Reactivity studies on [Cp′FeI]2: From iron hydrides to P4-activation†
Marc D.
Walter
*a,
Jörg
Grunenberg
b and
Peter S.
White
c
aInstitut für Anorganische und Analytische Chemie, Technische Universität Braunschweig, Hagenring 30, 38106, Braunschweig, Germany. E-mail: mwalter@tu-bs.de; Fax: +49 531-391-5309; Tel: +49 531-391-5312
bInstitut für Organische Chemie, Technische Universität Braunschweig, Hagenring 30, 38106, Braunschweig, Germany
cDepartment of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599-3290, USA
First published on 12th August 2011
Abstract
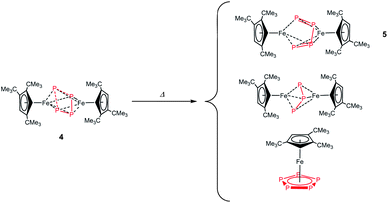
Metathesis of [Cp′FeI]2 (1) with KHBEt3 affords the polyhydride iron complexes [Cp′FeH2]2 (2) and [Cp′2Fe2H3] (3). The ratio in which both 2 and 3 are obtained correlates to the applied H2 pressure during synthesis. Complex 2 activates CH- or CD- bonds in aromatic compounds and shows slow H/D exchange in the presence of D2 at room temperature in cyclohexane solvent. [Cp′FeH2]2 acts as a Cp′Fe(I)-synthon when reacted with white phosphorus (P4) to give [Cp′Fe]2(μ-P4) (4) as the only P-containing product. This complex is best described as a triple-decker complex with a planar arrangement of a severely distorted kite-like cyclo-P4 unit. This distortion persists in solution and solid state as evidenced by a small PP coupling constant in the 31P{1H} NMR spectrum and a long P–P distance of 2.53 Å. Complex 4 is an isomer to the long-known [{Cp′Fe}2(μ-η4:η4-P4)] (5) with a cis-tetraphosphabutadiene moiety and it thermally rearranges to 5, [{Cp′Fe}2(μ-η3:η3-P3)] and [Cp′Fe(P5)]. All complexes described in this paper have been completely characterized including X-ray crystallography, variable temperature NMR studies and DFT calculations. Relaxed force constants (inverse compliance constants) are used as bond strength descriptors.
Introduction
The exploration of low-coordinate iron complexes as possible models systems for the active sites in metalloenzymes has been the focus of many groups.1 A common motif to all these investigations is the stabilization of low-coordinate iron fragments by sterically demanding ligands which allow highly unusual molecules to be synthesized and their chemistry to be explored.2–10 Despite the fact that ferrocene, (C5H5)2Fe, is one of the iconic complexes in organometallic chemistry, low-coordinate, open-shell mono(cyclopentadienyl) iron complexes have not been explored extensively. One reason is the competitive formation of thermodynamically favored Cp2Fe. Sterically demanding alkylcyclopentadienyl ligands are a prerequisite for the isolation of high-spin mono(cyclopentadienyl) iron(II) complexes and their kinetic stability results from the effective blocking of the dismutation pathway. In this context the synthesis of [(nCp)FeBr]2 (5Cp = C5(CH2Me2)5, 4Cp = C5H(CHMe2)4 and Cp′ = 1,2,4-C5H2(CMe3)3) without additional donor ligands has been reported.11,12 These species can be obtained as stable compounds and react with substituted phenolates to yield monomeric and dimeric complexes depending on the steric bulk of the phenolate ligand.12 More recently σ/π-rearrangements of aryl ligands connected to the Cp′Fe-fragment have been reported,13–15 whereas the related 5CpFe(C5H3-2,6-(iPr)2) derivative is a single-molecule magnet.16The synthesis of organophosphorus compounds directly from elemental phosphorus is a challenging process for chemists and the chemical industry, and involves the hazardous chlorination of P4 to PCl3.17,18 Therefore, it would be environmentally beneficial to develop mild metal-mediated reactions which allow direct P4 functionalization to form new P–C and/or P–H bonds based on abundant and environmentally benign metals.19 Hence, iron hydrides might be an intriguing starting material for this transformation. The only example of an iron polyhydride cluster supported exclusively by cyclopentadienyl ligands is [(C5Me5)FeH2]2 (prepared from (C5Me5)FeCl(tmeda) and LiAlH4),20 which exhibits a rich reaction chemistry with borane,21,22 silanes20,23 and phosphines.20 However, the use of sterically demanding ligands such as Cp′ can alter reactivity patterns significantly as previously shown for other metals.24–29
We have set out investigating the reactivity of [Cp′FeI]2 (1) focusing on synthetically attractive target molecules. In the course of these studies we have recently shown that 1 is a good synthon for the [Cp′Fe]+ fragment which has been successfully transferred to (POCOP)Ir pincer complexes.30 In this contribution the reaction chemistry of 1 with KHBEt3 is reported to give either [Cp′FeH2]2 (2) or [Cp′2Fe2H3] (3) depending on the reaction conditions. Subsequent activation of white phosphorus (P4) by [Cp′FeH2]2 is discussed.
Results and discussions
Iron polyhydrides
The reaction of 1 with KHBEt3 in a pentane/THF mixture under an Ar atmosphere did not yield the expected molecule possessing “[Cp′FeH]” units, instead a mixture of two different Fe-polyhydrides was isolated and identified as the diamagnetic [Cp′FeH2]2 (2) and paramagnetic mixed-valent [Cp′2Fe2H3] (3). The formation of these complexes was rather surprising, and spurred us on to investigate this reaction in more detail.The ratio in which complexes 2 and 3 are formed strongly depends on the reaction conditions: Under 1 atm of Ar the mixed-valent complex 3 is obtained as the major product (in a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 ratio of 3
60 ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). However, the product distribution is inverted, when the synthesis is carried out in the presence of H2 (1 atm), and a nearly complete formation of 2 (∼95%) may be achieved at elevated hydrogen pressures (15 atm pressure of H2). In general, the reaction proceeds less smoothly under Ar (28% total yield) compared to reactions carried out under H2 (52% total yield). An isolated mixture of 2 and 3 is stable under an H2 atmosphere and the relative ratio of 2 and 3 does not change. These observations suggest that “[Cp′FeH]” is formed initially, but it is unstable and converted to 2 and 3.‡ The formation of 2 is a consequence of trapping of the reactive intermediate “[Cp′FeH]” with H2, while 3 is formed as the major degradation product of “[Cp′FeH]” (in the absence of a suitable trap) (Scheme 1). Fig. 1 shows the linear relationship between the ratio in which complexes 2 and 3 are formed and the applied pressure of H2. It also reflects the high efficiency with which H2 traps the unobserved “[Cp′FeH]” intermediate. Complexes 2 and 3 can be isolated independently and are very soluble in all common organic solvents to give deep purple and deep green solutions, respectively. Solutions of both complexes are thermally stable at ambient temperature for an extended period of time, and can be exposed to vacuum without apparent degradation. Variable temperature NMR studies on 2 and 3 have been undertaken (see ESI for details†). The chemical shifts of 3 exhibit a linear temperature dependence consistent with a simple Curie-paramagnet. When heated in C6D6 at 65 °C, 3 shows a significantly higher thermal stability than 2, which is probably due to a relatively facile loss of H2 from this molecule and hence generation of the unstable “CpFeH” fragment. However, this behavior might have interesting synthetic applications (vide infra).
2). However, the product distribution is inverted, when the synthesis is carried out in the presence of H2 (1 atm), and a nearly complete formation of 2 (∼95%) may be achieved at elevated hydrogen pressures (15 atm pressure of H2). In general, the reaction proceeds less smoothly under Ar (28% total yield) compared to reactions carried out under H2 (52% total yield). An isolated mixture of 2 and 3 is stable under an H2 atmosphere and the relative ratio of 2 and 3 does not change. These observations suggest that “[Cp′FeH]” is formed initially, but it is unstable and converted to 2 and 3.‡ The formation of 2 is a consequence of trapping of the reactive intermediate “[Cp′FeH]” with H2, while 3 is formed as the major degradation product of “[Cp′FeH]” (in the absence of a suitable trap) (Scheme 1). Fig. 1 shows the linear relationship between the ratio in which complexes 2 and 3 are formed and the applied pressure of H2. It also reflects the high efficiency with which H2 traps the unobserved “[Cp′FeH]” intermediate. Complexes 2 and 3 can be isolated independently and are very soluble in all common organic solvents to give deep purple and deep green solutions, respectively. Solutions of both complexes are thermally stable at ambient temperature for an extended period of time, and can be exposed to vacuum without apparent degradation. Variable temperature NMR studies on 2 and 3 have been undertaken (see ESI for details†). The chemical shifts of 3 exhibit a linear temperature dependence consistent with a simple Curie-paramagnet. When heated in C6D6 at 65 °C, 3 shows a significantly higher thermal stability than 2, which is probably due to a relatively facile loss of H2 from this molecule and hence generation of the unstable “CpFeH” fragment. However, this behavior might have interesting synthetic applications (vide infra).
![Reactivity of [Cp′FeI]2 with KHBEt3.](/image/article/2011/SC/c1sc00413a/c1sc00413a-s1.gif) | ||
| Scheme 1 Reactivity of [Cp′FeI]2 with KHBEt3. | ||
![Product ratio of [Cp′FeH2]2 and [Cp′2Fe2H3] as a function of applied H2 pressure during synthesis.](/image/article/2011/SC/c1sc00413a/c1sc00413a-f1.gif) | ||
| Fig. 1 Product ratio of [Cp′FeH2]2 and [Cp′2Fe2H3] as a function of applied H2 pressure during synthesis. | ||
The diamagnetism of 2 facilitates characterization and reactivity studies by NMR spectroscopy, e.g. measurements of the inversion-recovery T1 relaxation time of the Fe–H resonances (δ −21.9) at variable temperatures are consistent with the description of a classic hydride complex (0.76 ms at −98 °C, 500 MHz). The bonding in 2 is similar to that in [(C5Me5)RuH2]2 which has been analyzed by ab initio studies on the model complex [(C5H5)RuH2]2.31,32 Slow H/D exchange occurs between D2 and the iron hydride ligands in 2 at ambient temperature in C6H12 solvent. This exchange was fitted to a pseudo first-order kinetic and the rate constant was determined to be 3.4(4) × 10−5 s−1 at 298 K which corresponds to a ΔG‡(298 K) = 23.3(2) kcal mol−1 (see Experimental Section and ESI for details†). The exchange is slower than in [(C5Me5)FeH2]2,20 presumably a consequence of the increased steric bulk of the Cp′ ligand, and consistent with an associative ligand exchange mechanism as proposed for [(C5Me5)RuH2]2 based on experimental33 and computational studies.34 In agreement with previous observations for [(C5Me5)FeH2]220 no aliphatic C–H bond activation of the solvent or the CMe3-groups of the Cp′ ligand was observed. However, slow H/D exchange occurs at room temperature between deuterated aromatic solvents such as C6D6 and C7D8 and the Fe–H functionality, while the exchange is fast at 90 °C. In the case of toluene-d8 only the less sterically hindered aromatic meta- and para-positions are activated with roughly the same rate (as judged by the amount of H incorporation). The decay of the Fe–H resonance on H/D exchange in C7D8 was monitored over time by 1H NMR spectroscopy and fit to a first-order decay model. The rate constants were determined over a temperature range 62–96 °C; and the values ranged from 3.9(2) × 10−5 to 7.8(3) × 10−4 s−1. The Eyring plot for this process is depicted in Fig. 2 and corresponds to ΔH‡ = 21.2(9) kcal mol−1 and ΔS‡ = −17(3) eu (see ESI for details†). The negative sign and large magnitude of ΔS‡ indicate an associative exchange process.
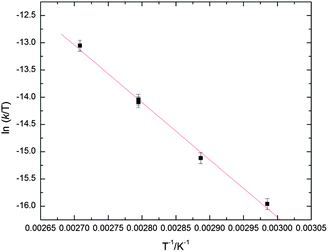 | ||
| Fig. 2 Eyring plot for the H/D exchange in 2 recorded in C7D8 (error bars shown at 95% confidence interval) yielding the estimated parameters ΔH‡ = 21.2(9) kcal mol−1 and ΔS‡ = −17(3) eu. | ||
Complex 2 acts as a slow hydrogenation catalyst for 2,2-dimethylbutene (TBE) in the presence of 1 atm of H2 in C6D6 at 65 °C (7 TON after 50 h). However, this reaction is accompanied by significant catalyst degradation to give a mixture of 3 and HCp′. Under an argon atmosphere complete degradation of 2 occurs in the presence of an excess of TBE in C6H12 at 65 °C within a few hours, while no degradation is observed for 3 under these conditions. This reactivity difference allows the isolation of 3 in pure crystalline form, albeit in low yield. No conversion of 3 to 2 was observed in the presence of a H-radical source such as 9,10-dihydroanthracene. The solution magnetic moment of 2.0(2) μB for complex 3 is consistent with one unpaired electron (S = ½) per molecule. The bonding in 3 can be explained by the frontier orbital diagram developed for [(L3)2M2H3] complexes.35,36
The molecular structures of 2 and 3 were determined by X-ray crystallography (Table 1) and are shown in Fig. 3 and 4. Selected bond distances and angles are given in the Figure captions. The methodology used for locating metal hydrides in Fourier density maps was previously described by Ibers and Bau.37,38 In both cases the hydride ligands were located in the Fourier density map as peaks of the expected height and the R1-factor dropped significantly when the hydrides were included in the least-square refinement cycles and refined isotropically (see ESI for details†). Further trust was gained by the fact that the Fe–H distance in 2 and 3 agree well with M–H bond distances determined by neutron or X-ray diffraction for related molecules.20,39–41
| Compound reference | 2 | 3 | 4 |
|---|---|---|---|
| Chemical formula | C34H62Fe2 | C34H61Fe2 | C34H58Fe2P4 |
| Formula Mass | 582.54 | 581.53 | 702.38 |
| Crystal system | Tetragonal | Triclinic | Orthorhombic |
| a/Å | 9.0398(2) | 10.2629(2) | 13.3878(3) |
| b/Å | 9.0398(2) | 12.4476(3) | 13.7768(3) |
| c/Å | 40.2263(12) | 14.5233(3) | 19.6471(4) |
| α (°) | 90.00 | 112.514(2) | 90.00 |
| β (°) | 90.00 | 91.655(2) | 90.00 |
| γ (°) | 90.00 | 105.741(2) | 90.00 |
| Unit cell volume/Å3 | 3287.21(14) | 1631.16(7) | 3623.73(14) |
| T/K | 100(2) | 100(2) | 100(2) |
| Space group | P 41212 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P 212121 |
| No. of formula units per unit cell, Z | 4 | 2 | 4 |
| Radiation type | Cu-Kα | Mo-Kα | Mo-Kα |
| Absorption coefficient, μ/mm−1 | 7.191 | 0.908 | 0.999 |
| No. of reflections measured | 22777 | 12986 | 51726 |
| No. of independent reflections | 3095 | 6865 | 7410 |
| R int | 0.0375 | 0.0389 | 0.0447 |
| Final R1 values (I > 2σ(I)) | 0.0253 | 0.0459 | 0.0388 |
| Final wR(F2) values (I > 2σ(I)) | 0.0648 | 0.0930 | 0.0845 |
| Final R1 values (all data) | 0.0262 | 0.0839 | 0.0476 |
| Final wR(F2) values (all data) | 0.0653 | 0.1076 | 0.0879 |
| Goodness of fit on F2 | 1.055 | 1.005 | 1.025 |
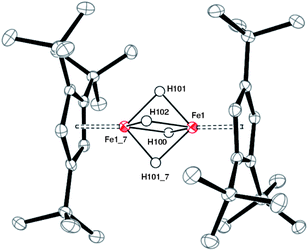 | ||
| Fig. 3 ORTEP diagram of 2. ‘_7’ indicate symmetry related positions. Selected bond distances (Å) and angles (deg): Cp1(cent)-Fe(1) 1.66, Fe(1)⋯Fe(1_7) 2.1989(5), Fe(1)-H(100) 1.61(3), Fe(1)-H(101) 1.57(3), Fe(1)-H(102) 1.63(3), H(100)-H(101) 1.65(4), H(101)-H(102) 1.62(4), H(102)-H(101_7) 1.62(4), H(101_7)-H(100) 1.65(4), H(100)-H(101)-H(102) 93.3, H(101)-H(102)-H(101_7) 87.9, H(102)-H(101_7)-H(100) 93.3. | ||
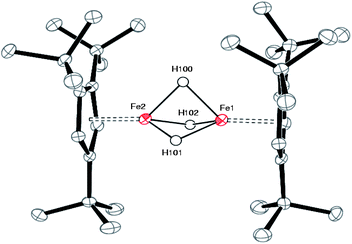 | ||
| Fig. 4 ORTEP diagram of 3. Selected bond distances (Å) and angles (deg): Cp1(cent)-Fe(1) 1.71, Cp2(cent)-Fe(1) 1.71, Fe(1)⋯Fe(2) 2.2205(6), Fe(1)-H(100) 1.64(4), Fe(1)-H(101) 1.54(4), Fe(1)-H(102) 1.56(4), Fe(2)-H(100) 1.66(4), Fe(2)-H(101) 1.57(4), Fe(2)-H(102) 1.49(4), H(100)-H(101) 1.87(6), H(101)-H(102) 1.94(6), H(102)-H(100) 1.96(6), H(100)-H(101)-H(102) 61.8, H(101)-H(102)-H(100) 57.4, H(102)-H(100)-H(101) 60.8. | ||
Reactivity towards P4
With complexes 2 and 3 in hand, we decided to explore their reactivity toward P4. Recent studies have mainly focused on the activation of P4 in the coordination sphere of coordinatively unsaturated metal fragments42–52 and in the presence of organic substrates,17–19e.g. the photochemical or thermal conversion of [Cp′Fe(CO)2]2 in the presence of P4 results in a surprisingly broad range of different Pn-containing molecules by successive lose of coordinated CO, such as [{Cp′(OC)2Fe}2(μ-η1:η1-P4)], [Cp′Fe(P5)], [{Cp′Fe}2(μ-η4:η4-P4)], [{Cp′Fe}(μ-η4:η1-P4){Fe(CO)2Cp′}], [{Cp′Fe}2(μ-CO)(μ-η2:η2-P2)] and [{Cp′Fe}2(μ-P)2].53–55 In a more recent approach low-valent transition metallates are used for P4 activation.56,57 For the latest reviews on P4 activation the reader may refer to refs. 58–60. However, with very few exceptions such as [(triphos)RhH3]61,62 and [(C5Me5)(C5H4tBu)ZrH2]63 reactions with transition metal hydrides and P4 have received very little attention. This is rather surprising considering that the reaction of metal hydrides with dinitrogen is known to be a very attractive route to dinitrogen complexes with different levels of N2 activation.64,65 It was therefore of interest to explore the reactivity of 2 and 3 toward P4 and to compare the results with those of [Cp′Fe(CO)2]2.No reaction of 3 was observed with an excess of P4 at 100 °C in C7D8, which is probably related to its inability to act as a Cp′Fe(I)-synthon and its significantly enhanced thermal stability when compared to 2. However, 2 reacts under the same conditions to a single P-containing complex 4, while H2 is evolved accompanied by a color change from purple to dark red (Scheme 2). No new P–H or Fe–H bonds were formed as shown by 1H and 31P NMR spectroscopy. This suggested that complex 2 can act as a masked Fe(I) synthon which offers interesting perspectives for further transformations.§ Dark red crystals of 4 suitable for a single crystal X-ray diffraction experiment were obtained from a saturated toluene solution at −38 °C (Table 1). Selected bond distances are given in the Figure caption (Fig. 5).
![Reactivity of [Cp′FeH2]2 with P4.](/image/article/2011/SC/c1sc00413a/c1sc00413a-s2.gif) | ||
| Scheme 2 Reactivity of [Cp′FeH2]2 with P4. | ||
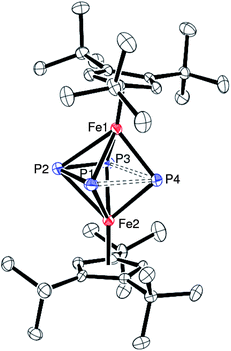 | ||
| Fig. 5 ORTEP diagram of 4 (thermal ellipsoids set at 50% probability). Hydrogen atoms have been omitted for clarity. Selected bond distances (Å) and angles (deg): Cp1(cent)-Fe(1) 1.71, Cp2(cent)-Fe(2) 1.71, Fe(1)-P4(cent) 1.62, Fe(2)-P4(cent) 1.62, Fe(1)-P(1) 2.3167(9), Fe(1)-P(2) 2.4526(10), Fe(1)-P(3) 2.2829(9), Fe(1)-P(4) 2.1913(10), Fe(2)-P(1) 2.2889(9), Fe(2)-P(2) 2.4526(10), Fe(2)-P(3) 2.3201(9), Fe(2)-P(4) 2.1893(10), P(1)-P(2) 2.12311(13), P(2)-P(3) 2.1371(13), P(3)-P(4) 2.5322(12), P(1)-P(4) 2.5314(12), Fe(1)⋯Fe(2) 3.1822(6), Cp1(cent)-Fe(1)-P4(cent) 172.96, Cp2(cent)-Fe(2)-P4(cent) 172.45, Fe(1)-P4(cent)-Fe(2) 157.3. | ||
The 31P{1H} NMR data of 4 confirmed the formation of an A2MX-spin system (PA = P(1),P(3); PM = P(2); PX = P(4)). The PA nucleus appears as a doublet of doublets at δ 625.5 (1JAM = 380 Hz, 1JAX = 126 Hz). The small PA-PX coupling constant of 1JPP = 126 Hz reflects the long distances between the atoms PA and PX observed in the crystal structure. The PX and PM resonances are both split into a doublet of triplets and reveal remarkable high-field shifts at δ −163.1 and δ −336.5, respectively (see ESI for details†). The crystal structure of 4 reveals a planar cyclo-P4 unit capped by two Cp′Fe moieties (Fe⋯Fe distance: 3.1822(6) Å) However, the P4-unit shows a significant kite-like distortion, and consequently two short 2.13 Å and two long P–P distances (2.53 Å) are observed. The longer distances are intermediate between a P–P bond and a van-der-Waals contact.66,67 In combination with the very small 1JAX coupling constant of 126 Hz the P–P bonding appears to be weak. Interestingly, the isoelectronic [{(C5H4CMe3)Co}2(μ2-η4-As4)]2+ complex shows a nearly square planar cyclo-As4 ring.68 However, complexes with cyclo-P4 do exist, and show different degrees of distortion, e.g. a rather small distortion was observed for mononuclear complexes of the second- and third-row transition metals such as (C5Me5)M(CO)2(η-P4) (M = Nb69 and Ta70), whereas a severe kite-like distortion was reported for the trimetallic Co-cluster [Cp′′Co(P4){(Cp′′Co)2(μ-CO)}] (Cp′′ = 1,3-(Me3C)2C5H3). Conversely, multi-decker complexes with a cyclo-P4 middle deck have been elusive.45,58–60 This is rather surprising considering that Tremel and Hoffmann predicted the existence of triple-decker complexes with μ-cyclo-P4 middle deck based on frontier orbital argument more than 20 years ago,71 and showed that the 30/34 electron rule is of special importance for the stability of triple-decker complexes in general.72 However, the combined constraints of M–P distance, P–P contacts in the bridge, and M–M separation leaves only a small “window” for possible structures. Cyclo-P42− can bridge two uranium fragments in μ2:η4:η4-fashion (see (μ2:η4:η4-P4)[U(N[tBu]Ar)3]2)73 and μ2:η2:η2-fashion (see [U(C5Me5)(C8H6(SiiPr3)2−1,4)]2(μ2:η2:η2-P4)),74 and Fryzuk and co-workers described an inverse Zr sandwich complex with a formal cyclo-P44− unit.50 Recently, a novel binding mode for cyclo-E42− (E = P4, As4, AsP3) has been reported in which it forms terminal E4-decks bridging two Cr-centers.75 However, it was suggested that a kite-like distortion of the cyclo-P4 might point to a reaction pathway of the tetrahedral P4 in the coordination sphere of transition metal complexes with an odd number of valence electrons. The first step includes the cleavage of two P–P bonds to give a square-planar cyclo-P4 unit followed by a kite-like distortion, and finally disproportionation into P1 and cyclo-P3 complexes.44,48 Hence, 4 might be regarded as a “snapshot” of such a disproportionation process.
However, 4 is also an isomer to the long-known [{Cp′Fe}2(μ-η4:η4-P4)] (5). Complex 5 contains a cis-tetraphosphabutadiene moiety (isolobal to the cis-butadiendiyl-fragment) sandwiched by the two Cp′Fe-fragments (Fe⋯Fe distance: 2.6430(8) Å) and should therefore only be considered as a pseudo-triple-decker.53 In addition, dynamic 31P{1H} NMR studies for 5 and [{Cp′′′Fe}2(μ-η4:η4-P4)] revealed that the P-atoms of the cis-tetraphosphabutadiene chain undergo rapid exchange on the NMR time scale at ambient temperature. Therefore only one broad resonance is observed for the AA‘XX’-spin system in 5 (294 K: ν1/2 = 910 Hz). This interchange process of the different P-atoms in the less-substituted derivative [{Cp′′Fe}2(μ-η4:η4-P4)] can be frozen out at 185 K, whereas 5 shows fast exchange on the NMR time scale at this temperature.53,54 One possible transition state accounting for this exchange process is the 30VE-triple-decker complex with a cyclo-P4 middle-deck (closo-octaeder), and this makes isolation and characterization of 4 even more remarkable.
It was therefore of interest to establish the relationship between the two isomers 4 and 5, and also to probe the ability of 4 to act as source for “P1”/“P3” fragments. Complex 5 was prepared from [Cp′Fe(CO)2]2 and P4 under rather harsh reaction conditions, i.e. thermolysis at 190 °C for 3 h, and [Cp′Fe(η5-P5)] was also formed as a side-product. In contrast the synthesis of 4 is accomplished under relatively mild conditions (100 °C, 10 min) and obtained as sole P-containing product. Consequently, the thermal stability of 4 was investigated. Complex 4 is stable in solution at ambient temperature for at least a week. However, the thermal behavior of 4 in C7D8 solution is very informative. After heating a C7D8 solution of 4 for 7 days at 75 °C the 31P{1H} NMR spectrum shows a complete conversion of 4 into 5, [{Cp′Fe}2(μ-η3:η3-P3)] and [Cp′Fe(η5-P5)] in an approximate ratio of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 3).¶ The phosphaallyl complex [{Cp′Fe}2(μ-η3:η3-P3)] was identified by 31P{1H} and 1H NMR spectroscopy, and it was previously reported as a side-product in the reaction of [{Cp′(OC)2Fe}2(μ-η1:η1-P4)] with tBuC
1 (Scheme 3).¶ The phosphaallyl complex [{Cp′Fe}2(μ-η3:η3-P3)] was identified by 31P{1H} and 1H NMR spectroscopy, and it was previously reported as a side-product in the reaction of [{Cp′(OC)2Fe}2(μ-η1:η1-P4)] with tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P.76 The phosphaallyl [η3-P3] moiety displays an AA′X spin system with a large chemical shift difference between the two resonances PA,A′ (δ 677.8) and PX (δ −380.9). These chemical shifts are very similar to the A2M part of the A2MX spin system of 4 (PA: δ 625.5, PM: δ −336.5) supporting the earlier contention that the bonding between the A2M and X part in 4 is extremely weak, and it might be more appropriately described as cyclo-P42− consisting of an allylic-like μ-[P3]− anion coordinated to a μ-[P]− anion. However, the major degradation pathway of 4 is the formation of 5 (80%), but the elimination of a P1-fragment is also feasible as demonstrated by the formation of [{Cp′Fe}2(μ-η3:η3-P3)] and [Cp′Fe(η5-P5)]. The addition of a P1-fragment to 5 would formally give the paramagnetic, 31VE triple-decker complex [{Cp′Fe}2(μ-η5:η5-P5)] with a cyclo-P5 middle deck, but this species was not detected by 1H or 31P NMR spectroscopy. Interestingly, isolated 5 can be converted to [Cp′Fe(η5-P5)] in the presence of excess P4 at 190 °C.54 This observation might point to an inherent instability of [{Cp′Fe}2(μ-η5:η5-P5)]. In conclusion, this study provides experimental evidence for the original proposal that the kite-like distorted cyclo-P4 precedes the formation of P1- and P3-fragments, but also yields a cis-tetraphosphabutadiene system.
P.76 The phosphaallyl [η3-P3] moiety displays an AA′X spin system with a large chemical shift difference between the two resonances PA,A′ (δ 677.8) and PX (δ −380.9). These chemical shifts are very similar to the A2M part of the A2MX spin system of 4 (PA: δ 625.5, PM: δ −336.5) supporting the earlier contention that the bonding between the A2M and X part in 4 is extremely weak, and it might be more appropriately described as cyclo-P42− consisting of an allylic-like μ-[P3]− anion coordinated to a μ-[P]− anion. However, the major degradation pathway of 4 is the formation of 5 (80%), but the elimination of a P1-fragment is also feasible as demonstrated by the formation of [{Cp′Fe}2(μ-η3:η3-P3)] and [Cp′Fe(η5-P5)]. The addition of a P1-fragment to 5 would formally give the paramagnetic, 31VE triple-decker complex [{Cp′Fe}2(μ-η5:η5-P5)] with a cyclo-P5 middle deck, but this species was not detected by 1H or 31P NMR spectroscopy. Interestingly, isolated 5 can be converted to [Cp′Fe(η5-P5)] in the presence of excess P4 at 190 °C.54 This observation might point to an inherent instability of [{Cp′Fe}2(μ-η5:η5-P5)]. In conclusion, this study provides experimental evidence for the original proposal that the kite-like distorted cyclo-P4 precedes the formation of P1- and P3-fragments, but also yields a cis-tetraphosphabutadiene system.
 | ||
| Scheme 3 Thermal degradation of 4. | ||
Computational studies
Density functional theory (DFT) calculations in combination with experimental data have become a valuable combination of tools in organometallic chemistry since DFT accounts, in part, for electron correlation effects. Especially for large molecular systems and clusters of low symmetry, the low computational expense in comparison with post-Hartree–Fock methods makes DFT nearly always the only choice when it comes to electronic structure calculations of such complex systems. However, given the fact that the exact functional of the electron density is not known and only approximate expressions are at hand, an exhaustive evaluation of different Density Functionals (DFs) is a prerequisite for computational chemistry studies. Concerning our phosphorus compounds, this is of particular importance: It is known that the potential energy surfaces (PES) involving P4 are very flat and minute changes in the nature of the surrounding metal fragments —and hence in the nature of the describing DF— can modify the structure of the P4 unit, which is due to the low lying P–P σ*-orbitals, the number of lone pairs and the ability of phosphorus to assume a large variety of different coordination modes.47 We evaluated several DFs with the focus on optimizing the complex geometries and on comparing these results to the available X-ray crystallographic data of 2, 3, 4 and 5. Surprisingly, most of the modern hybrid and hybrid meta DFs such as B3PW91, M05, M052x and M06 did not reproduce the kite-like distortion of the central cyclo-P4 unit in 4; instead a triple-decker complex with an aromatic, square-planar cyclo-P4 bridge was also predicted to be thermodynamically more stable than 5. The reason for this inconsistency might be twofold: 1) pronounced packing effects in the solid state, and/or 2) uncertainties and several, in part diametrical, intrinsic problems of the modern DFs. The molecular systems 4 and 5 for example comprise a variety of physical phenomena which until today present test cases for modern computational chemistry methods: steric effects (that means long range dispersion), delocalization vs. localization (that means dynamic correlation) and state mixing (that means static correlation). Several DFT functionals have been employed to rationalize (transition) metal mediated P4 activation and explain the bonding in Pn-fragment containing complexes.48,74,76–80 The best agreement between experimental and calculated structures have been achieved with the dispersion-corrected B97 functional, B97D81 in combination with the 6-311G(d,p) basis set. Selected experimental and calculated bond angles and distances are compared in Table 2.| [2] (exp) | [2] (calc) | [3] (exp) | [3] (calc) | [4] (exp) | [4] (calc) | [4′] (calc) | [5] (exp) | [5] (calc) | |
|---|---|---|---|---|---|---|---|---|---|
| Cp1(cent)-Fe1 | 1.66 | 1.61 | 1.71 | 1.67 | 1.71 | 1.65 | 1.68 | 1.74 | 1.71 |
| Cp2(cent)-Fe2 | 1.66 | 1.61 | 1.71 | 1.67 | 1.71 | 1.65 | 1.68 | 1.74 | 1.71 |
| Fe1⋯Fe2 | 2.1989 | 2.162 | 2.2005 | 2.219 | 3.1822 | 3.335 | 3.266 | 2.6430 | 2.568 |
| Fe1–H1 | 1.61(3) | 1.67 | 1.64(4) | 1.65 | |||||
| Fe2–H1 | 1.61(3) | 1.66 | 1.66(4) | 1.65 | |||||
| Fe1–H2 | 1.57(3) | 1.66 | 1.54(4) | 1.66 | |||||
| Fe2–H2 | 1.57(3) | 1.66 | 1.57(4) | 1.64 | |||||
| Fe1–H3 | 1.63(3) | 1.64 | 1.56(4) | 1.66 | |||||
| Fe2–H3 | 1.63(3) | 1.67 | 1.49(4) | 1.65 | |||||
| Fe1–H4 | 1.61(3) | 1.65 | |||||||
| Fe2–H4 | 1.61(3) | 1.65 | |||||||
| Fe1- P1 | 2.3167 | 2.331 | 2.343 | 2.2649 | 2.256 | ||||
| Fe2–P1 | 2.2889 | 2.296 | 2.328 | 2.2678 | 2.254 | ||||
| Fe1–P2 | 2.4526 | 2.484 | 2.497 | 2.3337 | 2.317 | ||||
| Fe2–P2 | 2.4526 | 2.484 | 2.491 | 2.3346 | 2.337 | ||||
| Fe1–P3 | 2.2829 | 2.296 | 2.323 | 2.3337 | 2.337 | ||||
| Fe2–P3 | 2.3201 | 2.331 | 2.344 | 2.3346 | 2.317 | ||||
| Fe1–P4 | 2.1913 | 2.212 | 2.212 | 2.2649 | 2.254 | ||||
| Fe2–P4 | 2.1893 | 2.212 | 2.209 | 2.2678 | 2.256 | ||||
| H1⋯H2 | 1.65(4) | 1.77 | 1.87(6) | 2.08 | |||||
| H2⋯H3 | 1.62(4) | 1.77 | 1.94(6) | 2.13 | |||||
| H3⋯H4 | 1.62(4) | 1.77 | 1.96(6) | 2.15 | |||||
| H4⋯H1 | 1.65(4) | 1.77 | |||||||
| P1–P2 | 2.1311 | 2.188 | 2.131 | 2.0877 | 2.112 | ||||
| P2–P3 | 2.1371 | 2.188 | 2.137 | 2.368 | 2.391 | ||||
| P3–P4 | 2.5322 | 2.369 | 2.532 | 2.0877 | 2.112 | ||||
| P4–P1 | 2.5314 | 2.369 | 2.531 | 3.555 | 3.570 | ||||
| H1-H2-H3 | 93.3 | 90.1 | 61.8 | 61.3 | |||||
| H2-H3-H4 | 87.9 | 90.1 | 57.4 | 58.4 | |||||
| H3-H4-H1 | 93.3 | 89.8 | 60.8 | 60.3 |
However, before discussing the results concerning the P4 activation product in detail, the iron hydride complexes 2 and 3 were investigated. Experimental and computational geometries are in good agreement (Table 2). As suggested above 2 and 3 are most likely derived from a “[Cp′FeH]” intermediate that could not be isolated, but trapped H2 to give 2 in good yields. DFT calculations were undertaken to predict a possible structure for the intermediate [Cp′FeH]2 assuming a diamagnetic ground state (S = 0). Addition of H2 or H• to [Cp′FeH]2 is exergonic with ΔG(298 K) = −22.4 and −62.3 kcal mol−1 to give 2 and 3, respectively. This explains the intrinsic instability of [Cp′FeH]2 with respect to the formation of 2 and 3, but also the enhanced thermal stability of 3 compared to 2. Complex 2 undergoes H/D exchange presumably via an associative reaction mechanism, and the calculated structure of the proposed reaction intermediate [Cp′Fe(H)(η2-H2)]2 is shown in Fig. 6. [Cp′FeH(H2)]2 lies about 21.8 kcal mol−1 higher in energy than 2, and therefore it is in reasonable agreement with the experimental data.
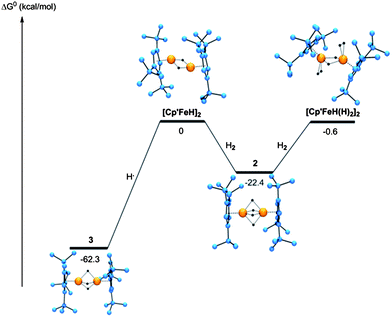 | ||
| Fig. 6 Gibbs Free Enthalpy of Iron Hydride Species. | ||
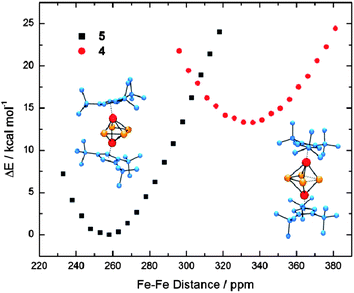 | ||
| Fig. 7 PES Scan along the Fe–Fe coordinate of 4 and 5. | ||
B97D also reproduces the kite-like distortion of the central cylclo-P4 middle deck and predicts isomer 5 to be more stable than 4 by 10.4 kcal mol−1 (free enthalpy) or 12.6 kcal mol−1 (electronic energy). However, the distortion is less pronounced than observed in solid state.|| Freezing the experimentally observed pronounced kite-like distortion of the cyclo-P4 middle-deck in the DFT it increases the electronic energy of 4′ relative to 4 by ca. 4.1 kcal/mol. A PES scan along the Fe⋯Fe reaction coordinate for 4 and 5 shows how flat the potential energy surface actually is in the proximity of the calculated minima structures (Fig. 8). Unfortunately, every attempt to locate the transition state between complexes 4 and 5 has been unsuccessful so far.
We also addressed the question if the distortion of the cyclo-P4 unit is a consequence of the steric demand of the Cp′ ligand. Maybe surprisingly, replacing the Cp′ ligand against C5H5 has no effect on the kite-like distortion, but the thermodynamic isomer 5a is significantly stabilized compared to 4a by 15.1 kcal mol−1 (see ESI for details†). We therefore conclude that the kite-like distortion is not a consequence of crystal packing and dispersion effects, but an intrinsic phenomenon for this system.
The long P–P bond distance and the small 1JPP coupling constant in the cyclo-P4 unit in 4 raise the question: How strong are the bonds within the cyclo-P4 unit or is it more appropriate to describe the distorted cyclo-P4 middle-deck as a P1/P3 moiety? In this context, local bond lengths in complex molecular systems are not necessarily good bond strength descriptors, since secondary interactions may lead to compressed (but weak; see the Ga–Ga discussion82) or elongated (because they are weak; see molecular recognition83) local bonds. Relaxed force constants can answer this type of question by measuring force due to a unit displacement, while all other coordinates are allowed to relax providing unique information about the intrinsic elasticity of bonds.84 There are more and more examples in the literature, where the significance of mechanical properties indeed helped to unravel otherwise quite obscure bonding situations.85–87Inter alia, different types of PP bonds have recently been evaluated by this method applying the DFT and coupled cluster methods. These studies have shown that relaxed force constants for P(III)-P(III) bonds correlate almost linearly to the formal P–P bond order. Furthermore, PP bonds are highly elastic, and can easily be deformed by inter- and intramolecular forces.88,89 Due to these calculations, the mechanical strength of a typical 2-electron P–P single bond is ∼1.5 N cm−1 while the value for a 4-electron P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P double bond averages out at ∼3.3 N cm−1. Therefore, we expected the value for an aromatic P–P bond to be between 2.1 and 2.4 N cm−1. Interestingly, our calculated P–P relaxed force constant for the isolated D4h symmetric P42− of just 1.50 N cm−1 (B97D/6-311G(d,p)) points to four equal P–P single bonds with no double bond character and therefore no π-contribution. This interpretation is supported by the positive sign of our computed compliance coupling constant between each germinal P–P bonds. Indeed, earlier computational analysis of the bonding in P5− and P42− has clearly shown that the charge is mainly localized via the P lone pairs, and therefore the term “lone-pair aromaticity” was suggested.90–92 This clearly demonstrates that care has to been taken when the well-established concept of (Hückel) aromaticity in classic hydrocarbons such as benzene, cyclopentadiene anion, C5H5−, or the cyclobutadiene diananion, C4H42−,93 is applied to inorganic compounds of main group elements. Consequently, not only the π-orbitals but also the eight in-plane σ-orbitals of the cyclo-P4 unit, four radial and four tangential ones, need to be considered in the interaction with the two CpFe fragments (vide infra).
P double bond averages out at ∼3.3 N cm−1. Therefore, we expected the value for an aromatic P–P bond to be between 2.1 and 2.4 N cm−1. Interestingly, our calculated P–P relaxed force constant for the isolated D4h symmetric P42− of just 1.50 N cm−1 (B97D/6-311G(d,p)) points to four equal P–P single bonds with no double bond character and therefore no π-contribution. This interpretation is supported by the positive sign of our computed compliance coupling constant between each germinal P–P bonds. Indeed, earlier computational analysis of the bonding in P5− and P42− has clearly shown that the charge is mainly localized via the P lone pairs, and therefore the term “lone-pair aromaticity” was suggested.90–92 This clearly demonstrates that care has to been taken when the well-established concept of (Hückel) aromaticity in classic hydrocarbons such as benzene, cyclopentadiene anion, C5H5−, or the cyclobutadiene diananion, C4H42−,93 is applied to inorganic compounds of main group elements. Consequently, not only the π-orbitals but also the eight in-plane σ-orbitals of the cyclo-P4 unit, four radial and four tangential ones, need to be considered in the interaction with the two CpFe fragments (vide infra).
Turning to the bonding situation in complex 4 the P–P interaction seems to become even softer compared to isolated cyclo-P42−: the kite-like distortion is accompanied by a pronounced weakening of the P–P bonds: The relaxed force constant values of 1.16 N cm−1 (short contact) and only 0.88 N cm−1 (long contact; both are the B97D/6-311G(d,p) values) illuminate the reason for the discrepancies between theory (gas phase) and experiment in terms of P–P distances: the exceptional softness of the P–P potential in 4. In addition, we preclude any pronounced Fe⋯Fe covalency or coupling in 4: the relaxed force constant of 0.80 N cm−1 is far from the value for a typical Fe–Fe two electron bond in, for example, Fe2(CO)9 (2.30 N cm−1,94). In contrast, the Fe–Fe contact in isomer 5 is characterized by a pronounced coupling: the relaxed force constants of 1.51 N cm−1 is in line with a molecular orbital analysis. To simplify the bonding discussion in 4 and 5 the Cp′ ligand was replaced by C5H5 and C2v symmetry was imposed on both molecules. These two model complexes 4′′ and 5′′ have very different Fe–Fe distances of 3.271 and 2.514 Å, respectively. Increasing the Fe–Fe distance (and decreasing the P–P distance) turns on a significant P–P repulsion. Consequently, two different orbitals react very sensitive to the variations of the Fe–Fe distance, that is the Fe–Fe σ*-orbital (b2) and the P–P σ*-orbitals (b1). The relevant Kohn–Sham orbitals are shown in Fig. 8. While the antibonding σ*-orbital is occupied in 4′′ (HOMO-2), it is unoccupied in 5′′ (LUMO) which is consistent with the relaxed force constant analysis. The driving force for the conversion of 4′′ to 5′′ may be traced to the formation of an Fe–Fe bond. Furthermore, the HOMO of 4′′ also suggests that the lone pairs at P will be accessible to electrophiles.
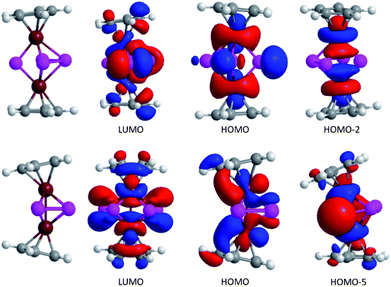 | ||
| Fig. 8 Selected Kohn–Sham orbitals for 4′′ and 5′′. | ||
Conclusions
Iron hydride complexes 2 and 3 were generated on salt metathesis of 1 with KHBEt3. Interestingly, the product distribution of 2 and 3 is directly proportional to the applied pressure of H2 during synthesis, suggesting that H2 is a highly efficient trap for the “[Cp′FeH]”-intermediate. The diamagnetic tetrahydride complex 2 is an excellent Cp′Fe(I)-synthon, and reacts smoothly with P4 to triple-decker complex 5, in which the two Cp′Fe-fragments are bridged by a severe kite-like distorted [cyclo-P4] unit. This distortion persists in solution and solid state, however, on prolonged heating 4 converts to the well-known pseudo-triple-decker complex 5 in which the Cp′Fe-units are bridged by a cis-tetraphosabutadiendiyl chain, but also to [{Cp′Fe}2(μ-η3:η3-P3)] and [Cp′Fe(η5-P5)]. This established 4 as intermediate in the thermodynamically favored formation of 5, and as a precursor for delivery P1-fragments. Due to our computations, the interatomic forces within the P4 moiety are weak and comparable to other P–P two-electron sigma bonds (in the case of isolated P4) or electron deficient clusters, leading to facile distortions of the quadratic planer D4h geometry in 4 and 5 without a pronounced energy penalty. The term “aromaticity” might be overstressed in this case, and we predict that the distortion (kite-like or butadiene-like) of the P4 fragment in triple-decker systems will be observed more frequently in the future. While any coupling in the metastable isomer 4 can be excluded, a considerably Fe–Fe interaction in 5 is observed, leading to a pronounced stabilization of this very isomer.In summary, the Cp-fragment has once again shown that it allows for much more than only ferrocene formation. These initial studies also suggest that this ligand set might exhibit complementary reactivity patterns to the trisphosphinoborato and β-diketiminato ligands. Therefore we are currently actively pursuing the synthesis of other synthetically attractive target functionalities for low-coordinate Cp′Fe-half-sandwich complexes. Further investigations regarding the reaction chemistry and electronic structure of the molecules presented here, are subject of ongoing work and will be reported in due course. In addition the reactivity of the complex 5 towards electrophiles with the aim to functionalize P4 in the iron coordination sphere will be evaluated.
Experimental section
General comments
All reactions and product manipulations were carried out under an atmosphere of dry, oxygen free argon using standard high-vacuum, Schlenk, or drybox techniques. Argon was purified by passage through BASF R3-11 catalyst (Chemalog) and 4 Å molecular sieves. Dry, oxygen-free solvents were employed throughout. NMR spectra were recorded on a Bruker DRX 500 MHz, a Bruker DRX 400 MHz, or a Bruker 400 MHz AVANCE spectrometer. All chemical shifts are reported in δ units with reference to the residual protons of the deuterated solvents, which are internal standards, for proton chemical shifts. The elemental analyses were performed by the analytical facilities at the University of California at Berkeley or by Robertson Microlit Laboratories of Madison, NJ.Materials
All solvents were deoxygenated and dried by passage over columns of activated alumina.95,96 Tetrahydrofuran dried over sodium/benzophenone and freshly distilled prior to use. Deuterated solvents, C6D6, C7D8, cyclohexane-d12 and methylcyclohexane-d14, were purchased from Cambridge Laboratories, Inc., dried over sodium metal, vacuum transferred to a Teflon sealable Schlenk flask containing 4 Å molecular sieves, and degassed via three freeze-pump-thaw cycles.KHBEt3 (1M in THF) was purchased from Aldrich and used as received. [Cp′FeI]2 was prepared according to ref. 30. White phosphorus (P4) was crystallized from toluene prior to use.
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (see ESI for details†) (0.08 g, 28%). These crystals were dissolved in hexane (15 mL) and t-butylethylene (TBE) (2 mL) was added. This mixture was heated to 60 °C for 6 h, and the color changed from purple to olive green. The solvent was removed and the slightly oily residue was extracted with a minimum amount of pentane (ca. 1 mL). Cooling of the pentane extracts to −38 °C yielded green plate like crystals of [Cp′2Fe2H3] in low yield (0.03 g, 0.052 mmol, 10%). 1H NMR spectrum revealed the purity of 3 (see ESI for details†). 1H NMR (C6D6, RT, 400 MHz): δ 32.9 (2H, ν1/2∼ 350 Hz), −8.0 (18 H, ν1/2 = 53 Hz), −9.9 (9 H, ν1/2 = 53Hz). Anal. calcd. for C34H61Fe2: C, 70.2; H, 10.57. Found: C, 70.1; H, 10.63.
60 (see ESI for details†) (0.08 g, 28%). These crystals were dissolved in hexane (15 mL) and t-butylethylene (TBE) (2 mL) was added. This mixture was heated to 60 °C for 6 h, and the color changed from purple to olive green. The solvent was removed and the slightly oily residue was extracted with a minimum amount of pentane (ca. 1 mL). Cooling of the pentane extracts to −38 °C yielded green plate like crystals of [Cp′2Fe2H3] in low yield (0.03 g, 0.052 mmol, 10%). 1H NMR spectrum revealed the purity of 3 (see ESI for details†). 1H NMR (C6D6, RT, 400 MHz): δ 32.9 (2H, ν1/2∼ 350 Hz), −8.0 (18 H, ν1/2 = 53 Hz), −9.9 (9 H, ν1/2 = 53Hz). Anal. calcd. for C34H61Fe2: C, 70.2; H, 10.57. Found: C, 70.1; H, 10.63.
Computational methods
All calculations employed the long-range dispersion-corrected Grimme's functional (B97D).81 The calculations were carried out with Gaussian 0997 and no symmetry restrictions imposed (C1). C, H, P and Fe were represented by an all-electron 6-311G(d,p) basis set. The nature of the extrema (minima) was established with analytical frequencies calculations. The zero point vibration energy (ZPE) and entropic contributions were estimated within the harmonic potential approximation. The Gibbs free energy, ΔG, was calculated for T = 298.15 K and 1 atm. Geometrical parameters were reported within an accuracy of 10−3 Å and 10−1 degrees.Acknowledgements
We thank the Alexander-von Humboldt Foundation for a Feodor-Lynen Fellowship (MDW), Prof. Maurice Brookhart for providing financial support (through NSF grant CHE-0615704) and laboratory facilities (MDW) during the initial phase of this research program, and Prof. Helmut Sitzmann, Prof. Richard A. Andersen and Prof. Odile Eisenstein for very helpful discussions. MDW gratefully acknowledges the current financial support by Deutsche Forschungsgemeinschaft (DFG) through the Emmy-Noether program (WA 2513/2-1).References
- J. C. Peters and M. P. Mehn, in Activation of Small Molecules, ed. W. B. Tolman, Wiley-VCH, Weinheim, 2006, pp. 81–120 Search PubMed.
- P. L. Holland, Acc. Chem. Res., 2008, 41, 905–914 CrossRef CAS.
- M. P. Hendrich, W. Gunderson, R. K. Behan, M. T. Green, M. P. Mehn, T. A. Betley, C. C. Lu and J. C. Peters, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 17107 CrossRef CAS , and references cited therein.
- C. Vogel, F. W. Heinemann, J. Sutter, C. Anthon and K. Meyer, Angew. Chem., Int. Ed., 2008, 47, 2681–2684 CrossRef CAS.
- J. J. Scepaniak, M. D. Fulton, R. P. Bontchev, E. N. Duesler, M. L. Kirk and J. M. Smith, J. Am. Chem. Soc., 2008, 130, 10515–10517 CrossRef CAS.
- J. J. Scepaniak, J. A. Young, R. P. Bontchev and J. M. Smith, Angew. Chem., Int. Ed., 2009, 48, 3158–3160 CrossRef CAS.
- J. J. Scepaniak, C. S. Vogel, M. M. Khusniyarov, F. W. Heinemann, K. Meyer and J. M. Smith, Science, 2011, 331, 1049–1052 CrossRef CAS.
- J. J. Scepaniak, T. D. Harris, C. S. Vogel, J. Sutter, K. Meyer and J. M. SMith, J. Am. Chem. Soc., 2011, 133, 3824–3827 CrossRef CAS.
- I. Nieto, F. Ding, R. P. Bontchev, H. Wang and J. M. Smith, J. Am. Chem. Soc., 2008, 130, 2716–2717 CrossRef CAS.
- F. A. Jové, C. Pariya, M. Scoblete, G. P. A. Yap and K. H. Theopold, Chem.–Eur. J., 2011, 17, 1310–1318 CrossRef.
- H. Sitzmann, T. Dezember, W. Kaim, F. Baumann, D. Stalke, J. Kärcher, E. Dormann, H. Winter, C. Wachter and M. Kelemen, Angew. Chem., Int. Ed. Engl., 1996, 35, 2872 CrossRef CAS.
- M. Wallasch, G. Wolmershäuser and H. Sitzmann, Angew. Chem., 2005, 117, 2653 CrossRef.
- M. W. Wallasch, F. Rudolphi, G. Wolmershäuser and H. Sitzmann, Z. Naturforsch. B, 2009, 64, 11–17 CAS.
- M. W. Wallasch, G. Vollmer, A. Kafiyatullina, G. Wolmershäuser, P. G. Jones, M. Mang, W. Meyer and H. Sitzmann, Z. Naturforsch. B, 2009, 64, 18–24 CAS.
- M. W. Wallasch, D. Weismann, C. Riehn, S. Ambrus, G. Wolmershäuser, A. Lagutschenkov, G. Niedner-Schatteburg and H. Sitzmann, Organometallics, 2010, 29, 806–813 CrossRef CAS.
- D. Weismann, Y. Sun, Y. Lan, G. Wolmershäuser, A. K. Powell and H. Sitzmann, Chem.–Eur. J., 2011, 17, 4700–4704 CrossRef CAS.
- M. Perruzini, L. Gonsalvi and A. Romerosa, Chem. Soc. Rev., 2005, 34, 1038–1047 RSC.
- M. Peruzzini, R. R. Abdreimova, Y. Budnikova, A. Romerosa, O. J. Scherer and H. Sitzmann, J. Organomet. Chem., 2004, 689, 4319–4331 CrossRef CAS.
- B. M. Cossairt and C. C. Cummins, Angew. Chem., Int. Ed., 2008, 47, 8863–8866 CrossRef CAS.
- Y. Ohki and H. Suzuki, Angew. Chem., Int. Ed., 2000, 39, 3120–3122 CrossRef CAS.
- M. A. Peldo, A. M. Beatty and T. P. Fehlner, Organometallics, 2003, 22, 3698–3702 CrossRef CAS.
- M. A. Peldo, A. M. Beatty and T. P. Fehlner, Organometallics, 2002, 21, 2821–2823 CrossRef CAS.
- Y. Ohki, T. Kojima, M. Oshima and H. Suzuki, Organometallics, 2001, 20, 2654–2656 CrossRef CAS.
- H. Sitzmann, Coord. Chem. Rev., 2001, 214, 287–327 CrossRef CAS.
- G. F. Zi, L. Jia, E. L. Werkema, M. D. Walter, J. P. Gottfriedsen and R. A. Andersen, Organometallics, 2005, 24, 4251–4264 CrossRef CAS.
- F. Jaroschik, F. Nief and L. Ricard, Chem. Commun., 2006, 426–428 RSC.
- E. L. Werkema, E. Messines, L. Perrin, L. Maron, O. Eisenstein and R. A. Andersen, J. Am. Chem. Soc., 2005, 127, 7781–7795 CrossRef CAS.
- M. D. Walter, F. Weber, G. Wolmershäuser and H. Sitzmann, Angew. Chem., Int. Ed., 2006, 45, 1903–1905 CrossRef CAS.
- M. D. Walter, D. Bentz, F. Weber, O. Schmitt, G. Wolmershäuser and H. Sitzmann, New J. Chem., 2007, 31, 305–318 RSC.
- M. D. Walter and P. S. White, New J. Chem., 2011 10.1039/c1nj20399a.
- N. Koga and K. Morokuma, J. Mol. Struct., 1993, 300, 181–189 CrossRef CAS.
- M.-H. Baik, R. A. Friesner and G. Parkin, Polyhedron, 2004, 23, 2879–2900 CrossRef CAS.
- H. Suzuki, H. Omori, D. H. Lee, N. Yoshida, M. Fukushima, M. Tanaka and Y. Moro-oka, Organometallics, 1994, 13, 1129–1146 CrossRef CAS.
- S. Tussupbayev and S. F. Vyboishchikov, Organometallics, 2007, 26, 56–64 CrossRef CAS.
- A. Dedieu, T. A. Albright and R. Hoffmann, J. Am. Chem. Soc., 1979, 101, 3141–3351 CrossRef CAS.
- R. H. Summerville and R. Hoffmann, J. Am. Chem. Soc., 1979, 101, 3821–3831 CrossRef CAS.
- S. J. LaPlaca and J. A. Ibers, J. Am. Chem. Soc., 1963, 85, 3501–3502 CrossRef CAS.
- S. W. Kirtley, J. D. Olsen and R. Bau, J. Am. Chem. Soc., 1973, 95, 4532–4536 CrossRef CAS.
- R. G. Teller and R. Bau, Struct. Bonding, 1981, 44, 1–82 CrossRef CAS , and references cited therein.
- R. Bau and M. H. Drabnis, Inorg. Chim. Acta, 1997, 259, 27–50 CrossRef CAS ; and references cited therein.
- M. Bortz, R. Bau, J. J. Schneider and S. A. Mason, J. Cluster Sci., 2001, 12, 285–291 CrossRef CAS.
- M. Regitz and O. J. Scherer, ed., Georg Thieme Verlag, Stuttgart, 1990.
- M. Scheer and U. Becker, Phosphorus, Sulfur Silicon Relat. Elem., 1994, 93–94, 257–260 CrossRef CAS.
- M. Scheer, K. Schuster and U. Becker, Phosphorus, Sulfur Silicon Relat. Elem., 1996, 109–110, 141–144 CrossRef CAS.
- O. J. Scherer, Acc. Chem. Res., 1999, 32, 751–762 CrossRef CAS.
- O. J. Scherer, Chem. Unserer Zeit, 2000, 34, 374–381 CrossRef CAS.
- M. Ehses, A. Romerosa and M. Perruzini, Top. Curr. Chem., 2002, 220, 107–140 CrossRef CAS.
- F. H. Stephens, M. J. A. Johnson, C. C. Cummins, O. P. Kryatova, S. V. Kryatov, E. V. R. Rybak-Akimova, J. E. McDonough and C. D. Hoff, Angew. Chem. Int. Ed., 2005, 127, 15191–15200 CAS.
- J. S. Figueroa and C. C. Cummins, Dalton Trans., 2006, 2161–2168 RSC.
- W. W. Seidel, O. T. Summerscales, B. O. Patrick and M. D. Fryzuk, Angew. Chem., Int. Ed., 2009, 48, 115–117 CrossRef CAS.
- F. Dielmann, M. Sierka, A. V. Virovets and M. Scheer, Angew. Chem., Int. Ed., 2010, 49, 6860–6864 CrossRef CAS.
- S. Yao, Y. Xiong, C. Milsmann, E. Bill, S. Pfirrmann, C. Limberg and M. Driess, Chem.–Eur. J., 2010, 16, 436–439 CrossRef CAS.
- O. J. Scherer, T. Hilt and G. Wolmershäuser, Organometallics, 1998, 17, 4110–4112 CrossRef CAS.
- T. Hilt, Ph.D. Dissertation, Universität Kaiserslautern, 1999.
- C. Eichhorn, O. J. Scherer, T. Sögding and G. Wolmershäuser, Angew. Chem., Int. Ed., 2001, 40, 2859–2861 CrossRef CAS.
- E. Urnéžius, W. W. Brennessel, C. J. Cramer, J. E. Ellis, P. von and R. Schleyer, Science, 2002, 295, 832–834 CrossRef.
- E.-M. Schnöckelborg, J. J. Weigand and R. Wolf, Angew. Chem., Int. Ed., 2011, 50, 6657–6660 CrossRef.
- B. M. Cossairt and C. C. Cummins, Chem. Rev., 2010, 110, 4164–4177 CrossRef CAS.
- M. Caporali, L. Gonsalvi, A. Rossin and M. Perruzini, Chem. Rev., 2010, 110, 4178–4235 CrossRef CAS.
- M. Scheer, G. Balazs and A. Seitz, Chem. Rev., 2010, 110, 4236–4256 CrossRef CAS.
- M. Peruzzini, J. A. Ramirez and F. Vizza, Angew. Chem., Int. Ed., 1998, 37, 2255 CrossRef CAS.
- P. Barbaro, A. Ienco, C. Mealli, M. Perruzini, O. J. Scherer, O. Schmitt, F. Vizza and G. Wolmershäuser, Chem.–Eur. J., 2003, 9, 5195–5210 CrossRef CAS.
- J. A. Pool, E. Lobkovsky and P. J. Chirik, Angew. Chem., Int. Ed., 2002, 41, 3463 CrossRef.
- J. Ballmann, R. F. Munhá and M. D. Fryzuk, Chem. Commun., 2010, 46, 1013–1025 RSC.
- M. D. Walter, C. D. Sofield and R. A. Andersen, Organometallics, 2008, 27, 2959–2970 CrossRef CAS.
- D. E. C. Corbridge, The Structural Chemistry of Phosphorus, Elsevier, New York, 1974 Search PubMed.
- M. Scheer, U. Becker, M. H. Chisholm, J. C. Huffman, F. Lemoigno and O. Eisenstein, Inorg. Chem., 1995, 34, 3117–3119 CrossRef CAS.
- C. von Hänisch, D. Fenske, F. Weigend and R. Ahlrichs, Chem.–Eur. J., 1997, 3, 1494–1498 CrossRef.
- O. J. Scherer, J. Vondung and G. Wolmershäuser, Angew. Chem., Int. Ed. Engl., 1989, 28, 1355 CrossRef.
- O. J. Scherer, R. Winter and G. Wolmershäuser, Z. Anorg. Allg. Chem., 1993, 619, 827–835 CrossRef CAS.
- W. Tremel, R. Hoffmann and M. Kertesz, J. Am. Chem. Soc., 1989, 111, 2030–2039 CrossRef CAS.
- J. W. Lauher, M. Ellian, R. H. Summerville and R. Hoffmann, J. Am. Chem. Soc., 1976, 98, 3219–3224 CrossRef CAS.
- F. H. Stephens, Massachusetts Institute of Technology, 2004 Search PubMed.
- A. S. P. Frey, F. G. N. Cloke, P. B. Hitchcock and J. C. Green, New J. Chem., 2011 10.1039/c1nj20163h.
- C. Schwarzmaier, A. Noor, G. Glatz, M. Zabel, A. Y. Timoshkin, B. M. Cossairt, C. C. Cummins, R. Kempe and M. Scheer, Angew. Chem., Int. Ed., 2011, 50, 7283–7286 CrossRef CAS.
- M. Scheer, S. Deng, O. J. Scherer and M. Sierka, Angew. Chem., Int. Ed., 2005, 44, 3755–3758 CrossRef CAS.
- M. Lein, J. Frunzke and G. Frenking, Inorg. Chem., 2003, 42, 2504–2511 CrossRef CAS.
- J. Frunzke, M. Lein and G. Frenking, Organometallics, 2002, 21, 3351–3359 CrossRef CAS.
- Y. Peng, H. Fan, H. Zhu, H. W. Roesky, J. Magull and C. E. Hughes, Angew. Chem., Int. Ed., 2004, 43, 3443–3445 CrossRef CAS.
- N. A. Piro and C. C. Cummins, J. Am. Chem. Soc., 2008, 130, 9524–9535 CrossRef CAS.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS.
- J. Grunenberg and N. Goldberg, J. Am. Chem. Soc., 2000, 122, 6045–6047 CrossRef CAS.
- J. Grunenberg, Phys. Chem. Chem. Phys., 2011, 13, 10136–10146 RSC.
- K. Brandhorst and J. Grunenberg, Chem. Soc. Rev., 2008, 37, 1558 RSC.
- C. A. Pignedoli, A. Curioni and W. Andreoini, ChemPhysChem, 2005, 6, 1795–1799 CrossRef CAS.
- Y. Xie and H. F. Schaefer, Z. Phys. Chem., 2003, 217, 189–203 CrossRef CAS.
- P. R. Schreiner, H. P. Reisenauer, J. Romanski and G. Mloston, Angew. Chem., Int. Ed., 2009, 48, 8133–8136 CrossRef CAS.
- S. Katsyuba and R. Schmutzler, Dalton Trans., 2008 Search PubMed.
- S. Katsyuba, R. Schmutzler and J. Grunenberg, Dalton Trans., 2005, 1701–1706 RSC.
- F. Kraus, J. C. Aschenbrenner and N. Korber, Angew. Chem., Int. Ed., 2003, 42, 4030–4033 CrossRef CAS.
- F. Kraus and N. Korber, Chem.–Eur. J., 2005, 11, 5945–5959 CrossRef CAS.
- F. Kraus, T. Hanauer and N. Korber, Inorg. Chem., 2006, 45, 1117–1123 CrossRef CAS.
- L. Salem, The Molecular Orbital Theory of Conjugated Systems, W.A. Benjamin, Inc., Reading, Massachusetts, 1966 Search PubMed.
- J. Grunenberg, unpublished results.
- P. J. Alaimo, D. W. Peters, J. Arnold and R. G. Bergman, J. Chem. Educ., 2001, 78, 64 CrossRef CAS.
- A. B. Pangborn, M. A. Giardello, R. H. Grubbs, R. K. Rosen and F. J. Timmers, Organometallics, 1996, 15, 1518–1520 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, GAUSSIAN 09 (Revision A.1), Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and crystallographic details and crystallographic data. CCDC reference numbers 738662–738664. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1sc00413a |
| ‡ The reaction of 1 with KHBEt3 was also investigated in methylcyclohexane-d14 under Ar. Immediately upon addition of KHBEt3 gas evolution was observed accompanied by the formation of a colorless precipitate and a color change from red to purple and then red-brown. Three paramagnetic species 2, 3 and an unidentified paramagnetic complex were detected in solution. The solvent was removed and the residue dissolved in methylcyclohexane-d14 to give a green solution which was filtered. The 1H NMR spectrum of the crude reaction mixture showed resonances due to 3 (as major product), 2 and HCp′ (consistent with the bulk experiment). This clearly demonstrates the intrinsic instability of “[Cp′FeH]” with respect to formation of 2 and 3. No 2H-incorporation in the Fe–H functionality of 2 was observed. The decomposition of “[Cp′FeH]” is most likely the source of the excess hydride by acting as H2 and H-radical source. This also explains the low yield when the reaction is carried out under Ar. |
| § No reaction of 2 with N2 was observed under the same conditions. |
| ¶ One should note that [{Cp′Fe}2(μ-η3:η3-P3)] slowly decomposes on prolonged heating at this temperature. |
| || Changing the basis set from 6-311G(d,p) to TZVP leads to slightly better agreement of calculated and experimental structures (see ESI for details†). |
| This journal is © The Royal Society of Chemistry 2011 |
