Co–Gd phosphonate complexes as magnetic refrigerants†
Yan-Zhen
Zheng
a,
Marco
Evangelisti
b and
Richard E. P.
Winpenny
*ac
aSchool of Chemistry, The University of Manchester, Oxford Road, M13 9PL, Manchester, UK
bInstituto de Ciencia de Materiales de Aragón, CSIC-Universidad de Zaragoza, Departamento de Fisica de al Materia Condensada, 50009, Zaragoza, Spain
cThe Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: richard.winpenny@manchester.ac.uk; Fax: (+44)161-275-4616
First published on 24th September 2010
Abstract
Three 3d–4f phosphonate complexes, [CoII8GdIII8(μ3-OH)4(NO3)4(O3PtBu)8(O2CtBu)16], [CoII8GdIII4(O3PtBu)6(O2CtBu)16] and [CoII4GdIII6(O3PCH2Ph)6(O2CtBu)14(MeCN)2], have been synthesized and have structures that can be related to molecular grids. Magnetic studies show they have promise as low temperature magnetic refrigerants.
One of the most promising areas for application of molecular nanomagnets is in low temperature magnetic cooling.1–4 This could be important as a replacement for the current low temperature refrigerant, helium-3, which is both expensive and increasingly rare. The process uses adiabatic demagnetization, which essentially exploits the entropy change in a paramagnetic material caused by magnetizing a material in a magnetic field, then removing the field.5,6 Early examples of such studies looked at highly anisotropic single molecule magnets,7 but studies by McInnes and co-workers1 demonstrated that high spin, highly isotropic spin clusters are most promising. Very recent work by Karotsis et al. has shown remarkable results for a {Mn4Gd4} complex stabilized by calixarene ligands.4 Here we report a second family of 3d–4f complexes which show remarkable changes in entropy with changing field, which should be equally promising for magnetic cooling. While the structures are heterometallic, in many ways the closest structural analogues in the literature are the very beautiful homometallic paramagnetic grids reported by the Thompson group8 and by Lehn and Ruben.9 There are also {MnIII4LnIII4} cages recently reported by the Powell group.10 So far the largest grid from the group of Thompson reaches [5 × 5].7
Here we report a family of 3d–4f molecular squares [CoII8GdIII8(μ3-OH)4(NO3)4(O3PtBu)8(O2CtBu)16] 1, [CoII8GdIII4(O3PtBu)6(O2CtBu)16] 2 and [CoII4GdIII6(O3PCH2Ph)6(O2CtBu)14(MeCN)2] 3 which have structures related to, albeit distorted from, grids. These compounds were synthesized from two precursors, [CoII2(μ-OH2)(O2CtBu)4]·(HO2CtBu)4411 and [Gd2(O2CtBu)6(HO2CtBu)6] 5. The structure of 5 is identical with the reported [Ln2(O2CtBu)6(HO2CtBu)6] analogues (Ln = La, Ce, Nd, Sm and Eu).12
Compound 1 crystallises in the space groupC2/c. The structure is shown in Fig. 1 and there is a C2 axis along one diagonal of the square. The centre of the molecule features a [2 × 2] {Co4} square grid in which six-coordinate cobalt(II) ions are bridged by four 2.210 nitrates (Harris notation13) that are disordered about the C2 axis with alternating up and down orientations. The Co⋯Co distances within the [Co4] grid are in the range of 4.26 to 4.33 Å. Such [2 × 2] M4 grids are not rare and have been reported for cobalt(II), copper(II), nickel(II) and manganese(II) compounds.14
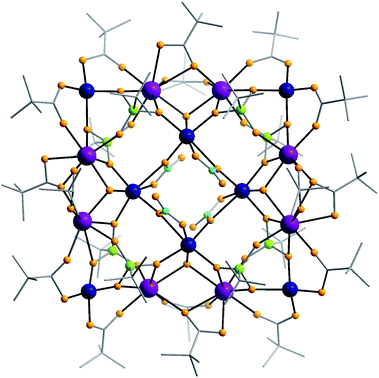 | ||
| Fig. 1 The structure of 1 in the crystal. Colour codes: Gd, purple; Co, blue; P, green; O, orange; N, cyan; C, grey. | ||
The phosphonate ligands show a 4.221-coordination mode that bridge between the inner {Co4} square and an outer {Gd8Co4} frame. Four μ3-OH groups bridge between each vertex of the {Co4} square and two adjacent Gd(III) ions, forming a [CoGd2(μ3-OH)] triangle. The Co⋯Gd separations in this triangle are in the range of 3.48 to 3.53 Å and the Gd⋯Gd separations are in the range of 3.58–3.81 Å. The four corner cobalt(II) ions of the outer [Gd8Co4] square are four-coordinate with two oxygen atoms from two phosphonates and the other two oxygen atoms from two pivalates. The Co⋯Gd separations in the outer [Gd8Co4] square are in the range of 3.646–3.920 Å, which are slightly longer than the inner Co⋯Gd separations.
Compared with a regular [4 × 4] grid the central {Co4} square is rotated through 45° with respect to the outer {Gd8Co4} frame. The metal core is slightly bowed rather than completely planar (for detail, see the side view in Fig. S1†).
The crystal structure of 2 features a [4 × 3] grid structure (Fig. 2). The outer rows of four metals are symmetry equivalent, and each contains two five-coordinate terminal Co(II) ions which are triply bridged to the two central Gd(III) ions by one 2.21 carboxylate, one 2.11 carboxylate and one oxygen from a 4.221 phosphonate. The Co⋯Gd separations are ca. 3.64 Å. The two central Gd(III) ions are also triply bridged by an oxygen from a 4.222 phosphonate and two 2.11 pivalates with a Gd⋯Gd separation of 4.21 Å. The central row of the grid contains four four-coordinate Co(II) ions and they are bridged by 4.221 and 4.222 phosphonates (Fig. 2). The three Co⋯Co separations within the central row are 4.322 or 4.782 Å. The metal–metal distances between the rows are shorter (3.343–3.356 Å). The metal sites in compound 2 lie in a single plane (Fig. S2†).
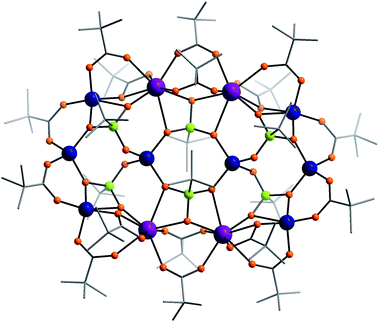 | ||
| Fig. 2 The structure of 2 in the crystal. Colours as Fig. 1. | ||
Compound 3 has a pair of octahedral Co(II) ions at the centre, surrounded by six Gd(III) ions and two tetrahedal Co(II) ions. If the central Co(II) dimer were regarded as a “node”, this molecule would be a [3 × 3] grid (Fig. 3). The central Co(II) dimer is part of a Co2O2 ring, with the oxygen atoms coming from two 5.222 phosphonates; the Co⋯Co separation is 3.190(2) Å. Each Gd⋯Gd edge is triply-bridged by an oxygen from a 4.221 phosphonate and two 2.11 pivalates with Gd⋯Gd separations of either 4.051 or 4.076 Å. The corner tetrahedral Co(II) ions are doubly-bridged to the adjacent Gd ions by one 2.11 pivalate and one O-atom from a 4.221 phosphonate with a Co⋯Gd separations of ca. 3.68 Å.
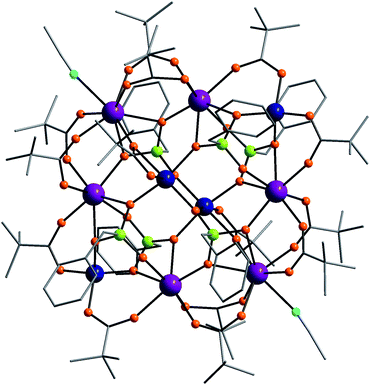 | ||
| Fig. 3 The structure of 3 in the crystal. Colours as Fig. 1. | ||
Each of the central Co(II) ions is bridged to two Gd ions by two O-atoms from two 4.221 phosphonates with Co⋯Gd separations of ca. 4.07 Å. The metal sites in compound 3 all lie in one plane (Fig. S3†), except for the central two cobalt sites which lie 1.18 Å out of the plane.
Compounds 2 and 3 are made from very similar reactions, differing only in the phosphonate used; this seems to have a significant impact on the structure. The tBuPO3 in both 1 and 2 adopt either the 4.221 or 4.222 binding mode, i.e. it binds to four metal centres. In contrast, the PhCH2PO3 used in 3 either 5.222 or 4.221 coordination modes. The difference is probably due to the different steric requirements of the benzyl group.
The magnetic behaviour of 1 to 3 has been studied on polycrystalline samples (Fig. 4–6). At room temperature in each case, the χMT value is larger than the calculated spin-only value (g = 2.00): for 1, observed 82.7 emu K mol−1 (calc. 78.0 emu K mol−1 for eight S = 3/2 and eight S = 7/2 centres); for 2, observed 54.6 emu K mol−1 (calc. 46.5 emu K mol−1 for eight S = 3/2 and four S = 7/2 centres); for 3, observed 61.6 emu K mol−1 (calc. 54.8 emu K mol−1 for S = 3/2 and six S = 7/2 centres). In each case the difference is due to orbital contributions from Co(II) ions.15
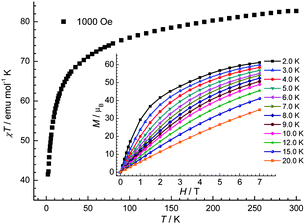 | ||
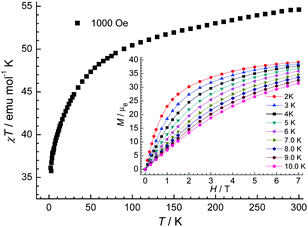
| Fig. 4 The χT vs. T plot of 1 under 1000 Oe dc field. Inset: the field-dependent magnetization plots at indicated temperatures. | ||
 | ||
| Fig. 5 The χTvs.T plot of 2 under 1000 Oe dc field. Inset: the filed-dependent magnetization plots at indicated temperatures. | ||
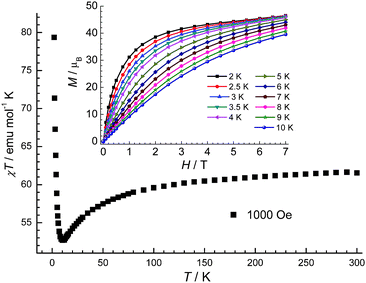 | ||
| Fig. 6 The χT vs. T plot of 3 under 1000 Oe dc field. Inset: the filed-dependent magnetization plots at indicated temperatures. | ||
Upon cooling, in each case the χT vs. T curve decreases steadily with decreasing temperature probably due single ion effects for the cobalt(II) centres.15 For 1, χT reaches a value around 41.5 emu K mol−1 at 2 K, indicating paramagnetic states are still populated. Magnetisation measurements on 1 at low temperatures (2 to 6 K) were also performed (insert of Fig. 4). The magnetisation (M) reaches 61.2 μB at 7 T at 2 K but does not saturate. For 2, χT vs. T curve reaches a value around 36.1 emu K mol−1 at 2 K. M vs. H plots at 2 and 4 K (insert of Fig. 5) show a steady increase that reaches 39.2 μB at 7 T at 2 K without saturation. The continuing fall at low temperature for 1 and 2 suggests that any exchange interaction present is predominantly anti-ferromagnetic.
For compound 3, χT decreases gradually to a minimum at 10 K and then increases sharply to 79.3 emu K mol−1 at 2 K; this is probably due to a ferromagnetic interaction between the metal centres. There is no sign of a downturn in the χT product at 2 K. The magnetisation reaches a value of 46.5 μB at 2 K (insert of Fig. 6), and the magnetisation appears to be closer to saturation than in compounds 1 and 2.
Studies of the ac susceptibility for 1–3 show no slow-relaxation behaviour down to 1.8 K, so these are not single molecule magnets. The large values for the magnetisation make these possible candidates for low temperature magnetic cooling, as the magnetocaloric effect (MCE) can be described as ΔS(T)ΔH = ∫[∂M(T,H)/∂T]HdH.6 Calculating the entropy changes obtained from the magnetisation data (Fig. 7) gives values at 4 K of: for 1, 20.4; for 2, 19.9; for 3, 22.3 J kg−1 K−1. These entropy changes are slightly larger than the value reported for the {Mn4Gd4} compound (19 J kg−1 K−1) at 4 K,4 and approach the highest value reported (25 J kg−1 K−1), for a {Mn14} cage.16 It is noticeable that the largest MCE is found for 3, which has a ferromagnetic component to the magnetic exchange (see rise in χT at low T); ferromagnetic exchange should lead to a higher MCE than an anti-ferromagnetic exchange.15,16
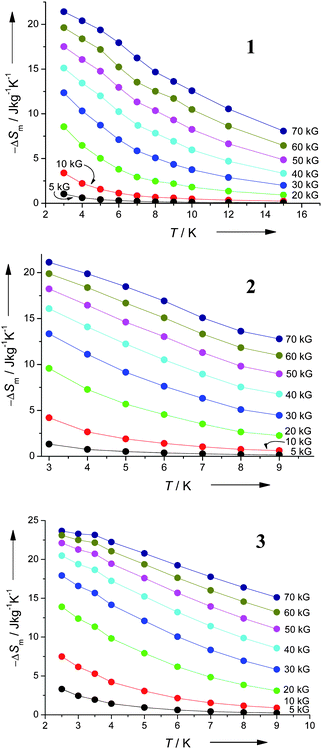 | ||
| Fig. 7 Experimental ΔS obtained from magnetisation data of 1–3 at various fields and temperatures. | ||
The highest possible entropy for a molecule with an isolated spin state is calculable from Rln(2Smax + 1);3 assuming SCo = 3/2 and SGd = 7/2, and that all the spins are parallel would give Smax = 40, 26 and 27 respectively for 1, 2 and 3. In turn, the calculated entropies would be 7.7, 9.3 and 9.0 J kg−1 K−1 for 1, 2 and 3, respectively. The much higher values found experimentally reflects the fact that there is not an isolated spin state in these compounds at low temperature and in zero external field. Therefore while the high values for MCE in 1–3 are due to the large number of coupled Gd(III) ions, a secondary factor is the weak coupling between these ions leading to a large number of populated paramagnetic states even at low temperature. Gd is often used in magnetic refrigerants;17 these results and those from the Brechin group,4 suggest that polymetallic Gd cages could have significant utility in low temperature cooling. Further studies such as heat capacity, and the magnetic and entropy behavior of analogous compounds of the other lanthanoids are progressing.
The presence of a highly anisotropic ion—cobalt(II)—in these compounds should have an adverse effect on the MCE.5 The lack of influence in 1 is, we believe, to anti-ferromagnetic exchange within the central {Co4} square leading to no contribution to the low temperature physics from the octahedral CoII ions; tetrahedral CoII is a spin only ion and therefore the other four cobalt centres should present no problem. In 2, the cobalt sites are either five- or four-coordinate, and again this removes the first order orbital contribution to the magnetism. In 3, the two octahedral sites are at the centre of the structure, and we can again assume that anti-ferromagnetic exchange between these sites eliminates their contribution to the MCE.
In summary, by using phosphonates, we have successfully synthesised a series of molecular squares which can be described as rotated-[4 × 4], [4 × 3] and pseudo-[3 × 3] grids. More importantly, these series of compounds show huge MCE at low temperatures. While there has been a great deal of recent work using phosphonates to make paramagnetic cages,18 there are only two previous examples of which we are aware where 3d–4f heterometallic cages have been reported using these ligands.19,20
We are grateful for financial support provided by a Marie Curie International Incoming Fellowship (to YZZ, EC Contract No: PIIF-GA-2008-219588), and by the EPSRC (UK), by the Spanish MICINN (to ME, contracts MAT2009-13977-C03 and CSD2007-00010) and the Royal Society for a Wolfson Merit Award (to REPW). We also thank Eric McInnes and David Collison for helpful discussions.
Notes and references
- (a) M. Evangelisti, A. Candini, A. Ghirri, M. Affronte, E. K. Brechin and E. J. L. McInnes, Appl. Phys. Lett., 2005, 87, 072504 CrossRef; (b) R. Shaw, R. H. Laye, L. F. Jones, D. M. Low, C. Talbot-Eeckelaers, Q. Wei, C. J. Milios, S. Teat, M. Helliwell, J. Raftery, M. Evangelisti, M. Affronte, D. Collison, E. K. Brechin and E. J. L. McInnes, Inorg. Chem., 2007, 46, 4968 CrossRef CAS.
- M. Manoli, R. D. L. Johnstone, S. Parsons, M. Murrie, M. Affronte, M. Evangelisti and E. K. Brechin, Angew. Chem., Int. Ed., 2007, 46, 4456 CrossRef CAS.
- M. Evangelisti, A. Candini, M. Affronte, E. Pasca, L. J. de Jongh, R. T. W. Scott and E. K. Brechin, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 104414 CrossRef.
- G. Karotsis, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 9928 CrossRef CAS.
- M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672 RSC.
- M. Evangelisti, F. Luis, L. J. de Jongh and M. Affronte, J. Mater. Chem., 2006, 16, 2534 RSC.
- (a) F. Torres, J. M. Hernández, X. Bohigas and J. Tejada, Appl. Phys. Lett., 2000, 77, 3248 CrossRef CAS; (b) Yu. I. Spichkin, A. K. Zvezdin, S. P. Gubin, A. S. Mischenko and A. M. Tishin, J. Phys. D: Appl. Phys., 2001, 34, 1162 CrossRef CAS; (c) X. X. Zhang, H. L. Wei, Z. Q. Zhang and L. Zhang, Phys. Rev. Lett., 2001, 87, 157203 CrossRef CAS.
- L. N. Dawe, K. V. Shuvaev and L. K. Thompson, Inorg. Chem., 2009, 48, 3323 CrossRef CAS.
- M. Ruben, J. Rojo, F. J. Romero-Salguero, L. H. Uppadine and J.-M. Lehn, Angew. Chem., Int. Ed., 2004, 43, 3644 CrossRef CAS.
- V. Mereacre, M. N. Akhtar, Y. Lan, A. M. Ako, R. Clérac, C. E. Anson and A. K. Powell, Dalton Trans., 2010, 39, 4918 RSC.
- G. Chaboussant, R. Basler, H.-U. Gudel, S. T. Ochsenbein, A. Parkin, S. Parsons, G. Rajaraman, A. Sieber, A. A. Smith, G. A. Timco and R. E. P. Winpenny, Dalton Trans., 2004, 2758 RSC.
- (a) I. G. Fomina, M. A. Kiskin, A. G. Martynov, G. G. Aleksandrov, Zh. V. Dobrokhotova, Yu. G. Gorbunova, Yu. G. Shvedenkov, A. Yu. Tsivadze, V. M. Novotortsev and I. L. Eremenko, Zh. Neorg. Khim., 2004, 49, 1463 CAS; (b) T. A. Zoan, N. P. Kuzmina, S. N. Frolovskaya, A. N. Rykov, N. D. Mitrofanova, S. I. Troyanov, A. P. Pisarevsky, L. I. Martynenko and Y. M. Korenev, J. Alloys Compd., 1995, 225, 396 CrossRef CAS.
- Harris notation describes the binding mode as [X.Y1Y2…Yn] where X is the overall number of metal bound by the whole ligand, and each value of Y refers to the number of metal atoms attached to the different donor atoms. See Supporting Information and also: R. A. Coxall, S. G. Harris, D. K. Henderson, S. Parsons, P. A. Tasker and R. E. P. Winpenny, J. Chem. Soc., Dalton Trans., 2000, 2349 Search PubMed.
- (a) L. N. Dawe, T. S. M. Abedin, T. L. Kelly, L. K. Thompson, D. O. Miller, L. Zhao, C. Wilson, M. A. Leech and J. A. K. Howard, J. Mater. Chem., 2006, 16, 2645 RSC; (b) H. Arora, F. Lloret and R. Mukherjee, Dalton Trans., 2009, 9759 RSC; (c) D. Wu, D. Guo, Y. Song, W. Huang, C. Duan, Q. Meng and O. Sato, Inorg. Chem., 2009, 48, 854 CrossRef CAS.
- O. Kahn, Molecular Magnetism, VCH: New York, 1993 Search PubMed.
- M. Manoli, A. Collins, S. Parsons, A. Candini, M. Evangelisti and E. K. Brechin, J. Am. Chem. Soc., 2008, 130, 11129 CrossRef CAS.
- (a) C. Zimm, A. Jastrab, A. Sternberg, V. Pecharsky, K. Gschneidner, Jr., M. Osborne and I. Anderson, Adv. Cryog. Eng., 1998, 43, 1759 Search PubMed; (b) V. K. Pecharsky and K. A. Gschneidner, Jr., J. Magn. Magn. Mater., 1999, 200, 44 CrossRef CAS.
- For example: (a) V. Chandrasekhar and S. Kingsley, Angew. Chem., Int. Ed., 2000, 39, 2320 CrossRef CAS; (b) V. Chandrasekhar, L. Nagarajan, R. Clérac, S. Ghosh and S. Verma, Inorg. Chem., 2008, 47, 1067 CrossRef CAS; (c) E. I. Tolis, M. Helliwell, S. Langley, J. Raftery and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2003, 42, 3804 CrossRef; (d) S. Maheswaran, G. Chastanet, S. J. Teat, T. Mallah, R. Sessoli, W. Wernsdorfer and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2005, 44, 5044 CrossRef CAS; (e) S. Khanra, M. Kloth, H. Mansaray, C. A. Muryn, F. Tuna, E. C. Sanũdo, M. Helliwell, E. J. L. McInnes and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2007, 46, 5568 CrossRef CAS; (f) S. Langley, M. Helliwell, R. Sessoli, S. J. Teat and R. E. P. Winpenny, Inorg. Chem., 2008, 47, 497 CrossRef CAS; (g) S. Konar, N. Bhuvanesh and A. Clearfield, J. Am. Chem. Soc., 2006, 128, 9604 CrossRef CAS; (h) S. Konar and A. Clearfield, Inorg. Chem., 2008, 47, 3492 CrossRef CAS; (i) S. Konar and A. Clearfield, Inorg. Chem., 2008, 47, 3489 CrossRef CAS; (j) S. Konar and A. Clearfield, Inorg. Chem., 2008, 47, 5573 CrossRef CAS; (k) Y.-S. Ma, Y. Song, Y.-Z. Li and L.-M. Zheng, Inorg. Chem., 2007, 46, 5459–5461 CrossRef CAS; (l) R. Murugavel, N. Gogoi, K. G. Suresh, S. Lavek and H. C. Verma, Chem.–Asian J., 2009, 4, 923 CrossRef CAS.
- V. Baskar, K. Gopal, M. Helliwell, F. Tuna, W. Wernsdorfer and R. E. P. Winpenny, Dalton Trans., 2010, 39, 4747 RSC.
- M. Wang, D.-Q. Yuan, C.-B. Ma, M.-J. Yuan, M.-Q. Hu, N. Li, H. Chen, C.-N. Chen and Q.-T. Liu, Dalton Trans., 2010, 39, 7276 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and selected bond lengths and angles for 1, 2, 3 and 5. CCDC reference numbers 784308–784310. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00371a |
| This journal is © The Royal Society of Chemistry 2011 |
