Oligofluorene-based electrophoretic nanoparticles in aqueous medium as a donor scaffold for fluorescence resonance energy transfer and white-light emission†
Chakkooth
Vijayakumar
,
Kazunori
Sugiyasu
and
Masayuki
Takeuchi
*
Macromolecules Group, Organic Nanomaterials Centre (ONC), National Institute for Materials Science (NIMS), 1-2-1 Sengen, Tsukuba, 305-0047, Japan. E-mail: TAKEUCHI.Masayuki@nims.go.jp; Fax: +81-29-859-2101; Tel: +81-29-859-2110
First published on 27th October 2010
Abstract
Self-assembled, surfactant-free organic nanoparticles of an oligofluorene derivative with high colloidal stability were prepared in aqueous medium. The blue emission of the nanoparticles was tuned to white through fluorescence resonance energy transfer (FRET) by encapsulating an orange–red emitting dye (1 mol%) within the nanoparticle scaffold.
White-light emitting organic materials is an area of intense research during the last decade because of their potential use in new classes of displays and various lighting applications.1 Most of the white emitting materials reported so far are based on polymers, which consist of a blue emitting polymer incorporated with green and red emitting moieties or orange emitting moiety either covalently attached with the polymer chain or doped to the polymer matrix.2 Another class of example includes self-assembled organogels consisting of π-conjugated oligomers incorporated with certain acceptor molecules.3 Partial energy transfer between the constituents, controlled by varying their relative concentrations is the basic mechanism for white emission in these systems. Several other methods including the use of single emitting molecules4 were also reported for the development of white emitting organic materials.5
In this context, nanoparticles derived from conjugated π-electron systems attract special attention because it forms a special class of materials with unique optical and electronic properties.6 Many of the organic nanoparticles find diverse applications such as in optoelectronic devices, optical data storage, chemo- and biosensors. It could be anticipated that white-light emitting, electrophoretic organic nanoparticles in aqueous medium would be suitable for most of the above mentioned applications. For instance, electrophoretic properties enable the particles to move quickly under the applied voltage and can be easily deposited onto electrically conducting plates. Such a coating of white-emitting particles could generate any color in the visible spectrum when combined with suitable color filters. On the other hand, aqueous dispersions of such nanoparticles are also suitable for various other applications ranging from bio-imaging to chemo- and biosensors. However, reports of white-light emitting organic nanoparticles are very rare in the literature,7 despite their various possible applications.
One of the best approaches towards this end is to make nanoparticles of blue emitting chromophores and use them as a scaffold for encapsulating molecules suitable for FRET, thereby achieving color tunable emission, including white. Recently, Schenning et al. have reported such a system using the above strategy in which the nanoparticles was derived from a mixture of blue, green, yellow and red emitting fluorene-based bolaamphiphiles mixed at an optimized molar ratio, allowing partial energy transfer, yielding white emission.7b However, the use of four components and controlling the energy transfer process is considered as a relatively complicated method. Herein, we report a simple and straightforward method for white-emitting, electrophoretic organic nanoparticles in aqueous medium. Nanoparticles having controllable size distribution with good stability and intense fluorescence were obtained through the self-assembly of an oligofluorene derivative (OF) in water. By utilizing the nanoparticle assembly as a donor scaffold for FRET, the blue emission was tuned to white with good intensity by encapsulating it with an orange–red emitting dye, 4-(Dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran (DCM). The judicious choice of donor–acceptor systems enabled us to obtain intense white emission by using a small amount (1 mol%) of the acceptor.
The oligofluorene derivative (OF) was synthesized according to literature procedures with some modifications and characterized by various analytical techniques.8 It was readily soluble in common organic solvents such as toluene, chloroform, THF, and so forth. OF readily self-assembled to form stable colloidal nanoparticles with narrow dispersion upon injection of a concentrated THF solution (50–200 μL, 0.5–1.0 × 10−3 M) into an excess of water (5 mL). Solvophobic interactions between the backbones and alkyl chains in water could be the main driving force for the self-assembly. No stabilizer or surfactant was added during the preparation of the nanoparticles. The suspension was macroscopically homogeneous, and was found to be stable without precipitation even over extended times (over six months so far), even at high concentrations.
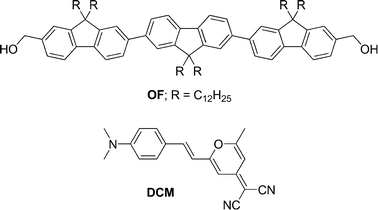
The nanoparticles were characterized by means of field-emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), dynamic light scattering (DLS) and X-ray diffraction (XRD) analysis. SEM and TEM images (Fig. 1a) revealed the particles are approximately spherical in shape. TEM images of the particles exhibited same contrast for the inner part and outer edge which ruled out the possibility of any vesicular morphologies. No significant peaks were observed in the XRD spectrum indicating the amorphous nature of the particles. DLS analysis showed a relatively narrow dispersion of the particles as shown in Fig. 1b. Hydrodynamic diameters determined by DLS correlated well with the results from SEM and TEM analysis. The average diameter of the particles could be effectively controlled between 70 and 180 nm by varying the volume (50–200 μL) and concentration (0.5–1.0 × 10−3M) of the THF solution.8
 | ||
| Fig. 1 (a) SEM image of the nanoparticles drop-casted from aqueous suspension (scale bar equivalent to 1 μm) and (b) the corresponding particle size distribution profile obtained from DLS analysis (average diameter is 95 nm). The inset of (a) shows the TEM image of a single nanoparticle (scale bar equivalent to 50 nm). | ||
Zeta (ξ) potential measurement showed that the surface of the nanoparticles is negatively charged.8 The ξ-potential of the nanoparticles with an average diameter of 95 nm was found to be −52 mV. A ξ-potential value above ±40 mV is an indication of good stability for the colloidal dispersion, which means that agglomeration and precipitation of the particles does not take place. The origin of the charge of the nanoparticles was not clear. However, most of the reported stable, surfactant free organic nanoparticles in aqueous solution contain some polar groups in the molecule.6c–i It is assumed that the polar groups provide some stability to the dispersion through interacting with the aqueous medium. In the present system, the hydroxyl end groups are relatively polar in nature and it is expected that the hydroxyl groups of the molecules at the periphery of the nanoparticles might be interacting with the polar aqueous medium to give sufficient stability to the nanoparticle assembly in water. The charged particles in water move under the influence of an applied external electric field (electrophoresis). Electrophoretic mobility (μe, the ratio of the velocity of the particles to the magnitude of the electric field strength) was also obtained from ξ-potential measurement.8 The particles showed excellent electrophoretic mobility (−2.76 × 10−4 cm2 V−1 s−1 for particles with 95 nm average diameter), which enabled them to move quickly under the applied voltage.
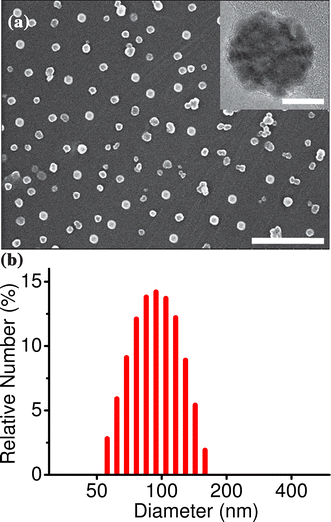
Absorption and emission spectra of OF in THF showed the characteristics of molecularly dissolved species (Fig. 2). The absorption maximum was observed at 354 nm (molar extinction coefficient, ε = 1.06 × 105 M−1 cm−1) and the emission in the blue region had two structured bands at 396 and 418 nm with a relative quantum yield of 72%. On the other hand, the absorption spectrum of nanoparticles in aqueous medium showed a slight decrease in ε and a marginal red shift in the absorption maximum. The nanoparticles emit bright, steady blue fluorescence upon excitation with UV light (λexc = 355 nm). Emission spectra exhibited two sharp peaks, as in the case of THF solution, but with a slight shift towards the red region (λem = 401 and 423 nm). Moreover, the emission becomes slightly broad with a higher relative intensity for the second peak and the quantum yield was reduced to 65% in the nanoparticle state, when compared to that of the THF solution. The reduction in molar extinction coefficient and quantum yield in the nanoparticle state is due to the aggregation of fluorene chromophores.
 | ||
| Fig. 2 (a) Absorption and (b) emission spectra of OF in THF solution (green, c = 5 × 10−6 M) and nanoparticle dispersion (blue, c = 5 × 10−6 M; red, c = 1 × 10−5 M). | ||
Fluorene derivatives are known to be good energy donors for FRET because of their suitable HOMO–LUMO energy levels and excellent quantum yield.9 In addition to this, the nanoparticle assembly can act as an effective scaffold for the organization of acceptor molecules. The presence of three fluorene units and six alkyl chains of OF makes the scaffold nonpolar, which is suitable for accommodating neutral dye molecules without phase separation. We have selected DCM, an orange–red emitting dye widely used in several optoelectronic devices,10 as the acceptor for this purpose. Significant spectral overlap (1.12 × 1015 M−1 cm−1 nm4) was observed between the donor emission and the acceptor absorption in the nanoparticle state.8 The UV-Vis absorption spectrum of the donor showed no additional peaks, or peak shift on addition of the acceptor, which is an indication of the absence of any ground-state interactions between donor and acceptor molecules. Absorption of the acceptor at the donor excitation wavelength (355 nm) was negligible, especially at low acceptor concentrations (a maximum of 4 mol% was used during the experiment), which ruled out the possibility for direct excitation of the acceptor on excitation of the donor.8 These features made the donor–acceptor system suitable for FRET studies.
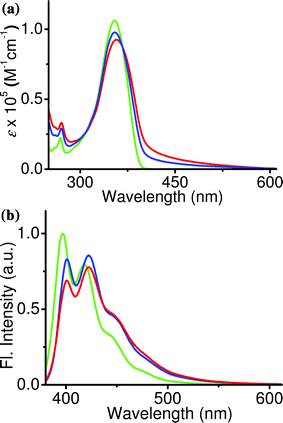
Encapsulation of the acceptor within the nanoparticle assembly of the donor was performed by dissolving small quantities of the former (0–4 mol%) in THF solution of the latter, while keeping the donor concentration at 0.5 × 10−3 M. 100 μL of this solution was injected into water (5 mL) to prepare the nanoparticles, as explained earlier. No changes in the size or shape of the nanoparticles were observed in the presence of the acceptor. Excitation of the nanoparticles at 355 nm in the presence of increasing amounts of DCM resulted in a gradual decrease in the blue emission (λmax = 401 and 423 nm) of the former with concomitant formation of an orange–red emission (λmax = 575 nm) of the latter (Fig. 3a). When the acceptor concentration was around 1 mol%, nearly 75% of the donor emission was quenched, resulting in an almost equal contribution from the donor and acceptor to the emission spectrum (Φf = 34%). Because of the balanced emission which covers the blue, green and red regions in the visible spectrum, the resulting emission was white.
 | ||
| Fig. 3 (a) Emission spectral changes of the nanoparticles in aqueous medium with increasing amounts of DCM. Inset shows the corresponding absorption spectral changes. Emission corresponding to blue (0 mol% of DCM), white (1 mol% of DCM) and orange–red (4 mol% of DCM) colors is indicated by blue, green and red lines respectively. (b) 1931 CIE coordinate diagram marked with coordinates corresponding to blue (0.16, 0.07), white (0.31, 0.34) and orange–red (0.50, 0.44) colors. | ||
The purity of the white emission is assigned in photometric terms, as standardized by the Commission Internationale de I'Eclairege (CIE).11 The CIE coordinates of the emission spectrum of nanoparticles containing 1 mol% of acceptor was found to be at (0.31, 0.34) which is in the white-emitting region according to the 1934 CIE coordinate diagram (Fig. 3b). When the acceptor concentration was further increased, the emission become orange–red due to the increase in contribution of the acceptor emission, along with a decrease in the donor emission. Nearly 98% quenching in the donor emission was observed when the acceptor concentration reaches 4 mol% (Φf = 23%). It was found that further increase in the acceptor concentration had no effect on the emission spectrum.
Photographs of the nanoparticle dispersion in aqueous medium containing different amounts of DCM under UV light (λex = 365 nm) provided visual evidence for the color tuning and white emission. As shown in Fig. 4, the emission was blue in the absence of the acceptor, while white emission was observed in presence of 1 mol% of DCM and orange–red emission was observed when the acceptor concentration became 4 mol%. The nanoparticle assembly in aqueous medium was easily transferable onto surfaces without losing its emission characteristics such as emission color and intensity, as shown by fluorescence microscopy analysis (Fig. 4, bottom).
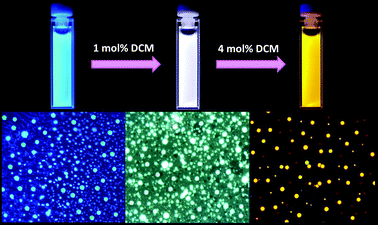 | ||
| Fig. 4 (Top) Photographs of the nanoparticle dispersion in aqueous medium with different concentrations of DCM (λexc = 365 nm) and (bottom) the corresponding fluorescence microscopic images (λexc = 330–380 nm). | ||
To study the importance of the scaffolding effect of nanoparticles on the energy transfer efficiency and color tuning, FRET studies were conducted in THF in which the donor and acceptor molecules were in a molecularly dissolved state. When increasing amounts of the acceptor (0–4 mol%) were added to the donor in THF solution followed by excitation at 355 nm, no acceptor emission was observed, indicating that the energy transfer from donor to acceptor molecules was negligible or absent in THF solution. This experiment clearly indicates that the nanoparticle scaffold is very important for organizing the acceptor molecules in such a way so as to maximize the interactions with donor molecules for efficient energy transfer.
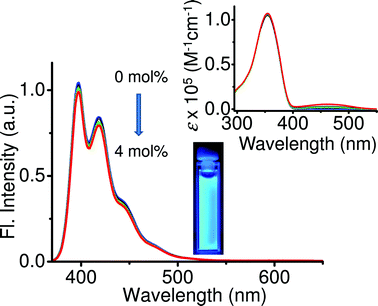 | ||
| Fig. 5 Emission spectral changes of OF in THF solution (c = 5 × 10−6 M, l = 1 cm, λexc = 355 nm) with increasing amounts of DCM (0–4 mol%). The inset shows the corresponding absorption spectral changes and photograph of the solution containing 4 mol% of DCM on excitation with 365 nm light. | ||
In conclusion, oligofluorene based stable, surfactant-free organic nanoparticles with steady, bright fluorescence were prepared in aqueous medium. Blue fluorescence of the nanoparticles was tuned to white with good intensity through FRET by encapsulating small amounts of an orange–red emitting dye. The supramolecular scaffolding ability of the nanoparticle assembly of the donor fluorene derivative played a key role in the FRET and, hence, the color tuning. This material might be suitable for potential applications in the fields of optoelectronics and bio-imaging due to the intense and tunable emission colors associated with excellent colloidal stability and electrophoretic properties in aqueous medium.
Acknowledgements
M. T. and C. V. thank Mr Michael Frank for valuable comments. This study was partially supported by KAKENHI on Innovative Areas (“coordination programming”, Area 2107, 21108010 to M. T.) from the Ministry of Education, Culture, Science, Sports, and Technology (Japan).Notes and references
- (a) B. W. D'Andrade and S. R. Forrest, Adv. Mater., 2004, 16, 1585 CrossRef; (b) K. T. Kamtekar, A. P. Monkman and M. R. Bryce, Adv. Mater., 2010, 22, 572 CrossRef CAS.
- (a) J. Luo, X. Li, Q. Hou, J. Peng, W. Yang and Y. Cao, Adv. Mater., 2007, 19, 1113 CrossRef CAS; (b) J. Liu, X. Guo, L. Bu, Z. Xie, Y. Cheng, Y. Geng, L. Wang, X. Jing and F. Wang, Adv. Funct. Mater., 2007, 17, 1917 CrossRef CAS.
- (a) C. Vijayakumar, V. K. Praveen and A. Ajayaghosh, Adv. Mater., 2009, 21, 2059 CrossRef CAS; (b) R. Abbel, R. van der Weegen, W. Pisula, M. Surin, P. Leclère, R. Lazzaroni, E. W. Meijer and A. P. H. J. Schenning, Chem.–Eur. J., 2009, 15, 9737 CrossRef CAS.
- (a) Y. Liu, M. Nishiura, Y. Wang and Z. Hou, J. Am. Chem. Soc., 2006, 128, 5592 CrossRef CAS; (b) Y. Yang, M. Lowry, C. M. Schowalter, S. O. Fakayode, J. O. Escobedo, X. Xu, H. Zhang, T. J. Jensen, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2006, 128, 14081 CrossRef CAS; (c) S. Park, J. E. Kwon, S. H. Kim, J. Seo, K. Chung, S.-Y. Park, D.-J. Jang, B. M. Medina, J. Gierschner and S. Y. Park, J. Am. Chem. Soc., 2009, 131, 14043 CrossRef CAS.
- (a) S. Li, G. Zhong, W. Zhu, F. Li, J. Pan, W. Huang and H. Tian, J. Mater. Chem., 2005, 15, 3221 RSC; (b) Y. S. Zhao, H. Fu, F. Hu, A. Peng, W. Yang and J. Yao, Adv. Mater., 2008, 20, 79 CrossRef; (c) R. Abbel, C. Grenier, M. J. Pouderoijen, J. W. Stouwdam, P. E. L. G. Leclère, R. P. Sijbesma, E. W. Meijer and A. P. H. J. Schenning, J. Am. Chem. Soc., 2009, 131, 833 CrossRef CAS; (d) S. Liu, F. Li, Q. Diao and Y. Ma, Org. Electron., 2010, 11, 613 CrossRef CAS; (e) S. Ye, J. Chen, C.-a. Di, Y. Liu, K. Lu, W. Wu, C. Du, Y. Liu, Z. Shuaic and G. Yu, J. Mater. Chem., 2010, 20, 3186 RSC.
- (a) D. Horn and J. Rieger, Angew. Chem., Int. Ed., 2001, 40, 4330 CrossRef CAS; (b) T. Tachikawa, H.-R. Chung, A. Masuhara, H. Kasai, H. Oikawa, H. Nakanishi, M. Fujitsuka and T. Majima, J. Am. Chem. Soc., 2006, 128, 15944 CrossRef CAS; (c) Y. Koizumi, S. Seki, S. Tsukuda, S. Sakamoto and S. Tagawa, J. Am. Chem. Soc., 2006, 128, 9036 CrossRef CAS; (d) B.-K. An, S.-K. Kwon and S. Y. Park, Angew. Chem., Int. Ed., 2007, 46, 1978 CrossRef CAS; (e) H.-J. Kim, J. Lee, T.-H. Kim, T. S. Lee and J. Kim, Adv. Mater., 2008, 20, 1117 CrossRef CAS; (f) X. Xu, S. Chen, L. Li, G. Yu, C. Dib and Y. Liu, J. Mater. Chem., 2008, 18, 2555 RSC; (g) R. M. Adhikari, B. K. Shah, S. S. Palayangoda and D. C. Neckers, Langmuir, 2009, 25, 2402 CrossRef CAS; (h) H. Li and H. Yan, J. Phys. Chem. C, 2009, 113, 7526 CrossRef CAS; (i) K. Panthi, R. M. Adhikari and T. H. Kinstle, J. Phys. Chem. A, 2010, 114, 4550 CrossRef CAS; (j) A. Kaeser and A. P. H. J. Schenning, Adv. Mater., 2010, 22, 2985 CrossRef CAS.
- (a) X. Zhang, S. Rehm, M. M. Safont-Sempere and F. Würthner, Nat. Chem., 2009, 1, 623 CrossRef CAS; (b) R. Abbel, R. van der Weegen, E. W. Meijer and A. P. H. J. Schenning, Chem. Commun., 2009, 1697 RSC.
- See ESI†.
- (a) R. Abbel, A. P. H. J. Schenning and E. W. Meijer, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4215 CrossRef CAS; (b) V. A. Montes, G. V. Zyryanov, E. Danilov, N. Agarwal, M. A. Palacios and P. Anzenbacher, Jr, J. Am. Chem. Soc., 2009, 131, 1787 CrossRef CAS; (c) F. Chen, W. Zhang, M. Jia, L. Wei, X.-F. Fan, J.-L. Kuo, Y. Chen, M. B. Chan-Park, A. Xia and L.-J. Li, J. Phys. Chem. C, 2009, 113, 14946 CrossRef CAS.
- (a) C. W. Tang, S. A. VanSlyke and C. H. Chen, J. Appl. Phys., 1989, 85, 3610 CrossRef; (b) Y. Divayana and X. W. Sun, Appl. Phys. Lett., 2007, 90, 203509 CrossRef; (c) A. Inoue, T. Hosokawa, M. Haishi and N. Ohtani, Phys. Status Solidi C, 2009, 6, 334 Search PubMed.
- http://hyperphysics.phy-astr.gsu.edu/hbase/vision/cie.html%23c2 (accessed on June 12, 2010).
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis, characterization, experimental techniques, dynamic light scattering analysis, zeta potential measurements and photophysical studies. See DOI: 10.1039/c0sc00343c |
| This journal is © The Royal Society of Chemistry 2011 |