N. S. Nandurkar and B. M. Bhanage, Can. J. Chem., 2009, 87, 401 CrossRef CAS; (f) Y. P. Patil, P. J. Tambade, K. M. Deshmukh and B. M. Bhanage, Catal. Today, 2009, 148, 355 CrossRef CAS; (g) Z. S. Qureshi, K. M. Deshmukh, P. J. Tambade, K. P. Dhake and B. M. Bhanage, Eur. J. Org. Chem., 2010, 6233 CrossRef CAS.
- (a) W. Wang, L. Shao, W. Cheng, J. Yang and M. He, Catal. Commu., 2008, 9, 337 CrossRef CAS; (b) A. R. Hajipour, L. Khazdooz and A. E. Ruoho, Catal. Commun., 2008, 9, 89 CrossRef CAS; (c) Y. Gu, F. Shi and Y. Deng, J. Mol. Catal. A. Chem., 2004, 212, 71 CrossRef CAS.
- (a) A. I. Kochnev, I. I. Oleynik, I. V. Oleynik, S. S. Ivanchev and G. A. Tolstikov, Russ. Chem. Bull., 2007, 56, 1125 CrossRef CAS; (b) G. L. Rouzo, M.-G. Marielle and J.-P. Simonato, J. Chem. Res. Synop., 2006, 8, 521 CrossRef; (c) H. Hoffmann, L. Roche and A. G. Berlin, DE, 615448, 1935 Search PubMed; (d) G. D. Yadav and G. S. Pathrea, Synth. Commun., 2008, 38, 2684 CrossRef CAS; (e) C. J. Yu, Y. Chong, J. F. Kayyem and M. Gozin, J. Org. Chem., 1999, 64, 2070 CrossRef CAS; (f) S. K. Das, R. Singh and G. Panda, Eur. J. Chem., 2009, 4757 CAS; (g) T. Itoh and T. Mase, Org. Lett., 2004, 6, 4587 CrossRef CAS; (h) L. Bauer and L. A. Gardella, J. Org. Chem., 1963, 28, 1323 CrossRef; (i) A. R. Katritzky, I. Takahashi and C. M. Marson, J. Org. Chem., 1986, 51, 4914 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
