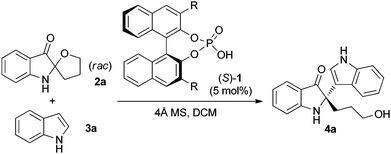
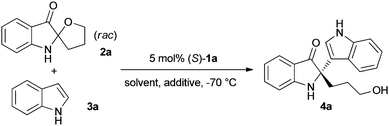
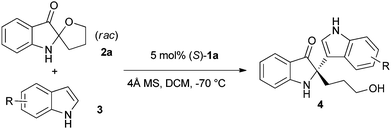
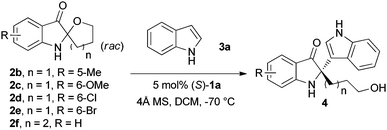
Chiral phosphoric acid-catalysed Friedel–Crafts alkylation reaction of indoles with racemic spiro indolin-3-ones†
Qin
Yin
and
Shu-Li
You
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032, China. E-mail: slyou@sioc.ac.cn; Fax: (+86) 21-5492-5087
First published on 10th May 2011
Abstract
Chiral phosphoric acid-catalysed Friedel–Crafts alkylation reactions of indoles, pyrrole and 3-(dimethylamino)phenol with racemic spiro indolin-3-ones have been realised. With 5 mol% (S)-TRIP, Friedel–Crafts adducts bearing a quaternary stereocentre were obtained in up to 99% yield and 99% ee. The reaction features readily available, stable starting materials and delivers synthetically useful but challenging products.
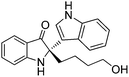
Construction of chiral quaternary carbon centres has been one of the most challenging subjects in asymmetric catalysis.1 On the other hand, chiral quaternary carbon centers can be found extensively in natural products and unnatural compounds with intriguing properties. For instance, indol-3-yl methanamines possess quaternary carbon centres and are a very useful motif, exemplified by isatisine A (Fig. 1) and its acetonide derivative that display very interesting antiviral properties.2,3 Retrosynthetically, indol-3-yl methanamines could be accessed directly from asymmetric Friedel–Crafts alkylation reactions.4 However, despite enormous progress made with asymmetric Friedel–Crafts reactions during the past decade,5 most of the Friedel–Crafts alkylation reactions of indoles with imines were limited to aldimine substrates.6,7 There are very few examples of asymmetric Friedel–Crafts alkylation reactions of indoles with ketimines, which would lead to the synthesis of indol-3-yl methanamines bearing a quaternary carbon center.8,9 In this regard, Zhou and co-workers developed an asymmetric Friedel–Crafts alkylation reaction of indoles with α-aryl enamides, which isomerized to ketiminesin situ, catalysed by chiral phosphoric acids.9 Chiral amine products bearing a quaternary carbon center were obtained in excellent ees but the substrates were limited to aryl methyl ketone derived enamides. In addition to the challenges faced in enantioselective control when using ketimines, their poor reactivity, stability and synthetic difficulty also impede progress toward asymmetric Friedel–Crafts alkylation reactions with ketimines. To address these problems, we recently envisaged that readily accessible racemic spiro indolin-3-ones might be suitable substrates in chiral phosphoric acid-catalysed Friedel–Crafts alkylation reaction.10 Under acid catalysis, the racemic spiro indolin-3-ones with quaternary stereocentres undergo ring opening affording highly functionalised ketiminesin situ (Scheme 1).11 In this paper, we report our results on the novel asymmetric Friedel–Crafts alkylation reaction of indoles, pyrrole and 3-(dimethylamino)phenol with racemic spiro indolin-3-ones, affording highly enantioenriched Friedel–Crafts adducts bearing a quaternary carbon centre. Given the fact that both indolin-3-one and indole are privileged structural units in biologically active compounds,12 the current methodology provides a facile access to novel scaffolds that would greatly benefit drug discovery research.
 | ||
| Fig. 1 Isatisine A, its acetonide derivative and indol-3-yl methanamines bearing a quaternary carbon center. | ||
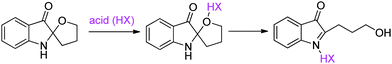 | ||
| Scheme 1 Acid-catalysed in situ formation of ketimine from racemic spiro indolin-3-one. | ||
We first examined the reaction between spiro indolin-3-one 2a and indole 3a catalysed by different chiral phosphoric acids 1 (Table 1).11 In the presence of 5 mol% (S)-TRIP (1a)13 and 4 Å molecular sieves (MS) in dichloromethane at room temperature, reaction of 2a with 2.0 equivalents of 3a proceeded smoothly in complete conversion to afford the desired product 4a in 43% ee (entry 1, Table 1). Lowering the reaction temperature significantly improved the enantioselectivity of this reaction, and the product was obtained in 98% ee at −70 °C (entry 4, Table 1). Various chiral phosphoric acids 1 were then tested at −70 °C (entries 5–10, Table 1). All the reactions went to completion with variable ees. Notably, catalyst 1b (R = 4-tBu-2,6-iPr2C6H2) also led to an excellent enantioselectivity comparable to catalyst 1a (98% ee, entry 5, Table 1).
| Entrya | 1 | R | T/°C | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reactions were performed with 2a (0.1 mmol), indole 3a (2 equiv.), 4 Å MS (10 mg) and 5 mol% of 1 in CH2Cl2. b Isolated yield. c Determined by HPLC. | |||||
| 1 | 1a | 2,4,6-iPr3C6H2 | rt | 96 | 43 |
| 2 | 1a | 2,4,6-iPr3C6H2 | 0 | 91 | 55 |
| 3 | 1a | 2,4,6-iPr3C6H2 | −40 | 92 | 91 |
| 4 | 1a | 2,4,6-iPr3C6H2 | −70 | 97 | 98 |
| 5 | 1b | 4-tBu-2,6-iPr2C6H2 | −70 | 92 | 98 |
| 6 | 1c | 4-NO2C6H4 | −70 | 90 | 75 |
| 7 | 1d | 1-Naphthyl | −70 | 90 | 77 |
| 8 | 1e | Biphenyl | −70 | 94 | 5 |
| 9 | 1f | 3,5-(CF3)2C6H3 | −70 | 93 | 5 |
| 10 | 1g | SiPh3 | −70 | 92 | 80 |
With (S)-TRIP (1a) as the catalyst, we further optimised the reaction conditions by tuning solvents (toluene, diethyl ether, and xylene), additives, and catalyst loadings, the results are summarised in Table 2. Overall, the reaction with 5 mol% (S)-TRIP and 4 Å MS in dichloromethane at −70 °C led to the best combination of isolated yield and enantioselectivity (97% yield, 98% ee, entry 1, Table 2).
| Entrya | Solvent | Additive | t/h | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reactions were performed with 2a (0.1 mmol), indole 3a (2 equiv.), additive (10 mg) and 5 mol% of 1a. b Isolated yield. c Determined by HPLC. d Reaction was performed with 2 mol% 1a. | |||||
| 1 | CH2Cl2 | 4 Å MS | 2 | 97 | 98 |
| 2 | Toluene | 4 Å MS | 30 | 75 | 75 |
| 3 | Diethyl ether | 4 Å MS | 48 | Trace | — |
| 4 | Xylene | 4 Å MS | 30 | 75 | 41 |
| 5 | CH2Cl2 | — | 15 | 88 | 97 |
| 6 | CH2Cl2 | 3 Å MS | 10 | 93 | 98 |
| 7 | CH2Cl2 | 5 Å MS | 4 | 90 | 98 |
| 8d | CH2Cl2 | 4 Å MS | 25 | 88 | 98 |
Under the above optimised reaction conditions, various substituted indoles were reacted with 2a to probe the generality of the reaction. The results are summarised in Table 3. Several substituted indoles 3b–j, containing either electron-donating (7-Me, 6-Me, 5-OMe, 6-OBn) or electron-withdrawing groups (6-Br, 5-Br, 6-F, 6-Cl, 5-Cl), were tested in the reaction with indolin-3-one 2a. In all cases, excellent yields and enantioselectivities were achieved (88–99% yield, 96–99% ee, entries 2–10, Table 3).
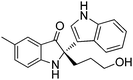
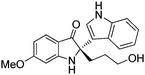
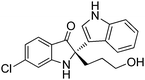
In addition, the reactions of various racemic indolin-3-ones bearing quaternary stereocentres with indole were also carried out to further examine the reaction scope. As summarised in Table 4, this asymmetric Friedel–Crafts reaction also works well for substituted indolin-3-ones. Excellent yields and enantioselectivities were obtained for indolin-3-ones bearing electron-donating groups (Me, OMe) (95–96% yield, 96–97% ee, entries 1 and 2, Table 4). When electron-withdrawing groups (Br, Cl) were introduced on the indolin-3-ones, the reactions proceeded with excellent yields but with slightly decreased enantioselectivities (92–95% yield, 88–89% ee, entries 3 and 4, Table 4). Six-membered ring containing substrate, 3′,4′,5′,6′-tetrahydrospiro[indoline-2,2′-pyran]-3-one 2f (Table 4, n = 2) was tolerated in the reaction, giving the Friedel–Crafts adduct in 98% yield and 88% ee (entry 5, Table 4).
To further broaden the substrate scope for the Friedel–Crafts reaction with spiro indolin-3-ones, other types of electron-rich arenes such as pyrrole and 3-(dimethylamino)phenol were tested, as shown in Scheme 2. With pyrrole as the substrate, under slightly modified reaction conditions, the asymmetric Friedel–Crafts reaction of spiro indolin-3-ones 2a–b led to the alkylation products 4p–q in excellent yields and ees (R = H, 92% yield, 96% ee; R = Me, 90% yield, 97% ee, Scheme 2, eq 1). However, when 3-(dimethylamino)phenol was used, the reaction proceeded much more slowly, even at room temperature. The optimal enantioselectivity for product 4r was obtained at only a moderate level (50% ee) in the presence of catalyst (S)-1b (Scheme 2, eq 2).
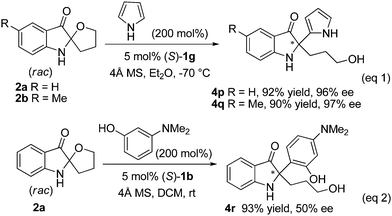 | ||
| Scheme 2 Asymmetric Friedel–Crafts reaction with pyrrole and 3-(dimethylamino)phenol. | ||
In order to determine the absolute configuration of the product, the crystal structure of enantiopure 4g was obtained and a single-crystal X-ray analysis determined its configuration as S (Fig. 2).
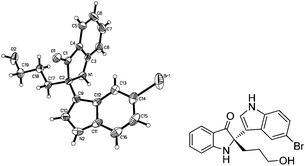 | ||
| Fig. 2 X-Ray crystal structure of (S)-4g. | ||
The products obtained here contain a free hydroxyl group that provides a versatile handle for performing subsequent transformations. Scheme 3 shows the synthetic utility of the products where treatment of 4a (4g) with PPh3/DIAD (diisopropyl azodicarboxylate) in THF delivers a dihydropyrrole ring yielding a tricycylic compound 5a (5g) with good stereochemical integrity. Moreover, when product 4a (4g) was subjected to Ley oxidation conditions,14 tricycylic compound 6a (6g) was formed in excellent ee. Notably, the latter tricyclic motif exists in isatisine A, thus potentially providing a basis for future asymmetric syntheses of isatisine A and related natural products.
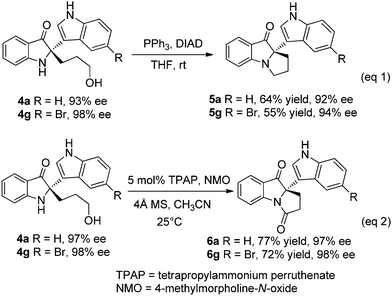 | ||
| Scheme 3 Transformation of 4a and 4g to tricyclic compounds. | ||
In summary, we have developed an efficient method for the construction of chiral quaternary stereocentres by enantioselective Friedel–Crafts alkylation reaction of indoles, pyrrole and 3-(dimethylamino)phenol with racemic indolin-3-ones having quaternary stereocenters. With (S)-TRIP as the catalyst, Friedel–Crafts adducts bearing quaternary carbon centres were obtained in excellent yields and ees. The ready availability of indolin-3-ones as efficient ketimine precursors in this asymmetric reaction will greatly facilitate access to enantiopure amines bearing a quaternary carbon centre at the α-position. Further expansion of this reaction and application of the current methodology are underway in our laboratory.
Acknowledgements
Financial support was provided by the National Natural Science Foundation of China (20732006, 20821002, 21025209) and National Basic Research Program of China (973 Program 2009CB825300). We thank Professor John S. Fossey (University of Birmingham) for proofreading the manuscript.Notes and references
- For recent reviews on the construction of chiral tetrasubstituted carbon centers, see: (a) K. Fuji, Chem. Rev., 1993, 93, 2037–2066 CrossRef CAS; (b) E. J. Corey and A. Guzman-Perez, Angew. Chem., Int. Ed., 1998, 37, 388–401 CrossRef; (c) J. Christoffers and A. Mann, Angew. Chem., Int. Ed., 2001, 40, 4591–4597 CrossRef CAS; (d) C. J. Douglas and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5363–5367 CrossRef CAS; (e) D. J. Ramón and M. Yus, Curr. Org. Chem., 2004, 8, 149–183 CrossRef CAS; (f) J. Christoffers and A. Baro, Adv. Synth. Catal., 2005, 347, 1473–1482 CrossRef CAS; (g) B. M. Trost and C. Jiang, Synthesis, 2006, 369–396 CrossRef CAS; (h) P. G. Cozzi, R. Hilgraf and N. Zimmermann, Eur. J. Org. Chem., 2007, 5969–5994 CrossRef CAS; (i) O. Riant and J. Hannedouche, Org. Biomol. Chem., 2007, 5, 873–888 RSC; (j) M. Bella and T. Gasperi, Synthesis, 2009, 1583–1614 CrossRef CAS.
- For first isolation and characterization of isatisine A, see: J.-F. Liu, Z.-Y. Jiang, R.-R. Wang, Y.-T. Zheng, J.-J. Chen, X.-M. Zhang and Y.-B. Ma, Org. Lett., 2007, 9, 4127–4129 Search PubMed.
- For recent total syntheses of isatisine A, see: (a) A. Karadeolian and M. A. Kerr, Angew. Chem., Int. Ed., 2010, 49, 1133–1135 CrossRef CAS; (b) A. Karadeolian and M. A. Kerr, J. Org. Chem., 2010, 75, 6830–6841 CrossRef CAS; (c) J. Lee and J. S. Panek, Org. Lett., 2011, 13, 502–505 CrossRef CAS.
- For recent reviews on asymmetric Friedel–Crafts alkyaltion reaction, see: (a) M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550–556 CrossRef CAS; (b) M. Bandini, A. Melloni, S. Tommasi and A. Umani-Ronchi, Synlett, 2005, 1199–1222 CrossRef CAS; (c) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903–2915 CrossRef CAS; (d) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9533–9644 CrossRef CAS; (e) M. Zeng and S.-L. You, Synlett., 2010, 1289–1301 CAS.
- (a) N. A. Paras and D. W. C. MacMillan, J. Am. Chem. Soc., 2001, 123, 4370–4371 CrossRef CAS; (b) J. F. Austin and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 1172–1173 CrossRef CAS; (c) N. A. Paras and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 7894–7895 CrossRef CAS.
- For selected asymmetric Friedel–Crafts alkylation reactions with aldimines catalyzed by chiral Brønsted acids, see: (a) D. Uraguchi, K. Sorimachi and M. Terada, J. Am. Chem. Soc., 2004, 126, 11804–11805 CrossRef CAS; (b) Y.-Q. Wang, J. Song, R. Hong, H. Li and L. Deng, J. Am. Chem. Soc., 2006, 128, 8156–8157 CrossRef CAS; (c) Q. Kang, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2007, 129, 1484–1485 CrossRef CAS; (d) M. Terada, S. Yokoyama, K. Sorimachi and D. Uraguchi, Adv. Synth. Catal., 2007, 349, 1863–1867 CrossRef CAS; (e) G. B. Rowland, E. B. Rowland, Y. Liang, J. A. Perman and J. C. Antilla, Org. Lett., 2007, 9, 4065–4068 CrossRef CAS; (f) M. Terada and K. Sorimachi, J. Am. Chem. Soc., 2007, 129, 292–293 CrossRef CAS; (g) G.-W. Zhang, L. Wang, J. Nie and J.-A. Ma, Adv. Synth. Catal., 2008, 350, 1457–1463 CrossRef CAS; (h) P. Yu, J. He and C. Guo, Chem. Commun., 2008, 2355–2357 RSC; (i) Q. Kang, X.-J. Zheng and S.-L. You, Chem.–Eur. J., 2008, 14, 3539–3542 CrossRef CAS; (j) D. Enders, A. A. Narine, F. Toulgoat and T. Bisschops, Angew. Chem., Int. Ed., 2008, 47, 5661–5665 CrossRef CAS; (k) X. Zeng, X. Zeng, Z. Xu, M. Lu and G. Zhong, Org. Lett., 2009, 11, 3036–3039 CrossRef CAS; (l) S. Nakamura, Y. Sakurai, H. Kakashima, N. Shibata and T. Toru, Synlett, 2009, 1639–1642 CrossRef CAS; (m) E. A. Peterson and E. N. Jacobsen, Angew. Chem., Int. Ed., 2009, 48, 6328–6331 CrossRef CAS; (n) Q. Kang, Z.-A. Zhao and S.-L. You, Tetrahedron, 2009, 65, 1603–1607 CrossRef CAS; (o) F.-X. Xu, D. Huang, C. Han, W. Shen, X.-F. Lin and Y.-G. Wang, J. Org. Chem., 2010, 75, 8677–8680 CrossRef CAS.
- For selected examples of Pictet–Spengler reaction, see: (a) M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 10558–10559 CrossRef CAS; (b) J. Seayad, A. M. Seayad and B. List, J. Am. Chem. Soc., 2006, 128, 1086–1087 CrossRef CAS; (c) I. T. Raheem, P. S. Thiara, E. A. Peterson and E. N. Jacobsen, J. Am. Chem. Soc., 2007, 129, 13404–13405 CrossRef CAS; (d) M. J. Wanner, R. N. S. vander Haas, K. R. deCuba, J. H. van Maarseveen and H. Hiemstra, Angew. Chem., Int. Ed., 2007, 46, 7485–7487 CrossRef CAS; (e) D. J. Mergott, S. J. Zuend and E. N. Jacobsen, Org. Lett., 2008, 10, 745–748 CrossRef CAS; (f) I. T. Raheem, P. S. Thiara and E. N. Jacobsen, Org. Lett., 2008, 10, 1577–1580 CrossRef CAS; (g) N. V. Sewgobind, M. J. Wanner, S. Ingemann, R. de Gelder, J. H. van Maarseveen and H. Hiemstra, J. Org. Chem., 2008, 73, 6405–6408 CrossRef CAS; (h) M. J. Wanner, R. N. A. Boots, B. Eradus, R. de Gelder, J. H. van Maarseveen and H. Hiemstra, Org. Lett., 2009, 11, 2579–2581 CrossRef CAS; (i) F. R. Bou-Hamdan and J. L. Leighton, Angew. Chem., Int. Ed., 2009, 48, 2403–2406 CAS; (j) M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796–10797 CrossRef CAS.
- (a) M. Rueping and B. J. Nachtsheimb, Synlett, 2010, 119–122 CrossRef CAS; (b) M. Rueping, S. Raja and A. Núñez, Adv. Synth. Catal., 2011, 353, 563–568 CrossRef CAS.
- Y.-X. Jia, J. Zhong, S.-F. Zhu, C.-M. Zhang and Q.-L. Zhou, Angew. Chem., Int. Ed., 2007, 46, 5565–5567 CrossRef CAS.
- (a) M. J. Buller, T. G. Cook and Y. Kobayashi, Heterocycles, 2007, 72, 163–166 CrossRef; (b) K. Higuchi, Y. Sato, S. Kojima, M. Tsuchimochi, K. Sugiura, M. Hatori and T. Kawasaki, Tetrahedron, 2010, 66, 1236–1243 CrossRef CAS.
- Reviews on chiral Brønsted acid catalysis, see: (a) M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520–1543 CrossRef CAS; (b) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713–5743 CrossRef CAS; (c) T. Akiyama, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS; (d) M. Terada, Chem. Commun., 2008, 4097–4112 RSC; (e) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190–2201 RSC; (f) J. N. Johnston, H. Muchalski and T. L. Troyer, Angew. Chem., Int. Ed., 2010, 49, 2290–2298 CAS; (g) M. Terada, Synthesis, 2010, 1929–1982 CrossRef CAS; (h) A. Zamfir, S. Schenker, M. Freund and S. B. Tsogoeva, Org. Biomol. Chem., 2010, 8, 5262–5276 RSC; (i) C. H. Cheon and H. Yamamoto, Chem. Commun., 2011, 47, 3043–3056 RSC.
- Selected references: (a) D. L. Boger, J. A. McKie, T. Nishi and T. Ogiku, J. Am. Chem. Soc., 1997, 119, 311–325 CrossRef CAS , and references cited therein; (b) N. Phay, T. Higashiyama, M. Tsuji, H. Matsuura, Y. Fukushi, A. Yokota and F. Tomita, Phytochemistry, 1999, 52, 271–274 CrossRef CAS; (c) F. P. Guengerich, J. L. Sorrells, S. Schmitt, J. A. Krauser, P. Aryal and L. Meijer, J. Med. Chem., 2004, 47, 3236–3241 CrossRef CAS; (d) P.-L. Wu, Y.-L. Hsu and C.-W. Jao, J. Nat. Prod., 2006, 69, 1467–1470 CrossRef CAS.
- For a review: G. Adair, S. Mukherjee and B. List, Aldrichimica Acta, 2008, 41, 31–39 Search PubMed.
- B. E. Maki and K. A. Scheidt, Org. Lett., 2009, 11, 1651–1654 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and analysis data for new compounds. CCDC reference number 808591 ((S)-4g). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1sc00190f |
| This journal is © The Royal Society of Chemistry 2011 |