DOI:
10.1039/C1RA00482D
(Paper)
RSC Adv., 2011,
1, 1799-1807
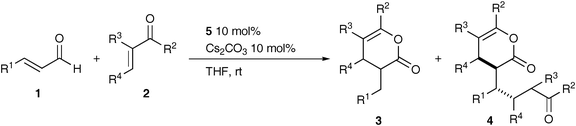
NHC-catalyzed reaction of enals with unactivated enones: substituent effect on the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 2 adducts†
2 adducts†
Received
19th July 2011
, Accepted 22nd August 2011
First published on 31st October 2011
Introduction
N-Heterocyclic carbenes have been utilized for many organic syntheses as versatile organocatalysts.1 Particularly, they can convert α,β-unsaturated aldehydes to various active species such as acyl anions A and homoenolate anions B, which serve as umpolung versions of enals (Scheme 1). Enolate C or enol is also generated from the enals by proton transfer or protonation of B. In the NHC-catalyzed reaction of enals with enones, products are variable depending on the enones and catalysts as well as the reaction conditions to generate the active species. The reaction with chalcone derivatives and dibenzylidene ketones gave cyclopentenes2 and cyclopentanones,3 respectively. It was proposed that these products were formed by Michael addition of the homoenolateB and subsequent aldol reaction2,3, or by cross-benzoin reaction of acyl anion A followed by oxy-Cope rearrangement.4,5 On the other hand, azadienes and internal enones reacted with the enolate C or enol to yield δ-lactams6 and lactones,7 respectively. The intermolecular reaction of C with activated enones was recently reported.8 High reaction selectivities usually have been achieved by the choice of amine. Strong amine bases such as DBU are suitable for the selective reaction of B,2,3 and in contrast, use of weak bases such as DIPEA and NMM is crucial for the reaction of C.7,8
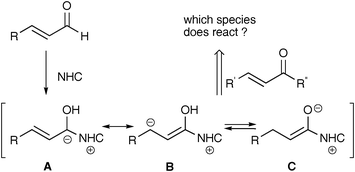 |
| | Scheme 1 | |
Although a lot of studies, including enantioselective reaction using chiral catalysts, have accumulated in this field, factors to control the reaction selectivity of various active species A–C are not always clear, because much of the work has been confined to aromatic enals and enones. Moreover, unsubstituted vinyl ketones, which are expected to be good Michael acceptors, have rarely been used. Thus, we carried out imidazole carbene-catalyzed reaction of aromatic and aliphatic enals with unactivated enones in order to investigate substituent effects on the reaction selectivity and pathway. We report here that the selectivity was mainly determined by the substituent effect of the enones, not so much by bases, and that primary products and intermediates reacted further with another molecule of the enone to yield two new kinds of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts.
2 adducts.
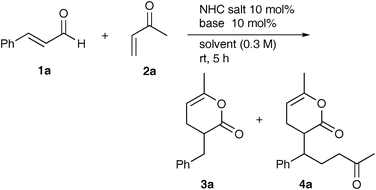
Results and discussion
Cinnamaldehyde (1a) and methyl vinyl ketone (2a) were selected as standard substrates and the reaction was carried out under various conditions (Table 1). Of the NHC precatalysts, mesitylimidazolium chloride 5 gave pyranone 3a quantitatively as a single product in the presence of Cs2CO3, while little 3a was formed with triazolium 6 and thiazolium salt 7 (entries 1–3). On substitution of the base by DBU, tBuOK and NaH, the other pyranone 4a, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct, was formed along with 3a (entries 4–9). THF and toluene were suitable solvents for this reaction (entries 1 and 10–13). Interestingly, the product ratio of 3a/4a was hardly affected by the molar ratio of 2a/1a in a range of 0.5 to 2, though it decreased to some extent with five equivalents of 2a (entries 1 and 14–16).
2 adduct, was formed along with 3a (entries 4–9). THF and toluene were suitable solvents for this reaction (entries 1 and 10–13). Interestingly, the product ratio of 3a/4a was hardly affected by the molar ratio of 2a/1a in a range of 0.5 to 2, though it decreased to some extent with five equivalents of 2a (entries 1 and 14–16).
|
|
| Entry |
NHC salt |
Base
|
Solvent
|
Equiv. of 2a |
Yield (%)a (3a + 4a) |
Ratioa (3a/4a) |
|
Determined by GC.
|
| 1 |
5
|
Cs2CO3 |
THF
|
2 |
Quant. |
100/0 |
| 2 |
6
|
Cs2CO3 |
THF
|
2 |
24 |
100/0 |
| 3 |
7
|
Cs2CO3 |
THF
|
2 |
Tr. |
— |
| 4 |
5
|
Et3N
|
THF
|
2 |
9 |
100/0 |
| 5 |
5
|
DMAP
|
THF
|
2 |
46 |
100/0 |
| 6 |
5
|
DBU
|
THF
|
2 |
84 |
86/14 |
| 7 |
5
|
KHMDS |
THF
|
2 |
28 |
100/0 |
| 8 |
5
|
tBuOK
|
THF
|
2 |
81 |
86/14 |
| 9 |
5
|
NaH
|
THF
|
2 |
43 |
44/56 |
| 10 |
5
|
Cs2CO3 |
Toluene
|
2 |
86 |
87/13 |
| 11 |
5
|
Cs2CO3 |
DCM
|
2 |
46 |
96/4 |
| 12 |
5
|
Cs2CO3 |
Et2O
|
2 |
36 |
100/0 |
| 13 |
5
|
Cs2CO3 |
DMF
|
2 |
49 |
65/35 |
| 14 |
5
|
Cs2CO3 |
THF
|
0.5 |
79 |
100/0 |
| 15 |
5
|
Cs2CO3 |
THF
|
1 |
Quant. |
100/0 |
| 16 |
5
|
Cs2CO3 |
THF
|
5 |
86 |
74/26 |
|
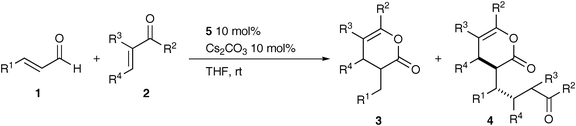
The reaction of aromatic enals 1 with various unactivated enones 2 was carried out under the standard conditions (Table 1, entry 1),9 and these results are summarized in Table 2. The reaction of cinnamaldehyde (1a) with 4-methoxyphenyl vinyl ketone (2b) gave two pyranones 3b and 4b in quantitative total yield with a ratio of 85/15 (entry 2). The product 4b was obtained as a mixture of two diastereomers (88/12). The structure of the major isomer of 4b was tentatively assigned to erythro by the analogy with that of 4m, which was determined by X-ray crystal analysis (Fig. 1). In contrast, cinnamaldehyde (1a) reacted with 2c and 2d to give the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4c and 4d as major products (entries 3 and 4). The reaction with α- and β-methylated vinyl ketones 2e and 2f afforded, however, the 1
2 adducts 4c and 4d as major products (entries 3 and 4). The reaction with α- and β-methylated vinyl ketones 2e and 2f afforded, however, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pyranones 3e and 3f exclusively (entries 5 and 6).10 No or little expected products 3 and 4 were obtained by the reaction with 2g–i (entries 7–9). Moreover, β-substituted vinyl ketones 2f and 2i produced the corresponding trans-cyclopentenes in 13% and 78% yield, respectively, as previously reported (entries 6 and 9).2 In the reaction of 3-(1-naphthyl)propenal (1b), the ratio of 3/4 was similarly affected by the enone substituents, except for the reaction with 2c wherein 4l was exclusively formed in good yield (entries 10–13). On the other hand, the aromatic enals 1c–d having electron-donating or electron-withdrawing aryl groups reacted with methyl vinyl ketone (2a) to predominantly yield the pyranone 3, suggesting that the selectivity of 3/4 was not altered by the enals 1 (entries 14–16).
1 pyranones 3e and 3f exclusively (entries 5 and 6).10 No or little expected products 3 and 4 were obtained by the reaction with 2g–i (entries 7–9). Moreover, β-substituted vinyl ketones 2f and 2i produced the corresponding trans-cyclopentenes in 13% and 78% yield, respectively, as previously reported (entries 6 and 9).2 In the reaction of 3-(1-naphthyl)propenal (1b), the ratio of 3/4 was similarly affected by the enone substituents, except for the reaction with 2c wherein 4l was exclusively formed in good yield (entries 10–13). On the other hand, the aromatic enals 1c–d having electron-donating or electron-withdrawing aryl groups reacted with methyl vinyl ketone (2a) to predominantly yield the pyranone 3, suggesting that the selectivity of 3/4 was not altered by the enals 1 (entries 14–16).
|
|
| Entry |
Enal
1 |
Enone
2 |
Time/h |
Product |
Total yield (%)b |
Ratiob |
Dr of 4b |
| |
R1 |
|
R2 |
R3 |
R4 |
|
3, 4 |
(3 + 4) |
(3/4) |
|
|
2/1 = 2.
Determined by 1H NMR of the crude mixture.
Carried out at 70 °C.
Dr = 78/22.
trans-1,3-Diphenyl-4-methylcyclopentene was also formed in 13% yield.
Dr = 71/29.
trans-1,3,4-Triphenylcyclopentene was formed in 78% yield.
Isolated yield.
Carried out at 50 °C.
|
| 1 |
1a
|
Ph |
2a
|
Me |
H |
H |
5 |
3a, 4a |
Quant. |
100/0 |
— |
| 2 |
1a
|
2b
|
C6H4OMe-4
|
H |
H |
12 |
3b, 4b |
Quant. |
85/15 |
88/12 |
| 3 |
1a
|
2c
|
Ph |
H |
H |
5 |
3c, 4c |
79 |
44/56 |
91/9 |
| 4 |
1a
|
2d
|
C6H4Cl-4
|
H |
H |
18 |
3d, 4d |
67 |
33/67 |
100/0 |
| 5 |
1a
|
2e
|
Ph |
Me |
H |
48 |
3e, 4e |
48 |
100/0 |
— |
| 6 |
1a
|
2f
|
Ph |
H |
Me |
3c |
3f
d, 4f |
55e |
100/0 |
— |
| 7 |
1a
|
2g
|
Me |
H |
Ph |
3c |
3g
f, 4g |
8 |
100/0 |
— |
| 8 |
1a
|
2h
|
Me |
H |
Me |
6c |
3h, 4h |
No reaction |
|
|
| 9 |
1a
|
2i
|
Ph |
H |
Ph |
12 |
3i, 4i |
0g |
|
|
| 10 |
1b
|
1-Naph
|
2a
|
Me |
H |
H |
5 |
3j, 4j |
82h |
100/0 |
— |
| 11 |
1b
|
2b
|
C6H4OMe-4
|
H |
H |
1i |
3k, 4k |
87 |
77/23 |
100/0 |
| 12 |
1b
|
2c
|
Ph |
H |
H |
12 |
3l, 4l |
71 |
0/100 |
77/23 |
| 13 |
1b
|
2d
|
C6H4Cl-4
|
H |
H |
19i |
3m, 4m |
29 |
41/59 |
100/0 |
| 14 |
1c
|
C6H4OMe-4
|
2a
|
Me |
H |
H |
3 |
3n, 4n |
84 |
74/26 |
100/0 |
| 15 |
1d
|
C6H4Cl-4
|
2a
|
Me |
H |
H |
3 |
3o, 4o |
83 |
100/0 |
— |
| 16 |
1e
|
C6H3Cl2-2,4
|
2a
|
Me |
H |
H |
14 |
3p, 4p |
75 |
100/0 |
— |
Next, the reaction of crotonaldehyde (1f) and acrolein (1g) was investigated (Table 3). Although the pyranone 3q was formed exclusively in 60% yield by the reaction of 1f with methyl vinyl ketone (2a) under standard conditions (entry 1), aliphatic enals such as 1f and 1g gave the products 3 and 4 in lower yields than aromatic ones. For example, the reaction of 1g with two equivalents of 4-chlorophenyl vinyl ketone (2d) gave the products 3z and 4z in 32% total yield with a 56/44 ratio based on 1g, which was probably caused by oligomerization and evaporation of the enal 1g. When the 2d/1g ratio decreased to 1.0 and 0.5, 3z and 4z were obtained in 59% (44/56) and 89% yield (53/47) based on 2d, respectively (entry 10). Thus, further reaction in Table 3 was carried out using two equivalents of enals 1. In analogy with the reaction of aromatic enals 1a and 1b, methyl vinyl ketone (2a) gave the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pyranones 3 exclusively, while the ratio of the 1
1 pyranones 3 exclusively, while the ratio of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4 increased as the substituent (R2) of the enones 2 changed from electron-donating groups to electron-withdrawing ones (entries 1–4 and 7–10). Moreover, the enones 2e and 2f having α- and β-methyl substituents on the vinyl moiety gave only the pyranones 3 (entries 5 and 6). Diastereomer ratios of 4r–t lowered considerably, compared with those of 4b–d and 4k–n (entries 2–4).
2 adducts 4 increased as the substituent (R2) of the enones 2 changed from electron-donating groups to electron-withdrawing ones (entries 1–4 and 7–10). Moreover, the enones 2e and 2f having α- and β-methyl substituents on the vinyl moiety gave only the pyranones 3 (entries 5 and 6). Diastereomer ratios of 4r–t lowered considerably, compared with those of 4b–d and 4k–n (entries 2–4).
|
|
| Entry |
Enal
1 |
Enone
2 |
Time/h |
Product |
Total yield (%)b,c |
Ratiob,c |
Dr of 4b |
| |
R1 |
|
R2 |
R3 |
R4 |
3, 4 |
(3 + 4) |
(3/4) |
|
|
2/1 = 0.5.
Determined by 1H NMR of the crude mixture.
Yield and ratio based on 2.
2/1 = 2.
Isolated yield.
Carried out at 50 °C.
A single diastereomer.
|
| 1d |
1f
|
Me |
2a
|
Me |
H |
H |
2 |
3q, 4q |
60e |
100/0 |
— |
| 2 |
1f
|
|
2b
|
C6H4OMe-4
|
H |
H |
4 |
3r, 4r |
84 |
48/52 |
53/47 |
| 3 |
1f
|
|
2c
|
Ph |
H |
H |
3 |
3s, 4s |
69 |
38/62 |
61/39 |
| 4 |
1f
|
|
2d
|
C6H4Cl-4
|
H |
H |
15 |
3t, 4t |
33e |
9/91 |
56/44 |
| 5 |
1f
|
|
2e
|
Ph |
Me |
H |
74f |
3u, 4u |
72 |
100/0 |
— |
| 6 |
1f
|
|
2f
|
Ph |
H |
Me |
25 |
3v
g, 4v |
55e |
100/0 |
— |
| 7 |
1g
|
H |
2a
|
Me |
H |
H |
25 |
3w, 4w |
26 |
100/0 |
— |
| 8 |
1g
|
|
2b
|
C6H4OMe-4
|
H |
H |
20 |
3x, 4x |
53 |
77/23 |
— |
| 9 |
1g
|
|
2c
|
Ph |
H |
H |
19 |
3y, 4y |
68 |
65/35 |
— |
| 10 |
1g
|
|
2d
|
C6H4Cl-4
|
H |
H |
2 |
3z, 4z |
89 |
53/47 |
— |
If the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4 were formed from the 1
2 adducts 4 were formed from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts 3 initially produced, it seemed curious that the 3/4 ratio was almost independent of the molar ratio of enal 1 to enone 2 as mentioned above. To address this point, the reaction of 3a with excess 2a was investigated (Scheme 2). Surprisingly, a different 1
1 adducts 3 initially produced, it seemed curious that the 3/4 ratio was almost independent of the molar ratio of enal 1 to enone 2 as mentioned above. To address this point, the reaction of 3a with excess 2a was investigated (Scheme 2). Surprisingly, a different 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct 8a was formed in good yields in the presence of the carbene precursor 5 and Cs2CO3 or DBU, but 4a was not detected at all. This reaction did not proceed with the base only under these conditions.11 The structure of 8a was confirmed by X-ray crystal analysis (Fig. 1). Although the product 8a was not detected in the reaction of 1a with 2a under the standard conditions because of a very slow reaction, it was certainly formed by use of an excess amount of 2a after a longer period together with a little 4a. In addition, the 1
2 adduct 8a was formed in good yields in the presence of the carbene precursor 5 and Cs2CO3 or DBU, but 4a was not detected at all. This reaction did not proceed with the base only under these conditions.11 The structure of 8a was confirmed by X-ray crystal analysis (Fig. 1). Although the product 8a was not detected in the reaction of 1a with 2a under the standard conditions because of a very slow reaction, it was certainly formed by use of an excess amount of 2a after a longer period together with a little 4a. In addition, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct 3c reacted with methyl vinyl ketone (2a) to give a mixture of phenyl- and methyl-substituted pyranones 8b and 8c in 41% and 12% yields, respectively, which may result from a reconstruction of the pyranone ring. Based on these results, it was confirmed that the pyranones 3 could react slowly with additional enones 2 to yield the different 1
1 adduct 3c reacted with methyl vinyl ketone (2a) to give a mixture of phenyl- and methyl-substituted pyranones 8b and 8c in 41% and 12% yields, respectively, which may result from a reconstruction of the pyranone ring. Based on these results, it was confirmed that the pyranones 3 could react slowly with additional enones 2 to yield the different 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 products 8, instead of 4.
2 products 8, instead of 4.
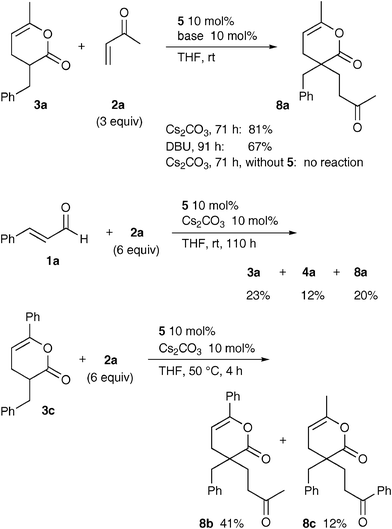 |
| | Scheme 2 Formation of 8 from 3. | |
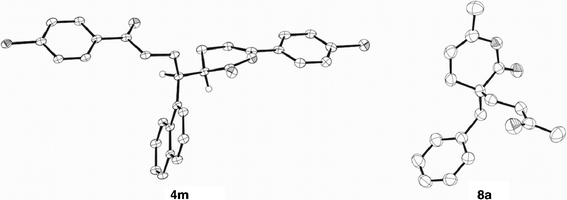 |
| | Fig. 1 ORTEP diagrams of 4m and 8a. H atoms are omitted except for those attached on the stereogenic carbons of 4m. | |
Although further work is necessary to elucidate the reaction mechanism, the scenario depicted in Scheme 3 would explain the formation of the three products 3, 4 and 8. It is well known that imidazole carbene 5’ converts the enal 1 to homoenolateB and then to enolate C or enol by proton transfer or by protonation. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pyranone 3 should be formed by the reaction of C with enone 2via a [4 + 2] process through hetero-Diels–Alder reaction,6,8 or Michael addition followed by acylation (cycle I).7 It is worthwhile to comment that selective reaction of C in competition with B has been shown to need less basic amines for protonation,6–8 whereas nearly all kinds of bases, except for NaH, showed selectivity for 3 in the reaction with methyl vinyl ketone (2a).
1 pyranone 3 should be formed by the reaction of C with enone 2via a [4 + 2] process through hetero-Diels–Alder reaction,6,8 or Michael addition followed by acylation (cycle I).7 It is worthwhile to comment that selective reaction of C in competition with B has been shown to need less basic amines for protonation,6–8 whereas nearly all kinds of bases, except for NaH, showed selectivity for 3 in the reaction with methyl vinyl ketone (2a).
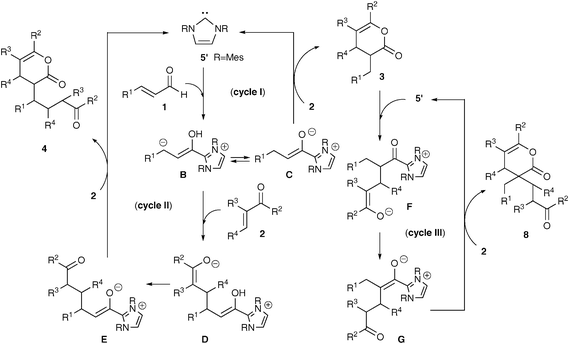 |
| | Scheme 3 Proposed reaction pathways. | |
With respect to the new 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4, Michael addition of B to the enone 2 yields the enolate D, which readily isomerizes to another enolate E (cycle II). Formation of cyclopentanone from D and cyclopentene from E has been reported.2,3 These known reactions, however, did not take place in the present case, except for the reactions with 2f and 2i. On the contrary, the enolate E reacts with another molecule of 2 faster than the aldol reaction, giving rise to 4. The difference would be caused by the substituent effect of 2, because unsubstituted vinyl ketones having electron-withdrawing groups could serve as good Michael acceptors.
2 adducts 4, Michael addition of B to the enone 2 yields the enolate D, which readily isomerizes to another enolate E (cycle II). Formation of cyclopentanone from D and cyclopentene from E has been reported.2,3 These known reactions, however, did not take place in the present case, except for the reactions with 2f and 2i. On the contrary, the enolate E reacts with another molecule of 2 faster than the aldol reaction, giving rise to 4. The difference would be caused by the substituent effect of 2, because unsubstituted vinyl ketones having electron-withdrawing groups could serve as good Michael acceptors.
The pathway to the other 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 8 would include the carbene-catalyzed ring opening reaction of the pyranone 3 to yield the intermediate F (cycle III).12Isomerization of F to G, followed by formal [4 + 2] reaction yields the products 8. Formation of the mixture of 8b and 8c in the reaction of 3c with 2a would provide additional support for this process, wherein the phenyl vinyl ketone moiety in the pyranone ring of 3c was substituted by methyl vinyl ketone in 8c.
2 adducts 8 would include the carbene-catalyzed ring opening reaction of the pyranone 3 to yield the intermediate F (cycle III).12Isomerization of F to G, followed by formal [4 + 2] reaction yields the products 8. Formation of the mixture of 8b and 8c in the reaction of 3c with 2a would provide additional support for this process, wherein the phenyl vinyl ketone moiety in the pyranone ring of 3c was substituted by methyl vinyl ketone in 8c.
Conclusions
We have found that the imidazole carbene-catalyzed reaction of aromatic and aliphatic enals 1 with unsubstituted vinyl ketones 2 gives new 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4 as well as 1
2 adducts 4 as well as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 products 3. The former products 4 would be formed from homoenolates, and the latter 3 from enolates. The selectivity of the reaction, i.e., ratio of 3/4, was determined mainly by the substituent effect of 2, not so much by the base nor the molar ratio of the two reactants. When the enones 2 acted as good Michael acceptors, 4 predominated over 3, though selectivities were not high. Furthermore, the 1
1 products 3. The former products 4 would be formed from homoenolates, and the latter 3 from enolates. The selectivity of the reaction, i.e., ratio of 3/4, was determined mainly by the substituent effect of 2, not so much by the base nor the molar ratio of the two reactants. When the enones 2 acted as good Michael acceptors, 4 predominated over 3, though selectivities were not high. Furthermore, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 products 3 proved to react very slowly with another molecule of the enones 2, giving rise to other 1
1 products 3 proved to react very slowly with another molecule of the enones 2, giving rise to other 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 products 8.
2 products 8.
Experimental
General
NMR spectra were recorded on 400 MHz or 500 MHz spectrometers using CDCl3 solvent. Mass spectra (EI) were obtained at 70 eV on a GC-MS apparatus. High resolution mass spectra were recorded by EI or ESI mode. Melting points were uncorrected. Solvents were distilled from sodium/benzophenone ketyl or CaH2 under N2. Amines such as Et3N, DMAP, and DBU were distilled from molecular sieves 4 Å. Cs2CO3 was dried under vacuum with heating. All enals 1 and enones 2 were distilled under N2 prior to use. Carbene precursors 5 and 6 were prepared according to the literature,13,14 and 7 was purchased. Other reagents were used as provided without further purification.
General procedure for the reaction of α,β-unsaturated aldehydes 1 with α,β-unsaturated ketones 2.
Imidazolium chloride
5 (36 mg, 0.1 mmol) and enone 2 (2 mmol) were placed in a 20 mL Schlenk tube under nitrogen, and dissolved in THF (3 mL). Then, enal 1 (1 mmol) and Cs2CO3 (34 mg, 0.1 mmol) were added to the tube. The mixture was stirred at room temperature for an appropriate time, monitoring by TLC or GC. The reaction was quenched with water (ca. 10 mL), and diphenylmethane was added to the mixture as an internal standard. The organic layer was extracted with diethyl ether, washed with brine, dried over MgSO4, and concentrated in vacuo. Yields and ratios of the products were determined using 1H NMR. Then, the crude products were purified by column chromatography on silica gel with hexane–ethyl acetate eluent (10/1 to 5/1). The two products 3 and 4 were easily isolated as pure compounds, judged by TLC and NMR spectra, but their diastereomers were inseparable from one other.
3-Benzyl-6-methyl-3,4-dihydro-2H-pyran-2-one (3a).
Yellow oil; Rf (hexane/EtOAc = 10/1) 0. 35; 1H NMRδ 1.77 (3H, s), 1.78–2.06 (2H, m), 2.54–2.67 (2H, m), 3.25 (1H, dd, J = 12.5, 3.0 Hz), 4.80–4.83 (1H, m), 7.01–7.23 (5H, m); 13C NMRδ 18.3, 23.5, 35.6, 40.0, 99.4, 126.4, 128.4, 128.9, 138.3, 149.4, 171.1; MSm/z (%) 202 (M+, 16), 174 (18), 131 (31), 110 (100) 104 (87); HRMS calcd for C13H14O2 (M+) 202.0994, found 202.0996.
3-Benzyl-6-(4-methoxyphenyl)-3,4-dihydro-2H-pyran-2-one (3b).
Yellow oil; 1H NMRδ 2.14–2.22 (2H, m), 2.59–2.72 (2H, m), 3.29 (1H, dd, J = 13.2, 3.6 Hz), 3.69 (3H, s), 5.48 (1H, dd, J = 5.9, 3.3 Hz), 6.71–7.73 (9H, m); 13C NMRδ 24.0, 35.7, 40.2, 55.3, 97.8, 113.8, 125.0, 125.8, 126.6, 128.6, 129.1, 138.4, 150.3, 160.1, 171.0; HRMS calcd for C19H18O3 (M+) 294.1256, found 294.1257.
3-Benzyl-6-phenyl-3,4-dihydro-2H-pyran-2-one (3c).
Yellow oil; 1H NMRδ 2.33 (1H, ddd, J = 17.4, 10.9, 3.6 Hz), 2.39 (1H, dt, J = 17.4, 6.1 Hz), 2.76 (1H, dd, J = 13.2, 9.9 Hz), 2.80–2.93 (1H, m), 3.43 (1H, dd, J = 13.2, 3.9 Hz), 5.74 (1H, dd, J = 6.1, 3.6 Hz), 7.20–7.61 (10H, m); 13C NMRδ 24.1, 35.7, 40.1, 99.8, 124.4, 126.7, 128.5, 128.6, 128.9, 129.1, 132.3, 138.3, 150.4, 170.8; HRMS calcd for C18H16O2 (M+) 264.1150, found 264.1148.
3-Benzyl-6-(4-chlorophenyl)-3,4-dihydro-2H-pyran-2-one (3d).
Yellow oil; 1H NMRδ 2.33 (1H, ddd, J = 17.4, 11.6, 3.5 Hz), 2.39 (1H, dt, J = 17.4, 6.2 Hz), 2.76 (1H, dd, J = 13.5, 9.7 Hz), 2.83–2.92 (1H, m), 3.42 (1H, dd, J = 13.5, 3.8 Hz), 5.76 (1H, dd, J = 6.2, 3.5 Hz), 7.20–7.54 (9H, m); 13C NMRδ 24.1, 35.8, 40.0, 100.3, 125.7, 126.7, 128.6, 128.7, 129.1, 130.8, 134.8, 138.2, 149.6, 170.4; HRMS calcd for C18H15ClO2 (M+) 298.0761, found 298.0761.
3-Benzyl-5-methyl-6-phenyl-3,4-dihydro-2H-pyran-2-one (3e).
Yellow oil; 1H NMRδ 1.80 (3H, s), 2.24 (2H, d, J = 9.8 Hz), 2.77 (1H, dd, J = 13.8, 9.8 Hz), 2.91 (1H, qd, J = 9.8, 4.6 Hz), 3.41 (1H, dd, J = 13.8, 4.6 Hz), 7.22–7.43 (10H, m); 13C NMRδ 18.2, 31.0, 35.7, 40.1, 110.5, 126.7, 128.1, 128.50, 128.57, 128.61, 129.1, 133.1, 138.5, 145.4, 171.0; MSm/z (%) 278 (M+, 4), 187 (100), 131 (25), 105 (72); HRMS calcd for C19H18O2 (M+) 278.1307, found 278.1312.
3-Benzyl-4-methyl-6-phenyl-3,4-dihydro-2H-pyran-2-one (3f).
Obtained as a mixture of two diastereomers (78/22). Yellow oil; Rf (hexane/EtOAc = 5/1) 0.6; 1H NMR (major) δ 1.13 (3H, d, J = 7.2 Hz), 2.45 (1H, br sextet, J = 6.2 Hz), 2.76 (1H, br q, J = 6.2 Hz), 2.92 (1H, dd, J = 13.8, 7.6 Hz), 3.05 (1H, dd, J = 13.8, 6.3 Hz), 5.73 (1H, d, J = 5.3 Hz), 7.21–7.64 (10H, m), (minor, distinguishable signals) δ 1.08 (3H, d, J = 7.2 Hz), 2.45–2.50 (1H, m), 2.74–2.80 (1H, m), 3.00–3.09 (1H, m), 3.43 (1H, dd, J = 14.4, 5.4 Hz), 5.89 (1H, br d, J = 7.2 Hz); 13C NMR (major) δ 20.1, 29.7, 35.2, 47.9, 104.5, 124.4, 126.7, 128.4, 128.5, 128.9, 129.1, 132.2, 137.8, 148.7, 170.3, (minor, distinguishable signals) δ 14.2, 28.0, 32.1, 45.6, 107.3, 170.8; MSm/z (%) 278 (M+, 4), 187 (100), 131 (25), 105 (72); HRMS calcd for C19H18O2 (M+) 278.1307, found 278.1302.
3-Benzyl-6-methyl-4-phenyl-3,4-dihydro-2H-pyran-2-one (3g).
Obtained as a mixture of two diastereomers (71/29). Yellow oil; 1H NMR (major) δ 2.00 (3H, s), 2.76–3.22 (3H, m), 3.27–3.36 (1H, m), 5.03 (1H, d, J = 4.6 Hz), 7.00–7.24 (10H, m), (minor, distinguishable signals) δ 1.96 (3H, s), 5.21 (1H, br d, J = 6.3 Hz); 13C NMR (major) δ 18.6, 35.8, 41.0, 48.5, 102.4, 126.4, 127.2, 128.2, 128.4, 128.8, 129.1, 138.0, 141.5, 149.2, 170.2, (minor, distinguishable signals) δ 32.2, 40.3, 45.5, 105.6, 170.7; HRMS calcd for C19H18O2 (M+) 278.1307, found 278.1299.
6-Methyl-3-(1-naphthylmethyl)-3,4-dihydro-2H-pyran-2-one (3j).
White solid; mp 68–69 °C; Rf (hexane/EtOAc = 10/1) 0. 35; 1H NMRδ 1.89 (3H, s), 1.98–2.12 (2H, m), 2.93 (1H, qd, J = 10.0, 3.0 Hz), 3.00 (1H, dd, J = 13.0, 10.0 Hz), 4.01 (1H, dd, J = 13.0, 3.0 Hz), 4.88 (1H, br s), 7.32 (1H, d, J = 7.0 Hz), 7.41 (1H, t, J = 7.0 Hz), 7.51 (1H, t, J = 7.0 Hz), 7.54 (1H, t, J = 7.0 Hz), 7.78 (1H, d, J = 7.0 Hz), 7.89 (1H, d, J = 7.0 Hz), 8.03 (1H, d, J = 7.0 Hz); 13C NMRδ 18.5, 24.0, 33.0, 39.2, 99.5, 123.4, 125.3, 125.7, 126.2, 127.45, 127.49, 128.9, 131.6, 133.9, 134.4, 149.6, 171.4; MSm/z (%) 252 (M+, 8), 154 (43), 141 (95), 111 (100); HRMS calcd for [C17H16O2 (M)+Na]+ 275.1043, found 275.1042.
6-(4-Methoxyphenyl)-3-(1-naphthylmethyl)-3,4-dihydro-2H-pyran-2-one (3k).
Yellow oil; Rf (hexane/EtOAc = 5/1) 0. 35; 1H NMRδ 2.19–2.23 (2H, m), 3.00–3.07 (2H, m), 3.79 (3H, s), 4.02–4.07 (1H, m), 5.50 (1H, t, J = 4.6 Hz), 6.87 (2H, d, J = 8.8 Hz), 7.32 (1H, d, J = 7.6 Hz), 7.42 (1H, t, J = 7.6 Hz), 7.47–7.54 (4H, m), 7.77 (1H, d, J = 8.4 Hz), 7.87 (1H, d, J = 8.8 Hz), 8.03 (1H, d, J = 8.0 Hz); 13C NMRδ 24.2, 32.8, 39.1, 55.1, 97.7, 113.6, 123.2, 124.7, 125.2, 125.6, 126.1, 127.38, 127.41, 128.8, 131.5, 133.8, 134.2, 150.0, 159.9, 170.9; HRMS calcd for [C23H20O3(M)+Na]+ 367.1305, found 367.1298.
6-(4-Chlorophenyl)-3-(1-naphthylmethyl)-3,4-dihydro-2H-pyran-2-one (3m).
White solid; mp 79–80 °C; Rf (hexane/EtOAc = 5/1) 0.46; 1H NMRδ 2.28–2.31 (2H, m), 3.01–3.10 (2H, m), 4.06 (1H, q, J = 9.7 Hz), 5.69 (1H, t, J = 4.8 Hz), 7.32–7.55 (8H, m), 7.79 (1H, d, J = 8.0 Hz), 7.89 (1H, d, J = 8.0 Hz), 8.02 (1H, d, J = 8.0 Hz); 13C NMRδ 24.4, 33.1, 39.1, 100.3, 123.3, 125.4, 125.7, 125.8, 126.4, 127.6, 127.7, 128.7, 129.0, 130.7, 131.6, 134.0, 134.1, 134.7, 147.6, 170.8; HRMS calcd for [C22H17ClO2 (M)+Na]+ 371.0809, found 371.0799.
3-(4-Methoxyphenylmethyl)-6-methyl-3,4-dihydro-2H-pyran-2-one (3n).
Colorless oil; Rf (hexane/EtOAc = 5/1) 0. 40; 1H NMRδ 1.86 (3H, s), 1.96–2.02 (1H, m), 2.10–2.16 (1H, m), 2.62–2.70 (2H, m), 3.26 (1H, dd, J = 13.0, 3.0 Hz), 3.78 (3H, s), 4.91 (1H, br s), 6.83 (2H, d, J = 8.5 Hz), 7.09 (2H, d, J = 8.5 Hz); 13C NMRδ 18.4, 23.5, 34.8, 40.2, 55.1, 99.5, 113.8, 130.0, 130.3, 149.5, 158.2, 171.3; HRMS calcd for [C14H16O3(M)+Na]+ 255.0992, found 255.0989.
3-(4-Chlorophenylmethyl)-6-methyl-3,4-dihydro-2H-pyran-2-one (3o).
Yellow oil; Rf (hexane/EtOAc = 5/1) 0. 43; 1H NMRδ 1.85 (3H, br s), 1.96–2.02 (1H, m), 2.11 (1H, dt, J = 17.0, 6.0 Hz), 2.65–2.73 (2H, m), 3.27 (1H, dd, J = 17.0, 8.5 Hz), 4.91 (1H, sextet, J = 1.5 Hz), 7.11 (2H, d, J = 8.0 Hz), 7.24 (2H, d, J = 8.0 Hz); 13C NMRδ 18.4, 23.6, 35.0, 39.9, 99.4, 128.5, 130.4, 132.3, 136.9, 149.6, 170.8; MSm/z (%) 236 (M+, 22), 131 (100), 125 (54), 111 (61); HRMS calcd for [C13H13ClO2(M)+Na]+ 259.0496, found 259.0494.
3-(2,4-Dichlorophenylmethyl)-6-methyl-3,4-dihydro-2H-pyran-2-one (3p).
White solid; mp 101–103 °C; Rf (hexane/EtOAc = 10/1) 0.5; 1H NMRδ 1.87 (3H, s), 2.08–2.16 (2H, m), 2.77–2.87 (2H, m), 3.42 (1H, dd, J = 13.5, 4.5 Hz), 4.94 (1H, br s), 7.18 (1H, d, J = 8.0 Hz), 7.22 (1H, d, J = 8.0 Hz), 7.36 (1H, s); 13C NMRδ 18.4, 24.0, 33.1, 38.4, 99.5, 127.1, 129.3, 132.3, 133.1, 134.8, 135.0, 149.7, 170.8; MSm/z (%) 270 (M+, 1), 236 (42), 165 (100), 159 (43), 101 (29); HRMS calcd for [C13H12Cl2O2(M)+Na]+ 293.0107, found 293.0102.
3-Ethyl-6-methyl-3,4-dihydro-2H-pyran-2-one (3q).
Colorless oil; Rf (hexane/EtOAc = 5/1) 0.5; 1H NMRδ 1.00 (3H, t, J = 7.5 Hz), 1.53 (1H, dquin, J = 14.5, 7.5 Hz), 1.86 (3H, d, J = 1.0 Hz), 1.92 (1H, dquin, J = 14.5, 7.5 Hz), 2.02–2.10 (1H, m), 2.33 (1H, dt, J = 16.5, 7.5 Hz), 2.41 (1H, dq, J = 11.0, 7.5 Hz), 5.00 (1H, m); 13C NMRδ 11.3, 18.5, 23.0, 24.0, 39.8, 99.5, 149.4, 171.7; MSm/z (%) 140 (M+, 21), 112 (12); HRMS calcd for [C8H12O2(M)+Na]+ 163.0730, found 163.0726.
3-Ethyl-6-(4-methoxyphenyl)-3,4-dihydro-2H-pyran-2-one (3r).
Colorless oil; Rf (hexane/EtOAc = 20/1) 0.15; 1H NMRδ 1.03 (3H, t, J = 7.5 Hz), 1.55–1.64 (1H, m), 1.93–2.02 (1H, m), 2.23–2.31 (1H, m), 2.48–2.58 (2H, m), 3.81 (3H, s), 5.65 (1H, dd, J = 5.5, 3.5 Hz), 6.87 (2H, d, J = 8.5 Hz), 7.53 (2H, d, J = 8.5 Hz); 13C NMRδ 11.3, 23.0, 24.4, 39.8, 55.3, 97.9, 113.7, 125.1, 125.7, 150.0, 160.0, 171.3; MSm/z (%) 232 (M+, 27), 162 (15), 135 (100); HRMS calcd for [C14H16O3(M)+H]+ 233.1172, found 233.1171.
3-Ethyl-6-phenyl-3,4-dihydro-2H-pyran-2-one (3s).
Yellow oil; Rf (hexane/EtOAc = 10/1) 0.4; 1H NMRδ 1.05 (3H, t, J = 7.6 Hz), 1.57-1.69 (1H, m),1.93–2.05 (1H, m), 2.26–2.37 (1H, m) 2.51–2.63 (2H, m), 5.80 (1H, dd, J = 5.6, 3.6 Hz), 7.31–7.39 (3H, m), 7.59–7.62 (2H, m); 13C NMRδ 11.4, 23.0, 24.5, 39.8, 99.8, 124.4, 128.4, 128.8, 132.5, 150.3, 171.1; MSm/z (%) 202 (M+, 35), 133 (41), 105 (100); HRMS calcd for [C13H14O2(M)+H]+ 203.1067, found 203.1067.
6-(4-Chlorophenyl)-3-ethyl-3,4-dihydro-2H-pyran-2-one (3t).
Colorless oil; Rf (hexane/EtOAc = 10/1) 0.3; 1H NMRδ 1.02 (3H, t, J = 7.3 Hz), 1.57–1.67 (1H, m), 1.94–2.03 (1H, m), 2.28–2.33 (1H, m), 2.51–2.61 (2H, m), 5.79 (1H, dd, J = 5.7, 3.7 Hz), 7.33 (2H, d, J = 8.5 Hz) 7.52 (2H, d, J = 8.5 Hz); 13C NMRδ 11.3, 23.0, 24.5, 39.7, 100.3, 125.7, 128.7, 130.9, 134.6, 149.3, 170.8; HRMS calcd for [C13H13ClO2(M)+Na]+ 259.0496, found 259.0495.
3-Ethyl-5-methyl-6-phenyl-3,4-dihydro-2H-pyran-2-one (3u).
Yellow oil; Rf (hexane/EtOAc = 30/1) 0.1; 1H NMRδ 1.05 (3H, t, J = 7.0 Hz), 1.61 (1H, dquin, J = 21.5, 7.0 Hz), 1.88 (3H, s), 1.98 (1H, dquin, J = 21.5, 7.0 Hz), 2.30 (1H, dd, J = 16.5, 11.5 Hz), 2.43 (1H, dd, J = 16.5, 7.0 Hz), 2.57 (1H, dq, J = 11.5, 7.0 Hz), 7.31–7.44 (5H, m); 13C NMRδ 11.4, 18.3, 22.8, 31.4, 39.8, 110.6, 128.0, 128.4, 128.5, 133.2, 145.1, 171.4; MSm/z (%) 216 (M+, 22), 147 (67), 105 (100); HRMS calcd for [C14H16O2(M)+Na]+ 239.1048, found 239.1036.
3-Ethyl-4-methyl-6-phenyl-3,4-dihydro-2H-pyran-2-one (3v).
Obtained as a single diastereomer. Yellow oil; Rf (hexane/EtOAc = 10/1) 0.4; 1H NMRδ 1.03 (3H, t, J = 7.5 Hz), 1.19 (3H, d, J = 7.0 Hz), 1.76 (2H, br quin, J = 7.0 Hz), 2.38 (1H, q, J = 7.0 Hz), 2.58 (1H, br sextet, J = 7.0 Hz), 5.71 (1H, d, J = 4.5 Hz), 7.33–7.62 (5H, m); 13C NMRδ 11.1, 20.1, 22.2, 30.4, 47.5, 105.5, 124.4, 128.4, 128.8, 132.2, 148.4, 170.8; MSm/z (%) 216 (M+, 13), 147 (36), 105 (100); HRMS calcd for [C14H16O2(M)+Na]+ 239.1048, found 239.1043.
3,6-Dimethyl-3,4-dihydro-2H-pyran-2-one (3w).
Colorless oil; Rf (hexane/EtOAc = 5/1) 0.5; 1H NMRδ 1.26 (3H, d, J = 7.0 Hz), 1.86 (3H, br s), 2.06 (1H, ddd, J = 16.5, 12.5, 2.5 Hz), 2.28 (1H, dt, J = 16.5, 7.0 Hz), 2.57 (1H, dquin, J = 12.5, 7.0 Hz), 4.96 (1H, br s); 13C NMRδ 15.4, 18.5, 26.9, 33.4, 99.7, 149.7, 172.4; MSm/z (%) 126 (M+, 11), 115 (65); HRMS calcd for [C7H10O2(M)+Na]+ 149.0573, found 149.0572.
6-(4-Methoxyphenyl)-3-methyl-3,4-dihydro-2H-pyran-2-one (3x).
Colorless oil; Rf (hexane/EtOAc = 5/1) 0.4; 1H NMRδ 1.34 (3H, d, J = 7.0 Hz), 2.28 (1H, ddd, J = 17.5, 12.5, 3.0 Hz), 2.51 (1H, dt, J = 17.5, 7.0 Hz), 2.70 (1H, dquin, J = 12.5, 7.0 Hz), 3.82 (3H, s), 5.66 (1H, dd, J = 7.0, 3.0 Hz), 6.88 (2H, d, J = 8.5 Hz), 7.52 (2H, d, J = 8.5 Hz); 13C NMRδ 15.4, 27.4, 33.6, 55.3, 98.1, 113.8, 125.8, 131.0, 150.4, 160.1, 172.1; HRMS calcd for [C13H14O3(M)+H]+ 219.1016, found 219.1010.
3-Methyl-6-phenyl-3,4-dihydro-2H-pyran-2-one (3y).
Yellow oil; 1H NMRδ 1.35 (3H, d, J = 7.0 Hz), 2.31 (1H, ddd, J = 17.0, 12.5, 3.5 Hz), 2.54 (1H, dt, J = 17.0, 7.0 Hz), 2.71 (1H, dquin, J = 12.5, 7.0 Hz), 5.81 (1H, dd, J = 7.0, 3.5 Hz), 7.31–7.38 (3H, m), 7.61 (2H, br d, J = 7.0 Hz); 13C NMRδ 15.4, 27.3, 33.5, 100.1, 124.4, 128.4, 128.8, 132.4, 150.5, 171.9; MSm/z (%) 188 (M+, 1), 160 (14), 120 (10), 105 (100); HRMS calcd for [C12H12O2(M)+H]+ 189.0910, found 189.0906.
6-(4-Chlorophenyl)-3-methyl-3,4-dihydro-2H-pyran-2-one (3z).
White solid; mp 83–84 °C; Rf (hexane/EtOAc = 5/1) 0.4; 1H NMRδ 1.35 (3H, d, J = 7.0 Hz), 2.31 (1H, ddd, J = 17.0, 12.5, 3.0 Hz), 2.55 (1H, dt, J = 17.0, 7.0 Hz), 2.72 (1H, dquin, J = 12.5, 7.0 Hz), 5.80 (1H, dd, J = 6.0, 3.0 Hz), 7.34 (2H, d, J = 8.5 Hz), 7.53 (2H, d, J = 8.5 Hz); 13C NMRδ 15.4, 17.4, 33.4, 100.5, 125.7, 128.7, 130.9, 134.7, 149.7, 171.6.
6-Methyl-3-(4-oxo-1-phenylpentyl)-3,4-dihydro-2H-pyran-2-one (4a).
Two diastereomers were formed in a ratio of ca. 76/24, but the minor was inseparable from the mixture. Major: yellow oil; Rf (hexane/EtOAc = 5/1) 0.15; 1H NMRδ 1.82 (3H, br s), 1.85–2.01 (3H, m), 1.95 (3H, s), 2.01–2.21(3H, m), 2.64 (1H, dd, J = 15.4, 8.7 Hz), 2.96 (1H, td, J = 10.2, 2.9 Hz), 4.76 (1H, br s), 7.06–7.25 (5H, m); 13C NMRδ 18.3, 22.6, 28.0, 29.8, 41.5, 43.9, 44.3, 99.7, 127.0, 128.3, 128.6, 140.3, 149.4, 170.7, 208.8; MSm/z (%) 272 (M+, 1), 201 (2), 168 (8), 142 (27), 131 (36), 111 (100); HRMS calcd for [C17H20O3(M)+Na]+ 295.1305, found 295.1305. Anal. Calcd for C17H20O3: C, 74.97; H, 7.40. Found: C, 74.83; H, 7.18. Minor (distinguishable signals): 1H NMRδ 1.75 (3H, br s), 1.99 (3H, s), 4.86 (1H, br s); 13C NMRδ 141.0, 149.1, 170.2, 208.4.
6-(4-Methoxyphenyl)-3-(4-(4-methoxyphenyl)-4-oxo-1-phenylbutyl)-3,4-dihydro-2H-pyran-2-one (4b).
Obtained as a mixture of two diastereomers (88/12). Yellow oil; 1H NMR (major) δ 1.95 (1H, ddd, J = 17.4, 8.4, 4.9 Hz), 2.07–2.15 (1H, m), 2.22–2.34 (2H, m), 2.58 (1H, ddd, J = 16.8, 10.4, 4.9 Hz), 2.70–2.76 (1H, m), 2.78–2.83 (1H, m), 3.12–3.16 (1H, m), 3.75 (3H, s), 3.76 (3H, s), 5.44 (1H, t, J = 2.5 Hz), 6.78 (2H, d, J = 8.9 Hz), 6.82 (2H, d, J = 8.9 Hz), 7.14–7.26 (5H, m), 7.45 (2H, d, J = 8.9 Hz), 7.70 (2H, d, J = 8.9 Hz); 13C NMR (major) δ 23.1, 28.9, 36.3, 44.2, 44.5, 55.26, 55.34, 97.4, 113.5, 113.8. 124.9, 125.8, 127.1, 128.5, 128.7, 130.1, 132.3, 140.4, 150.1, 160.1, 163.3, 170.3, 198.1, (minor, distinguishable signals) δ 21.5, 25.1, 36.5, 44.0, 45.2, 55.24, 55.36, 98.0. Anal. Calcd for C29H28O5: C, 76.30; H, 6.18. Found: C, 76.60; H, 5.77.
3-(1,4-Diphenyl-4-oxobutyl)-6-phenyl-3,4-dihydro-2H-pyran-2-one (4c).
Obtained as a mixture of two diastereomers (91/9). Yellow oil; 1H NMR (major) δ 1.94 (1H, ddd, J = 17.7, 8.2, 4.9 Hz), 2.07–2.14 (1H, m), 2.22 (1H, dt, J = 17.4, 5.7 Hz), 2.29–2.37 (1H, m), 2.61 (1H, ddd, J = 17.1, 9.8, 4.6 Hz), 2.73 (1H, dd, J = 9.8, 6.1 Hz), 2.76–2.81 (1H, m), 3.13 (1H, ddd, J = 11.0, 9.2, 4.0 Hz), 5.55 (1H, t, J = 4.9 Hz), 7.11–7.40 (11H, m), 7.49 (2H, d, J = 8.0 Hz), 7.68 (2H, d, J = 8.0 Hz); 13C NMR (major) δ 22.9, 28.4, 36.4, 43.8, 44.1, 99.2, 124.14, 127.0. 127.6, 128.18, 128.22, 128.3, 128.56, 131.98, 132.6, 136.5, 140.1, 150.0, 169.9, 199.2; (minor, distinguishable signals) δ 21.2, 24.7, 36.7, 43.7, 44.8, 99.9, 124.09, 126.9, 127.7. 128.10, 128.51, 128.60, 132.03, 132.7, 140.8, 149.7, 169.5, 199.4.
6-(4-Chlorophenyl)-3-(4-(4-chlorophenyl)-4-oxo-1-phenylbutyl)-3,4-dihydro-2H-pyran-2-one (4d).
Obtained as a single diastereomer. Yellow oil; 1H NMRδ 2.04 (1H, ddd, J = 17.4, 8.7, 4.8 Hz), 2.12–2.22 (1H, m), 2.30–2.44 (2H, m), 2.68 (1H, ddd, J = 17.4, 9.7, 4.8 Hz), 2.78–2.92 (2H, m), 3.15–3.21 (1H, m), 5.64 (1H, t, J = 4.8 Hz), 7.19–7.73 (13H, m); 13C NMRδ 23.2, 28.6, 36.5, 44.1, 44.3, 99.8, 125.2, 127.4. 128.4, 128.7, 128.8, 128.9, 129.3, 130.6, 134.8, 135.0, 139.3, 140.1, 149.5, 169.9, 198.2.
6-(4-Methoxyphenyl)-3-(4-(4-methoxyphenyl)-1-(1-naphthyl)-4-oxobutyl)-3,4-dihydro-2H-pyran-2-one (4k).
Obtained as a single diastereomer. Colorless oil; Rf (hexane/EtOAc = 5/1) 0.1; 1H NMRδ 1.85–1.90 (1H, m), 2.21–2.25 (1H, m), 2.33–2.38 (1H, m), 2.53–2.62 (2H, m), 2.71–2.77 (1H, m), 3.00–3.06 (1H, m), 3.72 (3H, br s), 3.82 (3H, br s), 4.12–4.18 (1H, m), 5.34 (1H, d, J = 4.4 Hz), 6.75 (2H, dd, J = 8.6, 3.4 Hz), 6.91 (2H, d, J = 8.6 Hz), 7.48–7.51 (4H, m), 7.55 (2H, d, J = 8.6 Hz), 7.64 (2H, dd, J = 8.6, 1.6 Hz), 7.79 (1H, d, J = 8.6 Hz), 7.87 (1H, m), 8.05–8.07 (1H, m); 13C NMRδ 23.6, 29.9, 35.8, 36.7, 45.2, 55.19, 55.21, 97.4, 113.3, 113.7, 122.8, 123.6, 124.8, 125.6, 125.7, 125.8, 126.2, 127.4. 129.0, 129.6, 130.0, 133.2, 133.7, 137.5, 149.9, 160.0, 163.0, 170.5, 198.0; HRMS calcd for [C33H30O5(M)+Na]+ 529.1985, found 529.1970.
3-(1-(1-Naphthyl)-4-oxo-4-phenylbutyl)-6-phenyl-3,4-dihydro-2H-pyran-2-one (4l).
Obtained as a mixture of two diastereomers (77/23). Yellow oil; Rf (hexane/EtOAc = 5/1) 0.2; 1H NMR (major) δ 1.90 (1H, dt, J = 17.6, 6.6 Hz), 2.25 (1H, dt, J = 17.2, 5.2 Hz), 2.34 (1H, q, J = 10.1 Hz), 2.57–2.66 (2H, m), 2.78 (1H, q, J = 10.8 Hz), 3.04 (1H, dt, J = 10.0, 6.4 Hz), 4.15 (1H, tm, J = 10.3 Hz), 5.49 (1H, t, J = 4.6 Hz), 7.25–7.31 (3H, m), 7.34–7.42 (3H, m), 7.46–7.52 (3H, m), 7.54–7.65 (3H, m), 7.18 (1H, d, J = 8.0 Hz), 7.86–7.89 (1H, m), 8.02–8.04 (1H, m), (minor, distinguishable signals) δ 4.46 (1H, d, J = 11.6 Hz), 5.73 (1H, dd, J = 6.8, 2.8 Hz); 13C NMR (major) δ 23.8, 29.7, 36.3, 36.8, 45.3, 99.5, 122.8, 123.7, 124.5, 125.7, 125.8, 126.3, 127.6. 127.8, 127.9, 128.3, 128.37, 128.44, 128.5, 128.9, 129.1, 132.7, 136.6, 137.5, 150.3, 170.4, 199.6, (minor, distinguishable signals) δ 22.6, 24.1, 31.6, 37.2, 43.8, 100.5, 199.9; HRMS calcd for [C31H26O3(M)+Na]+ 469.1774, found 469.1760.
6-(4-Chlorophenyl)-3-(4-(4-chlorophenyl)-1-(1-naphthyl)-4-oxobutyl)-3,4-dihydro-2H-pyran-2-one (4m).
Obtained as a single diastereomer. White solid; mp 91–92 °C; Rf (hexane/EtOAc = 5/1) 0.3; 1H NMRδ 1.85 (1H, dt, J = 17.6, 6.0 Hz), 2.18 (1H, dt, J = 17.6, 4.8 Hz), 2.29 (1H, t, J = 10.2 Hz), 2.51–2.71 (3H, m), 2.99 (1H, dd, J = 16.0, 6.4 Hz), 4.10 (1H, t, J = 9.4 Hz), 5.40 (1H, t, J = 4.2 Hz), 7.16 (2H, d, J = 8.0 Hz), 7.28 (2H, d, J = 8.0 Hz), 7.42–7.50 (8H, m), 7.74 (1H, d, J = 7.6 Hz), 7.83 (1H, d, J = 8.0 Hz), 7.95 (1H, d, J = 8.4 Hz), 13C NMRδ 23.6, 29.5, 36.0, 36.6, 44.9, 99.9, 122.5, 123.7, 125.6, 125.69, 125.72, 126.3, 127.6. 128.46, 128.51, 128.6, 129.7, 130.5, 133.1, 133.7, 134.6, 134.7, 137.1, 139.0, 149.2, 170.0, 198.2; HRMS calcd for [C31H24Cl2O3(M)+Na]+ 537.0995, found 537.0981.
3-(1-(4-Methoxyphenyl)-4-oxopentyl)-6-methyl-3,4-dihydro-2H-pyran-2-one (4n).
Obtained as a single diastereomer. Colorless oil; Rf (hexane/EtOAc = 5/1) 0.11; 1H NMRδ 1.72–1.79 (1H, m), 1.83 (3H, br s), 1.86–1.91 (1H, m), 1.98 (3H, s), 2.01–2.14 (2H, m), 2.16–2.20 (1H, m), 2.22–2.28 (1H, m), 2.64 (1H, q, J = 7.8 Hz), 2.97 (1H, td, J = 8.5, 4.0 Hz), 3.76 (3H, s), 4.80 (1H, t, J = 4.5 Hz), 6.82 (2H, d, J = 8.5 Hz), 7.03 (2H, d, J = 8.5 Hz); 13C NMRδ 18.3, 22.5, 28.1, 29.7, 41.6, 42.8, 44.4, 55.1, 98.9, 113.9, 129.3, 132.0, 149.3, 158.5, 170.7, 208.2; MSm/z (%) 302 (M+, 1), 181(27), 121 (100); HRMS calcd for [C18H22O4(M)+Na]+ 325.1416, found 325.1406.
6-(4-Methoxyphenyl)-3-(5-(4-methoxyphenyl)-5-oxo-2-pentyl)-3,4-dihydro-2H-pyran-2-one (4r).
Obtained as a mixture of two diastereomers (53/47). Yellow oil; Rf (hexane/EtOAc = 5/1) 0.22; 1H NMR (major) δ 1.09 (3H, d, J = 7.0 Hz), 1.62–1.69 (1H, m), 1.92–2.00 (1H, m), 2.15–2.24 (1H, m), 2.42–2.48 (3H, m), 2.90–3.06 (2H, m), 3.81–3.87 (6H, two s), 5.66–5.70 (1H, m), 6.88 (2H, d, J = 8.5 Hz), 6.93 (2H, br d, J = 8.5 Hz), 7.52 (2H, d, J = 8.5 Hz), 7.95 (2H, d, J = 8.5 Hz), (minor, distinguishable signals) δ 1.05 (3H, d, J = 7.0 Hz), 1.69–1.78 (1H, m), 1.81–1.87 (1H, m), 2.32–2.39 (1H, m), 2.58–2.69 (3H, m), 2.90–3.06 (2H, m); 13C NMR (major) δ 17.2, 21.2, 28.0, 32.1, 36.4, 44.0, 55.3, 98.1, 113.7, 125.0, 125.8, 129.8, 130.3, 149.83, 160.0. 163.41, 170.4, 198.6, (minor, distinguishable signals) δ 15.6, 19.6, 28.9, 31.7, 36.2, 43.0, 55.4, 98.3, 170.7, 198.4; MSm/z (%) 394 (M+, 1), 205 (13), 107 (16); HRMS calcd for [C24H26O5(M)+Na]+ 417.1672, found 417.1661.
3-(5-Oxo-5-phenyl-2-pentyl)-6-phenyl-3,4-dihydro-2H-pyran-2-one (4s).
Obtained as a mixture of two diastereomers (61/39). Yellow oil; Rf (hexane/EtOAc = 10/1) 0.2; 1H NMR (major) δ 1.10 (3H, d, J = 6.8 Hz), 1.63–1.80 (1H, m), 1.93–2.00 (1H, m), 2.21–2.26 (1H, m), 2.44–2.56 (3H, m), 2.94–3.12 (2H, m), 5.80–5.85 (1H, m),7.30–7.60 (8H, m), 7.94–8.00 (2H, m), (minor, distinguishable signals) δ 1.06 (3H, d, J = 6.8 Hz), 1.63–1.80 (1H, m), 1.83–1.91 (1H, m), 2.35–2.41 (1H, m), 2.59–2.69 (3H, m); 13C NMR (major) δ 17.1, 21.3, 27.8, 32.1, 36.6, 43.0, 100.2, 124.3, 128.0, 128.4, 128.6, 128.8, 132.3, 133.0, 136.8, 150.1 170.1, 199.7, (minor, distinguishable signals) δ 15.6, 19.7, 28.6, 31.7, 36.5, 43.9, 100.0, 170.4, 200.0; MSm/z (%) 334 (M+, 1), 170 (65), 155 (100), 129 (28), 105 (79); HRMS calcd for [C22H22O3(M)+Na]+ 357.1461, found 357.1460.
6-(4-Chlorophenyl)-3-(5-(4-chlorophenyl)-5-oxo-2-pentyl)-3,4-dihydro-2H-pyran-2-one (4t).
Obtained as a mixture of two diastereomers (56/44). Colorless oil; Rf (hexane/EtOAc = 10/1) 0.1; 1H NMR (major) δ 1.08 (3H, d, J = 7.0 Hz), 1.61–1.70 (1H, m), 1.92–2.03 (1H, m), 2.20–2.26 (1H, m), 2.41–2.53 (3H, m), 2.91–3.07 (2H, m), 5.80–5.83 (1H, m), 7.32 (2H, d, J = 8.5 Hz), 7.42 (2H, br d, J = 8.5 Hz), 7.51 (2H, d, J = 8.5 Hz), 7.89 (2H, d, J = 8.5 Hz), (minor, distinguishable signals) δ 1.04 (3H, d, J = 7.0 Hz), 1.70–1.77 (1H, m), 1.83–1.89 (1H, m), 2.32–2.39 (1H, m), 2.59–2.67 (3H, m); 13C NMR (major) δ 17.1, 21.1, 27.5, 32.0, 36.7, 43.7, 100.56, 125.62, 128.6, 128.88, 128.89, 129.42, 130.8, 134.6, 139.4, 149.1, 169.8, 198.6, (minor, distinguishable signals) δ 15.6, 19.8, 28.5, 31.6, 36.4, 42.9, 100.62, 170.0, 198.4; HRMS calcd for [C22H20Cl2O3(M)+Na]+ 425.0682, found 425.0673.
6-(4-Methoxyphenyl)-3-(4-(4-methoxyphenyl)-4-oxobutyl)-3,4-dihydro-2H-pyran-2-one (4x).
Colorless oil; Rf (hexane/EtOAc = 5/1) 0.1; 1H NMRδ 1.60–1.69 (1H, m), 1.81–2.05 (3H, m), 2.29–2.34 (1H, m), 2.58–2.67 (2H, m), 2.98 (2H, q, J = 7.0 Hz), 3.82 (3H, s), 3.87 (3H, s), 5.66–5.68 (1H, m), 6.88 (2H, d, J = 8.5 Hz), 6.93 (2H, d, J = 8.5 Hz), 7.53 (2H, d, J = 8.5 Hz), 7.94 (2H, d, J = 8.5 Hz); 13C NMRδ 21.7, 24.8, 29.5, 37.8, 38.5, 55.3, 55.5 , 97.9, 105.1, 113.7, 125.1, 125.8, 130.3, 141.2, 150.1, 160.1, 163.4, 171.3, 198.3; HRMS calcd for [C23H24O5(M)+Na]+ 403.1516, found 403.1518.
3-(4-Oxo-4-phenylbutyl)-6-phenyl-3,4-dihydro-2H-pyran-2-one (4y).
Yellow oil; Rf (hexane/EtOAc = 10/1) 0.1; 1H NMRδ 1.64–1.71 (1H, m), 1.83–1.98 (2H, m), 2.01–2.03 (1H, m), 2.33–2.39 (1H, m), 2.61–2.70 (2H, m), 3.06 (2H, t, J = 7.0 Hz), 5.82 (1H, dd, J = 6.0, 3.0 Hz), 7.33–7.39 (3H, m), 7.46 (2H, t, J = 7.9 Hz), 7.56 (1H, t, J = 7.9 Hz), 7.60 (2H, d, J = 7.9 Hz), 7.96 (2H, d, J = 7.9 Hz); 13C NMRδ 21.4, 24.9, 29.5, 29.7, 38.2, 99.8, 124.4, 128.0, 128.5, 128.6, 128.8, 132.3, 133.1, 136.8, 150.4, 171.1, 199.7; MSm/z (%) 320 (M+, 2), 111(19); HRMS calcd for [C21H20O3(M)+Na]+ 343.1305, found 343.1296.
6-(4-Chlorophenyl)-3-(4-(4-chlorophenyl)-4-oxobutyl)-3,4-dihydro-2H-pyran-2-one (4z).
White solid; mp 136–137 °C; Rf (hexane/EtOAc = 5/1) 0.2; 1H NMRδ 1.62–1.69 (1H, m), 1.81–1.95 (2H, m), 1.97–2.05 (1H, m), 2.31–2.37 (1H, m), 2.59–2.66 (2H, m), 3.01 (2H, t, J = 7.0 Hz), 5.80 (1H, br s), 7.32 (2H, d, J = 8.5 Hz), 7.42 (2H, t, J = 8.5 Hz), 7.52 (2H, d, J = 8.5 Hz), 7.89 (2H, d, J = 8.5 Hz); 13C NMRδ 21.3, 24.9, 29.4, 38.1, 38.3, 100.3, 125.7, 128.7, 128.9, 129.4, 130.8, 134.7, 135.1, 139.5, 149.4, 170.7, 198.3; MSm/z (%) 388 (M+, 4), 139 (100), 111(39); HRMS calcd for [C21H18Cl2O3(M)+Na]+ 411.0525, found 411.0519.
General procedure for the reaction of 3,4-dihydro-2H-pyran-2-ones 3 with methyl vinyl ketone (2a).
Imidazolium chloride
5 (72 mg, 0.2 mmol) and pyranone 3 (2 mmol) were placed in a 20 mL Schlenk tube under nitrogen, and dissolved in THF (7 mL). Then, the enone 2a (6∼12 mmol) and Cs2CO3 (65 mg, 0.2 mmol) were added to the mixture. The mixture was stirred at room temperature or 50 °C for an appropriate time, monitoring by TLC. The reaction was quenched with water (ca. 10 mL), and diphenylmethane was added to the mixture as an internal standard. The organic layer was extracted with diethyl ether, washed with brine, dried over MgSO4, and concentrated in vacuo. Yields and product ratios were determined using 1H NMR. The products were isolated by column chromatography on silica gel with hexane–ethyl acetate eluent (10/1 to 5/1).
3-Benzyl-6-methyl-3-(3-oxobutyl)-3,4-dihydro-2H-pyran-2-one (8a).
White solid; mp 82–83 °C; Rf (hexane/EtOAc = 5/1) 0.2; 1H NMRδ 1.78 (3H, d, J = 1.2 Hz), 1.80–1.90 (2H, m), 1.96 (1H, ddd, J = 17.2, 4.0, 1.8 Hz), 2.04–2.12 (1H, m), 2.12 (3H, s), 2.39 (1H, ddd, J = 17.6, 10.0, 5.6 Hz), 2.58 (1H, ddd, J = 17.6, 10.0, 6.0 Hz), 2.76 (1H, d, J = 13.8 Hz), 2.96 (1H, d, J = 13.8 Hz), 4.81 (1H, tm, J = 3.8 Hz), 7.06–7.23 (5H, m); 13C NMRδ 18.3, 28.4, 28.5, 29.9, 38.3, 41.2, 43.7, 98.4, 126.8, 128.1, 130.3, 135.7, 148.3, 172.0, 207.5; MSm/z (%) 272 (M+, 1), 181 (100), 131(30), 123 (44); HRMS calcd for [C17H20O3(M)+Na]+ 295.1305, found 295.1300.
3-Benzyl-3-(3-oxobutyl)-6-phenyl-3,4-dihydro-2H-pyran-2-one (8b).
White solid; mp 136–137 °C; Rf (hexane/EtOAc = 5/1) 0.23; 1H NMRδ 1.97 (2H, ddd, J = 10.0, 7.3, 2.5 Hz), 2.15 (3H, s), 2.28 (1H, dd, J = 17.5, 4.5 Hz), 2.37 (1H, dd, J = 17.5, 4.5 Hz), 2.47–2.54 (1H, m), 2.67–2.73 (1H, m), 2.90 (1H, d, J = 13.5 Hz), 3.10 (1H, d, J = 13.5 Hz), 5.71 (1H, t, J = 4.5 Hz), 7.16 (2H, d, J = 7.0 Hz), 7.22–7.30 (3H, m), 7.32–7.39 (3H, m), 7.60 (2H, d, J = 7.0 Hz); 13C NMRδ 28.6, 29.2, 30.1, 38.4, 41.3, 44.0, 98.8, 124.4, 127.1, 128.3, 128.5, 128.9, 130.5, 132.1, 135.6, 149.3, 171.6, 207.5; HRMS calcd for [C22H22O3(M)+Na]+ 357.1461, found 357.1458.
3-Benzyl-6-methyl-3-(3-oxo-3-phenylpropyl)-3,4-dihydro-2H-pyran-2-one (8c).
Yellow oil; Rf (hexane/EtOAc = 5/1) 0.35; 1H NMRδ 1.86 (3H, d, J = 1.5 Hz), 2.08–2.22 (4H, m), 2.90 (1H, d, J = 13.5 Hz), 2.93–2.99 (1H, m), 3.12 (1H, d, J = 14.0 Hz), 3.21–3.27 (1H, m), 4.91 (1H, tq, J = 4.4, 1.5 Hz), 7.17 (2H, d, J = 7.0 Hz), 7.23–7.30 (3H, m), 7.46 (2H, t, J = 7.8 Hz), 7.56 (1H, t, J = 7.5 Hz), 7.97 (2H, d, J = 7.0 Hz); 13C NMRδ 18.4, 28.7, 29.3, 33.6, 41.5, 44.1, 98.6, 127.0, 128.1, 128.3, 128.6, 130.5, 133.2, 135.8, 136.6, 138.5, 172.3, 199.3; HRMS calcd for [C22H22O3(M)+Na]+ 357.1461, found 357.1454.
References
- For reviews, see:
(a) P.-C. Chiang and J. W. Bode, TCI MAIL, 2011, 149, 2–17 Search PubMed;
(b) V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691–2698 RSC;
(c) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS;
(d) N. Marion, S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988–3000 CrossRef CAS;
(e) V. Nair, S. Bindu and V. Sreekumar, Angew. Chem., Int. Ed., 2004, 43, 5130–5135 CrossRef CAS;
(f) J. S. Johnson, Angew. Chem., Int. Ed., 2004, 43, 1326–1328 CrossRef CAS.
-
(a) V. Nair, S. Vellalath, M. Poonoth and E. Suresh, J. Am. Chem. Soc., 2006, 128, 8736–8737 CrossRef CAS;
(b) V. Nair, S. Vellalath, B. P. Babu, V. Varghese, R. R. Paul and E. Suresh, Org. Biomol. Chem., 2010, 8, 4861–4866 RSC.
- V. Nair, B. P. Babu, S. Vellalath and E. Suresh, Chem. Commun., 2008, 747–749 RSC.
-
(a) P.-C. Chiang, J. Kaeobamrung and J. W. Bode, J. Am. Chem. Soc., 2007, 129, 3520–3521 CrossRef CAS. For related reactions, see:
(b) J. Kaeobamrung and J. W. Bode, Org. Lett., 2009, 11, 677–680 CrossRef CAS;
(c) M. He and J. W. Bode, J. Am. Chem. Soc., 2008, 130, 418–419 CrossRef CAS.
- L. R. Domingo, R. J. Zaragozá and M. Arnó, Org. Biomol. Chem., 2010, 8, 4884–4891 CAS.
- M. He, J. R. Struble and J. W. Bode, J. Am. Chem. Soc., 2006, 128, 8418–8420 CrossRef CAS.
- E. M. Phillips, M. Wadamoto, A. Chan and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 3107–3110 CrossRef CAS.
- J. Kaeobamrung, M. C. Kozlowski and J. W. Bode, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20661–20665 CrossRef CAS.
- Although the same results were obtained with one or two equivalents of 2a (Table 1, entries 1 and 15), use of one equivalent of aromatic vinyl ketones sometimes decreased the yield.
- Determination of the stereochemistry of 3f, 3g and 3v failed, because these compounds were oils and their solid derivatives did not afford suitable crystals for X-ray analysis.
- Under severe conditions (70 °C, 24 h, DMF solvent), 8a was formed with the base in lower yield (30%) than with 5 and Cs2CO3 or DBU (86% and 81%).
- For cleavage of enol esters by NHC, see: S. J. Ryan, L. Candish and D. W. Lupton, J. Am. Chem. Soc., 2009, 131, 14176–14177 CrossRef CAS.
- M. H. Voges, C. Romming and M. Tilset, Organometallics, 1999, 18, 529–533 CrossRef CAS.
- M. S. Kerr, J. R. Alaniz and T. Rovis, J. Org. Chem., 2005, 70, 5725–5728 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 2 adducts†
2 adducts†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts, instead of cyclopentenes and cyclopentanones. The ratio of the two products was determined by the substituent effect of the enones, not by a molar ratio of the starting materials in general. Moreover, a longer reaction period caused the formation of another 1
2 adducts, instead of cyclopentenes and cyclopentanones. The ratio of the two products was determined by the substituent effect of the enones, not by a molar ratio of the starting materials in general. Moreover, a longer reaction period caused the formation of another 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct, which was derived from the 1
2 adduct, which was derived from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pyranones initially formed.
1 pyranones initially formed.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts.
2 adducts.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct, was formed along with 3a (entries 4–9). THF and toluene were suitable solvents for this reaction (entries 1 and 10–13). Interestingly, the product ratio of 3a/4a was hardly affected by the molar ratio of 2a/1a in a range of 0.5 to 2, though it decreased to some extent with five equivalents of 2a (entries 1 and 14–16).
2 adduct, was formed along with 3a (entries 4–9). THF and toluene were suitable solvents for this reaction (entries 1 and 10–13). Interestingly, the product ratio of 3a/4a was hardly affected by the molar ratio of 2a/1a in a range of 0.5 to 2, though it decreased to some extent with five equivalents of 2a (entries 1 and 14–16).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4c and 4d as major products (entries 3 and 4). The reaction with α- and β-methylated vinyl ketones 2e and 2f afforded, however, the 1
2 adducts 4c and 4d as major products (entries 3 and 4). The reaction with α- and β-methylated vinyl ketones 2e and 2f afforded, however, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pyranones 3e and 3f exclusively (entries 5 and 6).10 No or little expected products 3 and 4 were obtained by the reaction with 2g–i (entries 7–9). Moreover, β-substituted vinyl ketones 2f and 2i produced the corresponding trans-cyclopentenes in 13% and 78% yield, respectively, as previously reported (entries 6 and 9).2 In the reaction of 3-(1-naphthyl)propenal (1b), the ratio of 3/4 was similarly affected by the enone substituents, except for the reaction with 2c wherein 4l was exclusively formed in good yield (entries 10–13). On the other hand, the aromatic enals 1c–d having electron-donating or electron-withdrawing aryl groups reacted with methyl vinyl ketone (2a) to predominantly yield the pyranone 3, suggesting that the selectivity of 3/4 was not altered by the enals 1 (entries 14–16).
1 pyranones 3e and 3f exclusively (entries 5 and 6).10 No or little expected products 3 and 4 were obtained by the reaction with 2g–i (entries 7–9). Moreover, β-substituted vinyl ketones 2f and 2i produced the corresponding trans-cyclopentenes in 13% and 78% yield, respectively, as previously reported (entries 6 and 9).2 In the reaction of 3-(1-naphthyl)propenal (1b), the ratio of 3/4 was similarly affected by the enone substituents, except for the reaction with 2c wherein 4l was exclusively formed in good yield (entries 10–13). On the other hand, the aromatic enals 1c–d having electron-donating or electron-withdrawing aryl groups reacted with methyl vinyl ketone (2a) to predominantly yield the pyranone 3, suggesting that the selectivity of 3/4 was not altered by the enals 1 (entries 14–16).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pyranones 3 exclusively, while the ratio of the 1
1 pyranones 3 exclusively, while the ratio of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4 increased as the substituent (R2) of the enones 2 changed from electron-donating groups to electron-withdrawing ones (entries 1–4 and 7–10). Moreover, the enones 2e and 2f having α- and β-methyl substituents on the vinyl moiety gave only the pyranones 3 (entries 5 and 6). Diastereomer ratios of 4r–t lowered considerably, compared with those of 4b–d and 4k–n (entries 2–4).
2 adducts 4 increased as the substituent (R2) of the enones 2 changed from electron-donating groups to electron-withdrawing ones (entries 1–4 and 7–10). Moreover, the enones 2e and 2f having α- and β-methyl substituents on the vinyl moiety gave only the pyranones 3 (entries 5 and 6). Diastereomer ratios of 4r–t lowered considerably, compared with those of 4b–d and 4k–n (entries 2–4).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4 were formed from the 1
2 adducts 4 were formed from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts 3 initially produced, it seemed curious that the 3/4 ratio was almost independent of the molar ratio of enal 1 to enone 2 as mentioned above. To address this point, the reaction of 3a with excess 2a was investigated (Scheme 2). Surprisingly, a different 1
1 adducts 3 initially produced, it seemed curious that the 3/4 ratio was almost independent of the molar ratio of enal 1 to enone 2 as mentioned above. To address this point, the reaction of 3a with excess 2a was investigated (Scheme 2). Surprisingly, a different 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct 8a was formed in good yields in the presence of the carbene precursor 5 and Cs2CO3 or DBU, but 4a was not detected at all. This reaction did not proceed with the base only under these conditions.11 The structure of 8a was confirmed by X-ray crystal analysis (Fig. 1). Although the product 8a was not detected in the reaction of 1a with 2a under the standard conditions because of a very slow reaction, it was certainly formed by use of an excess amount of 2a after a longer period together with a little 4a. In addition, the 1
2 adduct 8a was formed in good yields in the presence of the carbene precursor 5 and Cs2CO3 or DBU, but 4a was not detected at all. This reaction did not proceed with the base only under these conditions.11 The structure of 8a was confirmed by X-ray crystal analysis (Fig. 1). Although the product 8a was not detected in the reaction of 1a with 2a under the standard conditions because of a very slow reaction, it was certainly formed by use of an excess amount of 2a after a longer period together with a little 4a. In addition, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct 3c reacted with methyl vinyl ketone (2a) to give a mixture of phenyl- and methyl-substituted pyranones 8b and 8c in 41% and 12% yields, respectively, which may result from a reconstruction of the pyranone ring. Based on these results, it was confirmed that the pyranones 3 could react slowly with additional enones 2 to yield the different 1
1 adduct 3c reacted with methyl vinyl ketone (2a) to give a mixture of phenyl- and methyl-substituted pyranones 8b and 8c in 41% and 12% yields, respectively, which may result from a reconstruction of the pyranone ring. Based on these results, it was confirmed that the pyranones 3 could react slowly with additional enones 2 to yield the different 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 products 8, instead of 4.
2 products 8, instead of 4.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pyranone 3 should be formed by the reaction of C with enone 2via a [4 + 2] process through hetero-Diels–Alder reaction,6,8 or Michael addition followed by acylation (cycle I).7 It is worthwhile to comment that selective reaction of C in competition with B has been shown to need less basic amines for protonation,6–8 whereas nearly all kinds of bases, except for NaH, showed selectivity for 3 in the reaction with methyl vinyl ketone (2a).
1 pyranone 3 should be formed by the reaction of C with enone 2via a [4 + 2] process through hetero-Diels–Alder reaction,6,8 or Michael addition followed by acylation (cycle I).7 It is worthwhile to comment that selective reaction of C in competition with B has been shown to need less basic amines for protonation,6–8 whereas nearly all kinds of bases, except for NaH, showed selectivity for 3 in the reaction with methyl vinyl ketone (2a).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4, Michael addition of B to the enone 2 yields the enolate D, which readily isomerizes to another enolate E (cycle II). Formation of cyclopentanone from D and cyclopentene from E has been reported.2,3 These known reactions, however, did not take place in the present case, except for the reactions with 2f and 2i. On the contrary, the enolate E reacts with another molecule of 2 faster than the aldol reaction, giving rise to 4. The difference would be caused by the substituent effect of 2, because unsubstituted vinyl ketones having electron-withdrawing groups could serve as good Michael acceptors.
2 adducts 4, Michael addition of B to the enone 2 yields the enolate D, which readily isomerizes to another enolate E (cycle II). Formation of cyclopentanone from D and cyclopentene from E has been reported.2,3 These known reactions, however, did not take place in the present case, except for the reactions with 2f and 2i. On the contrary, the enolate E reacts with another molecule of 2 faster than the aldol reaction, giving rise to 4. The difference would be caused by the substituent effect of 2, because unsubstituted vinyl ketones having electron-withdrawing groups could serve as good Michael acceptors.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 8 would include the carbene-catalyzed ring opening reaction of the pyranone 3 to yield the intermediate F (cycle III).12Isomerization of F to G, followed by formal [4 + 2] reaction yields the products 8. Formation of the mixture of 8b and 8c in the reaction of 3c with 2a would provide additional support for this process, wherein the phenyl vinyl ketone moiety in the pyranone ring of 3c was substituted by methyl vinyl ketone in 8c.
2 adducts 8 would include the carbene-catalyzed ring opening reaction of the pyranone 3 to yield the intermediate F (cycle III).12Isomerization of F to G, followed by formal [4 + 2] reaction yields the products 8. Formation of the mixture of 8b and 8c in the reaction of 3c with 2a would provide additional support for this process, wherein the phenyl vinyl ketone moiety in the pyranone ring of 3c was substituted by methyl vinyl ketone in 8c.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts 4 as well as 1
2 adducts 4 as well as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 products 3. The former products 4 would be formed from homoenolates, and the latter 3 from enolates. The selectivity of the reaction, i.e., ratio of 3/4, was determined mainly by the substituent effect of 2, not so much by the base nor the molar ratio of the two reactants. When the enones 2 acted as good Michael acceptors, 4 predominated over 3, though selectivities were not high. Furthermore, the 1
1 products 3. The former products 4 would be formed from homoenolates, and the latter 3 from enolates. The selectivity of the reaction, i.e., ratio of 3/4, was determined mainly by the substituent effect of 2, not so much by the base nor the molar ratio of the two reactants. When the enones 2 acted as good Michael acceptors, 4 predominated over 3, though selectivities were not high. Furthermore, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 products 3 proved to react very slowly with another molecule of the enones 2, giving rise to other 1
1 products 3 proved to react very slowly with another molecule of the enones 2, giving rise to other 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 products 8.
2 products 8.



