Effects of sputtering conditions on formation of gold nanoparticles in sputter deposition technique†
Yoshikiyo
Hatakeyama
,
Kei
Onishi
and
Keiko
Nishikawa
*
Graduate School of Advanced Integration Science, Chiba University, Chiba, 263-8522, Japan. E-mail: k.nishikawa@faculty.chiba-u.jp
First published on 31st October 2011
Abstract
Sputter deposition of metals in a capture medium with extremely low vapor pressure is a simple and convenient method to generate metal nanoparticles (NPs) without chemical reactions. By careful selection of the capture medium and/or temperature of the medium for the deposition, the size of the synthesized NPs can be controlled. Sputtering conditions also play an important role in determining the size of the NPs. We synthesized Au NPs in a standard ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate ([C4mim]BF4) by systematically varying the sputtering conditions, which influence the formation processes and/or the size and size distributions of Au NPs. The Au NPs were characterized by small-angle X-ray scattering immediately after the synthesis of NPs in the capture medium. It is concluded that the temperature of the target and applied voltages have a strong influence on the size of Au NPs generated in the capture media, while the working distance between the target and the surface of the capture media, sputtering time, and discharge current have little or no influence. Lower temperature of the target and higher applied voltage are desired for generating size-controlled smaller NPs.
1. Introduction
Contrary to the usual wet processes in which metal nanoparticles (NPs) are synthesized by chemical reactions in liquids, other physical vapor deposition processes have attracted much attention as unique methods to generate metal NPs. In these methods, atoms, clusters, or fragments of metals are ejected by sputtering,1thermal evaporation,2,3 or laser ablation4,5 and are captured in a medium to form NPs. Except for laser ablation, the capture medium used for this purpose must have an extremely low vapor pressure to endure vacuum operation. Moreover, if the medium has the stabilizing ability to prevent NPs from aggregating, we can generate clean NPs in it with neither chemical reaction nor additional stabilizing agents. The combination of sputtering to generate atoms or small clusters and deposition in ionic liquids (ILs) as the capture medium is an elegant method from the viewpoint of operation simplicity and possibility to generate NPs with relatively uniform sizes. Vapor pressures of most ILs, if any,6,7 are negligibly small. When ILs are used as the capture media, complicated devices8–10 are not necessary and metal NPs are obtained through a very simple operation. The constituent ions of ILs stabilize the generated NPs; therefore, no additional stabilizing agents are needed. Since the first report on the sputter deposition technique using ILs as capture media for NP synthesis,11 many investigations1,12–18 have been performed to develop the preparative techniques and elucidate the formation mechanisms and factors determining the size and shape of NPs.Other media apart from ILs have been used as the capture media in sputter deposition techniques. For environmentally friendly and easier treatments after the generation of NPs, Dupont et al. succeeded in the preparation of Au NPs in castor oil,19 and we also successfully generated Au NPs in liquid polyethylene glycol with a lower molecular weight.20 In both capture media, it is considered that oxygen atoms are coordinated to the surface of Au NPs and –CH2–CH2– chains worked as stabilizers by surrounding the NPs. This stabilization mechanism is the same as crown ethers for alkali metal ions. It is well known that thiol groups chemically bond with the surface atoms of Au NPs and stabilize NPs. Nishihara et al. generated Au NPs protected by thiol groups of compounds with low vapor pressure,21 and moreover synthesized resin dispersing Au NPs by their polymerization for use in optical devices.22 In such a way, sputter deposition of metals in various capture media has been recognized as a simple and useful method to generate clean metal NPs, which can be widely used in many applications.
Due to their unique properties, metal NPs have attracted much attention as functionalized materials for use in optical devices, electrical devices, biosensors, and catalysts.23,24 Because their properties and functions are strongly dependent on the size and shape of NPs, techniques of synthesizing size- and shape-controlled NPs are very important. Here we limit our subject to metal NPs synthesized by the sputter deposition technique. Torimoto et al. showed that the sizes of Au NPs are dependent on the types of ionic liquid.11 Although some groups have reported the size and size distribution of metal NPs in various media,1,11–18 the values are not consistent even when the same capture medium is used. This confusion is because of many complicated inter-related factors.
As pointed out by Torimoto et al.,11 selection of a capture medium makes it possible to synthesize size-controlled NPs because the capture media have different stabilization capabilities.16 Recently, we reported that the size and size distribution of Au NPs are greatly affected by the temperature of the capture medium.17 This is because the temperature change causes a drastic change in the viscosity of the medium and consequently affects the diffusion velocity of the sputtered particles. It is concluded that collision of the sputtered metal particles determines the size and size distribution of NPs generated by sputter deposition.17 Although it is reported that post heat-treatments cause aggregation of once-generated NPs and result in the formation of larger NPs,13,15,17 the temperature effects, which we are discussing, are the effects on nucleation and growth of Au NPs just at the sputtering operation.
The other important factors to determine the size and size distribution of NPs are the sputtering conditions such as working distance between the target and the capture medium, sputtering time, temperature of the target, applied voltage, discharge current, and gas pressure. In this study, we vary these conditions systematically to determine their effects on the size and size distribution of Au NPs generated by sputtering.
There are three familiar methods for the structural characterization of Au NPs: UV-Vis absorption spectroscopy, transmission electron microscopy (TEM), and small-angle X-ray scattering (SAXS).25 For studying the optical characteristics of Au NPs, UV-Vis absorption measurements are indispensable; however, the UV-Vis spectroscopy is not sensitive enough to determine the size and size distribution of the NPs. TEM observation is almost always used for the characterization of size, size distribution, and shape of the NPs of a size larger than about 1 nm; however, sputter deposition often generates NPs of a size smaller than 1 nm,16,17 which TEM cannot detect. Moreover, for smaller NPs, we have found that growth or aggregation occurs in the preparative stages for TEM observations. On the other hand, SAXS measurements are good for characterizing NPs in the range 0.4–10 nm, and this method requires no preparative steps and can be performed immediately after the generation of NPs. For these reasons, we used SAXS measurements to characterize the NPs.
2. Experiments and data analyses
2.1 Samples
The imidazolium-based ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([C4mim]BF4, > 98% purity) was purchased from Kanto Chemical Co. Ltd., and was dried for 24 h at 333 K under a vacuum of 10−3 Pa and kept under an argon atmosphere before its use in sputter deposition. As water affects the physical and chemical properties of ILs,26 the water content was checked using the Karl Fischer titration and was maintained at less than 20 ppm. The IL [C4mim]BF4 was chosen because the size of Au NPs generated in it changed most sensitively among our tested capture media depending on the experimental conditions.27 The Au foil target was 99.99% pure.2.2 Sputter deposition of Au on the capture medium
Fig. 1 shows the schematic diagram of the sputtering apparatus. A temperature-regulated water circulating device was attached to the base of the deposition chamber and another to the target area in a commercially available sputter coater (SC-704, SANYU Electron). As reported in the previous paper,17 the temperature-regulated water circulating device at the base of the chamber helped maintain the temperature of the capture medium constant at 20–80 °C within ± 1 °C. In addition, by the temperature-regulated water circulating device attached to the target, the temperature of the target is maintained at 20 °C; the temperature range exceeded 100 °C during the sputtering operation when the circulation of cooled water was turned off.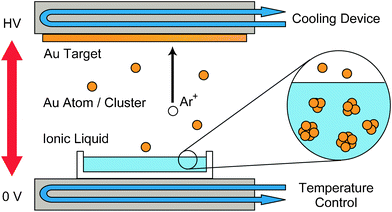 | ||
| Fig. 1 The schematic diagram of the sputtering apparatus. The water circulating device attached to the base of the deposition chamber regulates the temperature of a capture medium in the range of 20–80 °C. Another attached to the target area maintains the temperature of the target at 20 °C. | ||
To study how the working distances between the Au target and the surface of the capture medium affect the size of Au NPs, the apparatus was also remodeled so as for the distance to be changed in 25, 50, and 75 mm. With the sputter coater, the applied voltage and discharge current were varied in the ranges of 700–1000 V and 20–40 mA, respectively.
The IL (2 cm3) was spread on a plate of stainless steel (15.9 cm2) that was horizontally set in the sputter coater. The typical sputtering time was 50 min. At a working distance of 25 mm, the Au concentration was estimated to be about 40 mmol dm−3 as Au atoms, from density measurements. The Au concentration is almost proportional to the sputtering time.
To elucidate the effects of sputtering conditions on the formation of Au NPs, experiments were performed, varying (1) working distance between the Au target and the liquid surface, (2) sputtering time, (3) temperature of the target, and (4) discharge current and applied voltage.
2.3 SAXS measurements
To characterize the size and size distribution of Au NPs as they were, small-angle X-ray scattering intensities were measured with a SAXS apparatus (NANO-Viewer, RIGAKU Corporation) immediately after Au NP generation. In addition of some devises to obtain precise intensity data of SAXS experiments,28 details of the SAXS apparatus and experimental procedure are described in our previous papers.16,17 The theoretical curve fitting of the measured SAXS intensities were performed assuming that the NPs are spherical and the size distribution is expressed by Γ distribution. Further details are given in our previous paper.16 From this analysis, we extracted two parameters, the value of the diameter at the peak (dpeak), which corresponds to the diameter of the most abundant NPs, and the full width at half maxima (WFWHM) of the distribution curves.3 Results and discussion
3.1 Effect of working distance on the size of Au NPs
The working distance between the Au target and the surface of the capture medium was varied at 25, 50, and 75 mm. The experimental conditions were as follows: the temperature of the capture medium, 20 °C; sputtering time, 50 min; applied voltage, 1000 V; and discharge current, 20 mA. This experiment was performed without cooling of the target because the remodeling was not made at that stage.
Fig. 2(a) displays the scattering profiles of the Au NPs in [C4mim]BF4 that were generated at working distances of 25 (red points), 50 (green points), and 75 mm (blue points); after the corrections for the intensity fluctuations of incident X-rays, background intensities, and absorption effects.16 As we chose the scattering intensity of pure [C4mim]BF4 as the background, the profiles shown in Fig. 2(a) are the scattering intensities of the Au NPs themselves. The intensities depend on the working distance, because the amount of Au particles captured by the medium changes along with the working distance. However, the whole scattering patterns are the same keeping the constant ratios in intensity. The ratio was 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.47
0.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.26, corresponding to the distances of 25, 50, and 75 mm, respectively. The black curves in Fig. 2(a) are theoretical fittings, which were obtained with the assumption that the NPs are spherical and the size distribution is expressed by Γ distribution. The derived particle size distributions against the diameter for these scattering profiles are shown in Fig. 2(b). As shown in the inset of Fig. 2(b), the values of dpeak (closed symbols) and WFWHM (open symbols) for the three distribution curves almost perfectly agree with each other. This result indicates that the sputtered Au atoms or small clusters do not aggregate in the Ar gas area between the Au target and the surface of the capture medium under the present sputtering conditions and the working distance does not affect the size and size distribution of Au NPs generated in the capture medium.
0.26, corresponding to the distances of 25, 50, and 75 mm, respectively. The black curves in Fig. 2(a) are theoretical fittings, which were obtained with the assumption that the NPs are spherical and the size distribution is expressed by Γ distribution. The derived particle size distributions against the diameter for these scattering profiles are shown in Fig. 2(b). As shown in the inset of Fig. 2(b), the values of dpeak (closed symbols) and WFWHM (open symbols) for the three distribution curves almost perfectly agree with each other. This result indicates that the sputtered Au atoms or small clusters do not aggregate in the Ar gas area between the Au target and the surface of the capture medium under the present sputtering conditions and the working distance does not affect the size and size distribution of Au NPs generated in the capture medium.
![(a) The scattering profiles of the Au NPs in [C4mim]BF4 that were generated at working distances of 25 (red points), 50 (green points), and 75 mm (blue points). The black curves are theoretical fittings, which were obtained with the assumption that the NPs are spherical and the size distribution is expressed by Γ distribution. (b) The particle size distributions against the diameter for the scattering profiles of Fig. 2(a). The working distance dependences of dpeak (closed circles) and WFWHM (open circles) are shown in the inset.](/image/article/2011/RA/c1ra00688f/c1ra00688f-f2.gif) | ||
| Fig. 2 (a) The scattering profiles of the Au NPs in [C4mim]BF4 that were generated at working distances of 25 (red points), 50 (green points), and 75 mm (blue points). The black curves are theoretical fittings, which were obtained with the assumption that the NPs are spherical and the size distribution is expressed by Γ distribution. (b) The particle size distributions against the diameter for the scattering profiles of Fig. 2(a). The working distance dependences of dpeak (closed circles) and WFWHM (open circles) are shown in the inset. | ||
Including us,16 some groups thought that the nucleation and growth of NPs from the ejected Au particles occur on the surface of the capture medium.2–4,18 The present results are contrary to the inference. If the surface may be the main stage of nucleation and growth, the size and size distribution should be influenced by the population of the Au particles deposited on the surface. However, the above-mentioned experimental results showed that the NPs with the same size and the same size distribution were generated in spite of different populations of the deposited Au particles on the surface. Moreover, if the processes may mainly occur on the surface, the surface tension of the capture medium may hold a casting vote to determine the staying time of the deposited Au particles and affect the forming process of NPs. However, the surface model is also contradictory to our recent experimental results.17,20 Namely, larger NPs were generated in a capture medium with smaller surface tension (i.e. longer staying time), where the medium was fixed and its surface tension was regulated by the change of the temperature.
When the viscosity of capture medium is very high or deposition speed is rapid, it is considered that metal particles cannot diffuse into it and result in forming thin film on the surface.29 For the present case where viscosity of [C4mim]BF4 is 150 cP at 20 °C30 and the sputtered Au particles have considerably higher kinetic energies compared to those of the Au particles made by thermal evaporation,31 the nucleation and growth are thought to start in the beginning of the Au dispersion into the medium, taking the balance with the stabilization due to coordination by the functional groups of the capture medium.
3.2 Effect of sputtering time on the size of Au NPs
Since the Au concentration in the capture medium is proportional to the sputtering time, the study on sputtering-time dependence provides the information on the concentration dependence of the characteristics of Au NPs generated in the capture medium. In our previous paper on Au NP preparation by sputter deposition technique,16 we reported that the size and size distribution depended on the sputtering time. However, we did not control the temperature of the ionic liquids despite the temperature increase of the capture medium during the sputtering operation. Thereafter, we noted that the temperature of the capture medium greatly influences the size and size distribution of NPs.17 The sputtering-time dependence on the generation of Au NPs was affected by not only the concentration of Au but also the temperature increase of the capture media during the sputtering operation.16 In the present investigation, to examine the inherent concentration dependence only, we prepared various Au-dispersed [C4mim]BF4 solutions by varying the sputtering time, which is linearly related to Au concentration, and keeping the temperatures of the capture medium [C4mim]BF4 constant at either 20 °C or 50 °C. The temperature of 50 °C is somewhat lower than the temperature at which the generated Au NPs start to aggregate because of post heat treatment.17 As shown in Fig. 3, it is concluded that, within experimental and simulation errors, the particle size is independent of Au concentration in the present concentration range but dependent on the temperature of the capture medium. The apparent concentration dependence of Au particle size reported previously16 was because of the temperature increase during sputtering.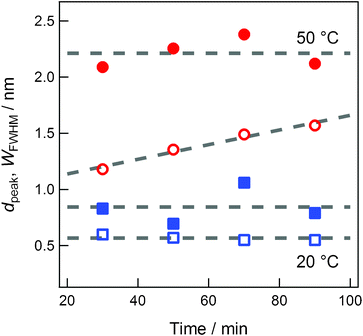 | ||
| Fig. 3 The sputtering-time dependences of dpeak (closed symbols) and WFWHM (open symbols) of Au NPs generated at 20 °C (blue squares) or 50 °C (red circles). | ||
Other groups reported that the concentration of Au does not seriously affect the size of Au NPs,11,14,18 although they did not pay special attention to the temperature of the ionic liquids. We infer that these observations occurred because their sputtering times were relatively shorter (for example, they were 5 min and 2.5 min for Torimoto et al. and Dupont et al., respectively) than that in the present study and the temperature increase was less.
3.3 Effect of target temperature on the size of Au NPs
During Ar+ sputtering, the temperature of the target increases sharply. To check this temperature effect on the generation of Au NPs, the sputter target temperature was regulated at 20 °C (Fig. 1). The experimental conditions were as follows: working distance, 25 mm; sputtering time, 50 min; applied voltage, 1000 V; and discharge current, 20 mA. The red solid curve in Fig. 4 indicates the size distribution of the Au NPs when the target was cooled to 20 °C and temperature of the capture medium [C4mim]BF4 was 50 °C. The distribution curve of Au NPs obtained without cooling is indicated by the blue broken curve. For comparison, the size distribution of Au NPs obtained in our previous paper17 is displayed by the black chain curve. All the distribution curves are normalized by area to highlight the width of the distribution. Although we cannot measure the temperature of the target, we infer that the temperature exceeds 100 °C during the sputtering operation when the circulating water is turned off. The previous target holder17 was smaller than the present remodeled one, and hence the temperature of the former is considered to increase higher than the latter. The difference in the distribution curves in Fig. 4 is certainly caused by the temperature difference of the target. Lower target temperatures resulted in smaller size and size distribution of the Au NPs.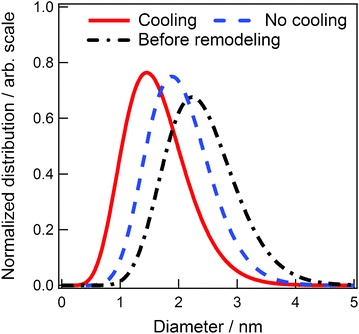 | ||
| Fig. 4 The size distributions of Au NPs synthesized at different target temperatures. | ||
The possible reasons for the effect of target temperature on the size and size distribution of the generated Au NPs are the change in kinetic energy of the sputtered Au particles and their degree of clustering. In our previous paper, we reported that highly mobile Au particles in the capture medium accumulate and grow to form larger NPs.17 In the previous case, the mobility was regulated by the change in diffusion caused by the temperature change of the capture medium. If we assume that the sputtered Au atoms/clusters grow at the beginning of Au dispersion into the capture medium17 and that the growth process is influenced by the mobility of the Au particles, then it is reasonable to infer that the smaller NPs are generated from sputtered Au particles with less kinetic energy.
From the fact that Ar+ bombardment of Au causes physical ejection of Au atoms and/or small clusters31 and our assumption that the population of the clusters against Au atoms increases with a rise of the target temperature, we can infer that smaller NPs are generated in the capture medium from Au particles sputtered from the lower temperature target.
3.4 Effects of discharge current and applied voltage on the size of Au NPs
To elucidate the effects of discharge current and applied voltage, we synthesized Au NPs under different current conditions of 20, 30, and 40 mA at a fixed voltage of 700 or 1000 V. Other experimental conditions were maintained the same; namely, the working distance was 25 mm, temperature of the capture medium was 50 °C, temperature of the target was 20 °C, and sputtering time was 50 min. In our sputter coater, the pressure of Ar is determined by the applied voltage and discharge current and is listed in Table 1. The blue and red curves in Fig. 5 indicate the distribution curves of the generated Au NPs at 700 and 1000 V, respectively. The color tones become deeper as the current increases from 20 to 40 mA. The distribution curves are normalized by area to highlight the width of the distribution. The inset indicates the values of dpeak (closed symbols) and WFWHM (open symbols) against the discharge current. The symbols of circle and triangle correspond to 1000 V and 700 V series, respectively.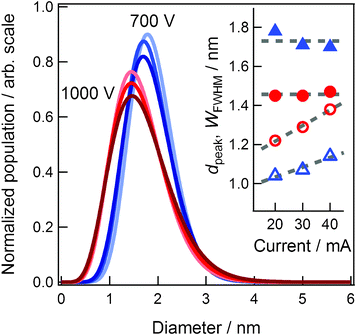 | ||
| Fig. 5 The size distributions of Au NPs synthesized under different current conditions of 20, 30, and 40 mA at fixed voltage of 700 or 1000 V. The values of dpeak (closed circles) and WFWHM (open circles) against the discharge current are shown in the inset. | ||
| Voltage/V | Current/mA | Pressure/Pa |
|---|---|---|
| 700 | 20 | 19 |
| 700 | 30 | 25 |
| 700 | 40 | 30 |
| 1000 | 20 | 13 |
| 1000 | 30 | 16 |
| 1000 | 40 | 20 |
First, let us discuss the discharge current effects on the size and size distribution of Au NPs. As indicated in the inset of Fig. 5, at 700 or 1000 V, the values of dpeak (closed symbols) are almost the same and independent of the discharge current. Although the values of WFWHM (open symbols) increase with the discharge current, the rate of increase is small. These results indicate that the discharge current mainly increases the amount of sputtered Au particles and has no influence on the size and negligible influence on size distribution of Au NPs.
Other groups obtained the opposite conclusion for the effects of discharge current on the mean size of metal NPs. According to the report by Suzuki et al., the size of Ag NPs generated in [C4mim]PF6 changes from 5.7 to 11 nm with an increase in the discharge current from 10 to 40 mA.14 Wender et al. also reported that the mean diameter of the Au NPs in 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([C4mim]NTf2) almost linearly increases from 3.2 to 4.6 nm with increase in the discharge current from 20 to 110 mA at applied voltages in the range 299–410 V and sputtering time 2.5 min.18 These experiments did not consider the temperature increase in the capture medium and the target with increasing discharge currents. As reported in our previous papers,17,20 the temperature increase of the capture media decreases the viscosities, which affects the formation of NPs and generates larger NPs. Although the capture medium [C4mim]NTf2 used by Wender et al. is different from our reported sample [C4mim]BF4,17 our preliminary results show that the temperature effect in [C4mim]NTf2 is the same and the size of Au NPs generated in it increases as the temperature of the capture medium increases.27 Moreover, as mentioned above, larger NPs are generated from the metal particles sputtered from the higher temperature target, which is a result of the increase in the discharge current. We believe that the size increase reported by the two groups14,18 may be attributed to the effects of temperature increases in both capture medium and target.
As listed in Table 1, the pressure of Ar almost linearly increases with the increase in discharge current at fixed applied voltages. Despite this pressure change, the sizes of the generated Au NPs are almost the same. At low pressures, Ar atoms and/or Ar+ ions do not collide with the sputtered Au particles in the space between the target and the capture medium, and therefore do not affect the deposition process of Au particles.
On the other hand, the influence of applied voltage significantly affects the size of the generated Au NPs. Smaller Au NPs with a wider size distribution are generated from the Au particles sputtered at high applied voltages. We cannot explain this applied-voltage effect, as discussed below. First, we focus on the effects from the viewpoint of the kinetic energies of ejected Au particles. The kinetic energies of sputtered neutral Au particles are of the order of 100 eV even at applied voltages of 43 kV.32 Moreover, no strong voltage dependence of the energy distribution was observed when the applied voltage varied from 80 to 1200 V for Cu particles sputtered by Kr+.33 Although no data on the energy distribution of sputtered Au particles by Ar+ at around 1000 V is found in the literature, from the above-mentioned experimental results, we may infer that the kinetic energy distributions of sputtered neutral Au particles do not vary with applied voltages of 700 or 1000 V. Therefore, we cannot obtain decisive explanation on the size dependence from the kinetic energies.
Second, let us discuss the applied-voltage effect from the viewpoint of clusters which are generated from the beginning by the Ar+ sputtering. Gerhard et al. reported that the ratios of Au2 and Au3 clusters to Au atoms in Ar+ sputtering at the applied voltage of 1000 V are 0.12 and 0.0016, respectively, and that the ratios of clusters increase with higher applied voltage.34 If larger NPs are generated from larger sputtered clusters, Au NPs generated at the voltage of 1000 V should be larger than those at 700 V. This, however, is contrary to our experimental observations.
From the kinetic energy and clustering of sputtered Au particles, we cannot explain the present experimental results regarding the dependence of size of NPs in the capture medium on applied voltage. However, larger Au NPs are certainly generated by a lower applied voltage. This is consistent with the recent report by Wender et al.,18 who generated Au NPs of size 3.6 ± 0.4 nm with applied voltage 335 V, discharge current 40 mA, and capture medium [C4mim]BF4.
4. Conclusions
Focusing on the sputtering conditions, we elucidated the factors that affect the size and size distribution of Au NPs generated by the sputter deposition technique. We summarize the results as follows.1) No or little influence of the size of Au NPs.
a) Sputtering time (i.e. concentration of injected Au particles in the capture medium).
b) Working distance between the metal target and the capture medium.
c) Discharge current.
d) Pressure of Ar.
2) Strong influence to determine the size of Au NPs.
e) Temperature of Au target.
f) Applied voltage.
g) Temperature of the capture medium. (This is the result of our previous studies. The size of generated NPs is strongly dependent on the temperature.17,20 To summarize the factors determining the size of NP, this item is listed up here.) For the generation of smaller NPs, lower temperature of the target, higher applied voltage and lower temperature of the capture medium are desired.
In continuation to our previous report on temperature effects of the capture medium,17 we have systematically extracted the factors related to sputtering conditions that influence the formation process and/or the size and size distribution of Au NPs. The nucleation and growth of the sputtered Au particles are thought to start in the beginning of the Au dispersion into the medium, taking the balance with the stabilization due to coordination by the functional groups of the capture medium. The effect of the types of capture medium, especially the choice of anions and cations in ILs, on the size and size distribution of Au NPs is the basis of our future work.
Acknowledgements
The present study was supported by a Grand-in-Aid for Scientific Research (No. 17073002 for K. N.) on Priority Area “Science on Ionic Liquids” (Area number: 452) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This work was also supported in part by “Research Support Program for Young Researchers” of Chiba University (Y. H.) and “Sasagawa Scientific Research Grant” from The Japan Science Society (Y. H.).References
- For example
(a) T. Torimoto, T. Tsuda, K. Okazaki and S. Kuwabata, Adv. Mater., 2009, 21, 1–26 Search PubMed
; (b) S. Kuwabata, T. Tsuda and T. Torimoto, J. Phys. Chem. Lett., 2010, 1, 3177–3188 CrossRef CAS
.
- K. Richter, A. Birkner and A-V. Mudring, Angew. Chem. Int. Ed., 2010, 49, 2431–2435 CAS
.
- K. Richter, A. Birkner and A-V. Mudring, Phys. Chem. Chem. Phys., 2011, 13, 7136–7141 RSC
.
- For example H. Wender, M. L. Andreazza, R. R. B. Correia, S. R. Teixeira and J. Dupont, Nanoscale, 2011, 3, 1240–1245 RSC
.
- Y. Kimura, H. Takata, M. Terazima, T. Ogawa and S. Isoda, Chem. Lett., 2007, 36, 1130–1131 CrossRef CAS
.
- M. J. Earle, J. M. S. S. Esperança, M. A. Gilea, J. N. C. Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS
.
- J. M. S. S. Esperança, J. N. C. Lopes, M. Tariq, L. M. N. B. F. Santos, J. W. Magee and L. P. N. Rebelo, J. Chem. Eng. Data, 2010, 55, 3–12 CrossRef
.
- I. Nakatani, T. Furubayashi, T. Takahashi and H. Hanaoka, J. Magn. Magn. Mater., 1987, 65, 261–264 CrossRef CAS
.
- M. Wagener and B. Gunther, J. Magn. Magn. Mater., 1999, 201, 41–44 CrossRef CAS
.
- S. Stoeva, K. J. Klabunde, C. M. Sorensen and I. Dragieva, J. Am. Chem. Soc., 2002, 124, 2305–2311 CrossRef CAS
.
- T. Torimoto, K. Okazaki, T. Kiyama, K. Hirahara, N. Tanaka and S. Kuwabata, Appl. Phys. Lett., 2006, 89 CrossRef CAS
, 243117/1–243117.
- K. Okazaki, T. Kiyama, K. Hirahara, N. Tanaka, S. Kuwabata and T. Torimoto, Chem. Commun., 2008, 691–693 RSC
.
- K. Okazaki, T. Kiyama, T. Suzuki, S. Kuwabata and T. Torimoto, Chem. Lett., 2009, 38, 330–331 CrossRef CAS
.
- T. Suzuki, K. Okazaki, T. Kiyama, S. Kuwabata and T. Torimoto, Electrochemistry, 2009, 77, 636–638 CAS
.
- T. Kameyama, Y. Ohno, T. Kurimoto, K. Okazaki, T. Uematsu, S. Kuwabata and T. Torimoto, T. Phys. Chem. Chem. Phys., 2010, 12, 1804–1811 CAS
.
- Y. Hatakeyama, M. Okamoto, T. Torimoto, S. Kuwabata and K. Nishikawa, J. Phys. Chem. C, 2009, 113, 3917–3922 CAS
.
- Y. Hatakeyama, S. Takahashi and K. Nishikawa, J. Phys. Chem. C, 2010, 114, 11098–11102 CAS
.
- H. Wender, L. F. Oliveira, P. Migowski, A. F. Feil, E. Lissner, M. H. G. Prechtl, S. R. Teixeira and J. Dupont, J. Phys. Chem. C, 2010, 114, 11764–11768 CAS
.
- H. Wender, L. F. Oliveira, A. F. Feil, E. Lissner, P. Migowski, M. R. Meneghetti, S. R. Teixeira and J. Dupont, Chem. Commun., 2010, 46, 7019–7021 RSC
.
- Y. Hatakeyama, T. Morita, S. Takahashi, K. Onishi and K. Nishikawa, J. Phys. Chem. C, 2011, 115, 3279–3285 CAS
.
- Y. Shishino, T. Yonezawa, H. Kawai and H. Nishihara, Chem. Commun., 2010, 46, 7211–7213 RSC
.
- Y. Shishino, T. Yonezawa, S. Udagawa, K. Hase and H. Nishihara, Angew. Chem., Int. Ed., 2011, 50, 703–705 CrossRef CAS
.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578–1586 CrossRef CAS
.
- R. W. Murray, Chem. Rev., 2008, 108, 2688–2720 CrossRef CAS
.
- B. Abécassis, F. Testard, O. Spalla and P. Barboux, Nano Lett., 2007, 7, 1723–1727 CrossRef
.
-
(a) A. M. O'Mahony, D. S. Silvester, L. Aldous, C. Hardacre and R. G. Compton, J. Chem. Eng. Data, 2008, 53, 2884–2891 CrossRef CAS
; (b) S. Zhang, X. Li, H. Chen, J. Wang, J. Zhang and M. Zhang, J. Chem. Eng. Data, 2004, 49, 760–764 CrossRef CAS
; (c) L. A. S. Ries, F. A. Amaral, K. Matos, E. M. A. Martini, M. O. Souza and R. F. Souza, Polyhedron, 2008, 27, 3287–3293 CrossRef CAS
.
-
Y. Hatakeyama, S. Takahashi, K. Onishi and K. Nishikawa
, unpublished work.
- T. Morita, Y. Tanaka, K. Ito, Y. Takahashi and K. Nishikawa, J. Appl. Crystallogr., 2007, 40, 791–795 CrossRef CAS
.
- E. F. Borra, O. Seddiki, R. Angel, D. Eisenstein, P. Hickson, K. R. Seddon and S. P. Worden, Nature, 2007, 447, 979–981 CrossRef CAS
.
- K. R. Harris, M. Kanakubo and L. A. Woolf, J. Chem. Eng. Data, 2007, 52, 2425–2430 CrossRef CAS
.
-
W. O. Hofer in Sputtering by Particle Bombardment III, ed. R. Behrisch and K. Wittmaack, Springer, Berlin, 1991, vol. 64, ch. 2, pp. 15 Search PubMed
.
- M. W. Thompson, Phil. Mag., 1968, 18, 337–414 Search PubMed
.
- R. V. Stuart and G. K. Wehner, J. Appl. Phys., 1964, 35, 1819–1824 CrossRef CAS
.
-
(a) H. Oechsner and W. Gerhard, Surf. Sci., 1974, 44, 480–488 CrossRef CAS
; (b) W. Gerhard, Z. Phys. B: Condens. Matter Quanta, 1975, 22, 31–39 CrossRef CAS
; (c) W. Gerhard and H. Z. Oechsner, Phys. B, 1975, 22, 41–48 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1ra00688f |
| This journal is © The Royal Society of Chemistry 2011 |
