Magnetically immobilized nanoporous giant proteoliposomes as a platform for biosensing†
Tse-Ming
Hsin
*,
Kan
Wu
and
Gowri
Chellappan
Department of Chemistry, University of Massachusetts Lowell, MA 01854, USA. E-mail: bobhsin@gmail.com
First published on 9th November 2011
Abstract
We report a biosensing method that is based on magnetically immobilized functional liposomes. The vesicles encapsulate magnetic nanoparticles (MNP) and enzymatic sensing reagents. Magnetic attraction between MNP and external magnets first immobilizes liposomes onto the surface of a coverglass. With the assistance from α-hemolysin (aHL), translocations of analytes through a lipid membrane trigger intravesicular enzymatic reactions. After 90 s incubation, the product from the sensing reactions, resorufin, was probed by laser-induced fluorescence. Detection of two analytes, glucose and ethanol, was demonstrated using two types of functional vesicles. The effects of MNP-containing vesicles and biotinylated vesicles on aHL's translocations of analytes were also compared. Unlike biotinylated lipids, MNP facilitate immobilization of liposomes without compromising the integrity of membrane and pore-forming activity of aHL.
Introduction
Liposomes are synthetic lipid vesicles whose membrane properties can be fine tuned using different reagents, for instance, derivatized lipids, cholesterol and proteins to mimic biological cells. In addition, liposomes are able to encapsulate a large variety of species, such as, ions, small organic molecules, biological macromolecules and nanoparticles.1–11 Therefore, liposome has been a very versatile tool in areas of research.6–11 Recently, magnetic nanoparticles (MNP) have been enclosed in giant vesicles for morphological investigation of lipid membranes and biomedical applications.7–11 These reports are important demonstrations, nevertheless, many applications of liposomes are based upon chemical reactions and transmembrane activities.1–5,12,13Several types of biological channels, for example ion channels, membrane receptors, and bacterial porins, have been adopted to prepare transmembrane pathways for biosensing.14–24 Among them, α-hemolysin (aHL), a bacterial pore-forming toxin, is one of the most popular choices because of its self-assembling capability to form ∼1.4 nm transbilayer channels and remarkable stability under various experimental conditions.17–22 In order to facilitate or improve manipulation of liposomes, chemically modified lipids are usually used for immobilization of vesicles. Specific interactions such as biotin–avidin,25 complimentary DNAs,26gold–thiol,27 functional nanoparticles28 and combinations of several linkers29–31 have been employed. Thanks to its high specificity and affinity, the biotin–avidin pair has been extensively applied in vesicular conjugation and immobilization. However, a few factors originated from the pair have shown adverse effects on lipids and transbilayer channels.32,33 Due to the extremely strong interaction between biotin and avidin, dispersions and reorganizations of local lipid molecules have been reported.32 Further, avidins can inhibit the formation of synthetic ion channels and/or block the entrance of the channels.33 Hence, vesicles that are modified by biotin or avidin could compromise activity of porins and translocation of analytes.
Herein, we present a facile liposomal biosensing method without undesired chemical effects on lipid bilayer. In this method, MNP and proteins in liposomes served as immobilizing and sensing agents respectively. The multifunctional vesicles were prepared by modified rotary evaporation methods.1,3,4 The sensing vesicles were immobilized by an external magnet. Analytes were then introduced extraliposomally along with aHL. Diffusion of analytes through the pores triggered intravesicular sensing reactions. The enzymatic reactions produced fluorescent products that were detected by laser-induced fluorescence microscopy.
Experimental section
1. Chemicals and materials
Soybean phosphatidylcholine (PC) was obtained from Sigma-Aldrich (St Louis, MO). The biotinylated lipid, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(cap biotinyl) (sodium salt), was purchased from Avanti Polar Lipids (Alabaster, AL). Chloroform, methanol, D-glucose, ethanol, sodium citrate, alcohol dehydrogenase, resazurin, β-nicotinamide adenine dinucleotide (NAD+) hydrate from yeast, diaphorase, glucose HK reagents (containing 1.5 mM NAD+, 1.0 mM ATP, 1.0 U mL−1 of hexokinase and 1.0 U mL−1 of glucose-6-phosphate dehydrogenase), iron(II) chloride tetrahydrate, iron(III) chloride hexahydrate and α-hemolysin were obtained from Sigma-Aldrich (St Louis, MO). Glass coverslips were purchased from Corning Inc. (Corning, NY) and Fisher Scientific Inc. (Pittsburgh, PA). PVDF membranes for dialysis (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWCO) were obtained from Spectrum Labs (Rancho Dominguez, CA). Press-to-Seal silicone spacers were purchased from Invitrogen Inc. (Carlsbad, CA).
000 MWCO) were obtained from Spectrum Labs (Rancho Dominguez, CA). Press-to-Seal silicone spacers were purchased from Invitrogen Inc. (Carlsbad, CA).
2. Synthesis of magnetic nanoparticles and functional liposomes
MNP were synthesized using reported methods.34 FeCl2·4H2O (498.6 mg) and FeCl3·6H2O (810.9 mg) were first dissolved in water (pH 1.5). The solution was deoxygenated by N2 and stirred vigorously. NH4OH (1.5 M) was added dropwise to this solution until pH 9 and the formation of black MNP (Fe3O4, magnetite) was observed. The particles were washed extensively by water and then ethanol. Residual ethanol was removed by lyophilization. The black dry powder was reconstituted in sodium citrate buffer (43 mM, pH 8.27) and sonicated for 1 h for better suspension.35,36 The concentration of MNP stock solution was 20 mg mL−1.Multifunctional vesicles were synthesized by the rotary evaporation method.1,3,4 Typically, 1000 μL of 25.8 mM soybean PC in chloroform and 100 μL of methanol were mixed with 2 mL of aqueous buffer (43 mM sodium citrate, pH 8.3) containing 0.1 mg mL−1MNP and reagents of interest. Reagents of interest for glucose sensor: 1 mL of glucose HK assay reagents (prepared according to vendor's instruction), 10 μM resazurin, and 1.0 U mL−1diaphorase were prepared in 2 mL citrate buffer. For ethanol sensor: 1.0 U mL−1alcohol dehydrogenase, 0.002 g NAD+, 5 μM resazurin, and 1.0 U mL−1diaphorase were prepared in 2 mL citrate buffer. The mixture was heated above 42 °C under reduced pressure (recirculating water aspirator, Brinkmann, B-169) and the organic solvents were removed in minutes. The aqueous phase then contained liposomes. The cloudy solution was dialyzed extensively to remove reactants outside of the liposomes. The concentration of aHL in biosensing experiments was 6.5 μM.
3. Manipulation and microscopy
Typically an aliquot (2 μL) that contained many vesicles is used for sensing. Such an aliquot was applied on a piece of coverglass against an external magnet. Due to the strong attraction between MNP and the external magnets, the MNP reside on the bottom of the vesicles and facilitate immobilization and aggregation of liposomes. Generally, a 3 min waiting time was applied to ensure complete immobilization of the vesicles, although precipitation occurred immediately. The magnet was removed prior to analyte introduction and imaging. After mixing with aHL, the solution of analytes (8 μL) was introduced to the immobilized layer and incubated for 90 s.The experimental setup for microimaging consisted of an inverted microscope equipped with three objectives, 5×, 10×, and 100× (Carl Zeiss MicroImaging Inc, Thornwood, NY), a HeNe (543 nm, 5 mW, JDS Uniphase Corporation, Milpitas, CA) laser for excitation, and a CCD camera (Photometrics, Tucson, AZ). A dichroic mirror and a long-pass filter (XF2015 and XF3085, Omega Optical, Brattleboro, VT) were employed to work with the laser.
Result and discussion
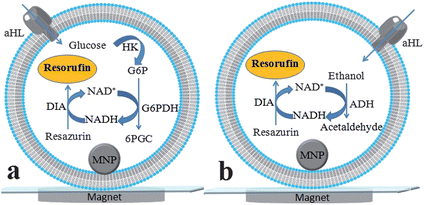
Detection of glucose is shown in Fig. 1a. Glucose first enters the vesicle through aHL pores and triggers a series of reactions. Hexokinase (HK) phosphorylates glucose to glucose-6-phosphate (G6P). G6P and NAD+ are transformed to 6-phosphogluconate (6PGC) and NADH by G6P dehydrogenase (G6PDH). Diaphorase (DIA) catalyzes the conversion from resazurin to resorufin, a fluorescent product, with concomitant oxidation of NADH to NAD+. The last two transformations constitute enzymatic cycling reactions. By employing different enzymes, ethanol can be detected in a similar manner (Fig. 1b). Alcohol dehydrogenase (ADH) was used for converting ethanol and NAD+ to acetaldehyde and NADH.The results of biosensing are shown in Fig. 2. Fig. 2a and b are the bright-field and fluorescence images of immobilized vesicles using a 10× objective. Due to the high concentrations of lipids (25.8 mM, 1 mL) and MNP (0.1 mg mL−1) used during synthesis, vesicles immobilized and aggregated under the influence of the external magnet (Fig. 2a). After analyte introduction and 90 second incubation, the immobilized liposomes were probed by the 543 nm HeNe laser. The fluorescence images were collected by a CCD camera (Fig. 2b). Although it is possible for small species to diffuse out of liposomes,37 most of the products remained inside the liposomes (Fig. 2c) due to such short incubation time and the use of giant vesicles.37,38Fig. 2d and e are the calibration curves for glucose and ethanol. The curves were constructed by obtaining the mean of fluorescence signals from micrographs. The calibration curves show good linearity with R2 values of 0.9814 and 0.9836 for glucose and ethanol sensors respectively. It has been reported that several inherent liposomal characteristics, for instance, average size, polydispersity, lamellarity, and encapsulation efficiency, could introduce large variations in concentration of encapsulants,39 especially in single vesicle-based experiments,13 and thus could undermine quantitative applications. Nevertheless, such bias did not affect our biosensors. We utilized large amounts of lipid vesicles and objectives of lower magnification for quantitation, which allowed imaging of a large number of liposomes at the same time. The standard deviations for glucose and ethanol detections are about 10%, which is attributed to many intravesicular events that were collected and fluorescence signals that were averaged. The detection limits of glucose and ethanol biosensors are 0.39 and 0.54 mM which were obtained based on two times of signal to noise ratio.
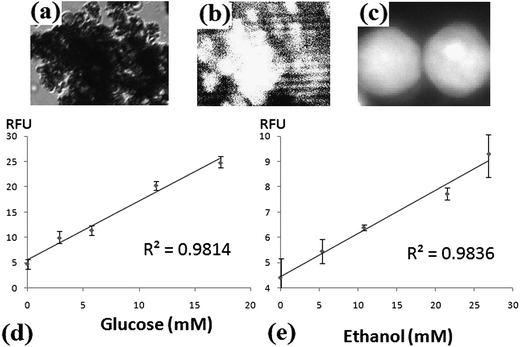 | ||
| Fig. 2 Results of glucose and ethanol biosensing. (a) Bright-field microimage of immobilized liposomes before introduction of analyte solution. (b) Fluorescent image of the same area after incubation. Both micrographs in (a) and (b) were imaged using a 10× objective and the corresponding areas are about 250 × 300 μm2. (c) Fluorescence image of two large liposomes obtained using a 100× objective. The area of this micrograph is 15 × 20 μm2. Most of the fluorophores remained in the porous reactors after 90 s incubation. (d) Calibration curve for glucose. (e) Calibration curve for ethanol. For (d) and (e), x-axes are the concentrations of analytes and y-axes are RFU, relative fluorescence units after background subtraction. | ||
During the development of the sensing method, we compared the effects of MNP and biotinylated lipids (biotin-lipids) on the transmembrane activity of aHL since the biotin–avidin linker has been used extensively in immobilization and surface modification of liposomes.13,25,29–31 The results from bulk measurements shown in Fig. 3 were obtained using fluorometers; their effects on glucose and ethanol sensors are shown in Fig. 3a and b respectively. The signals in each column represent the increase of fluorescence due to analyte-triggered enzymatic reactions after background subtraction. The error bars in each column are standard deviations. Liposomes without MNP or biotin (PC only) and MNP containing-liposomes (MNP-liposomes) showed identical increases in fluorescence within experimental errors. In contrast to MNP-liposomes, biotinylated vesicles strongly perturbed the activity of aHL and resulted in limited translocation of analytes and fluorescent products. For vesicles with 1% biotin-lipids, pronounced negative effects were observed with 60–70% deviation from unmodified or MNP-liposomes. The fluorescence intensities of 5% biotin-vesicles were further reduced, 75–83% lower than those of control experiments. Furthermore, it has been reported that 30 min incubation was required for aHL insertion on vesicles with 1% biotin-lipids in order to achieve reproducible transbilayer pores13 while results from our study and previous hemolysin literature37,38 have shown that aHL is able to prepare channels on the order of ten to tens of seconds. Thus, aHL's transmembrane activity is highly sensitive to biotinylation of the vesicles; biotin-lipids affect both equilibrium and kinetics of pore-forming behavior of aHL.
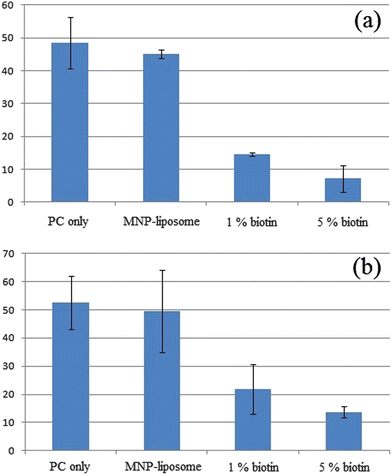 | ||
| Fig. 3 Comparison of unmodified liposomes, MNP-liposomes, 1% biotinylated-liposomes and 5% biotinylated-liposomes on aHL's transmembrane activity in (a) glucose and (b) ethanol sensors. The results from PC only and MNP-liposomes were identical. Introduction of 1% and 5% biotin-lipids to vesicles compromised the transbilayer activity of aHL and translocation of analytes. Effect of MNP and biotin-lipids on aHL's transmembrane activity was evaluated by obtaining the difference of signals before and after the addition of aHL. The increased signals were due to diffusion of analytes through pores of aHL. See ESI†. | ||
In order to further investigate the interaction between MNP and lipid membrane of vesicles, we entrapped a single MNP-liposome tightly by two pieces of coverglass. By moving the external magnet clockwise, we observed that the MNP aggregate moved accordingly, as illustrated in Fig. 4. Such behavior could be repeated many times. Combined with measurements obtained from fluorometry (Fig. 3), we conclude that MNP were indeed trapped within the inner space of vesicles and had very little or no effect on liposomal membrane and, therefore, the transmembrane activity of aHL.
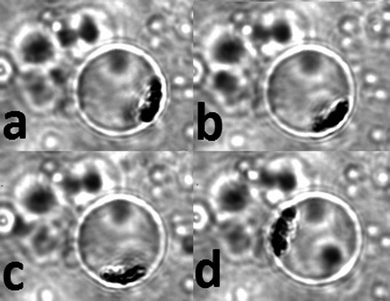 | ||
| Fig. 4 The movements of aggregated MNP in liposome under the influence of an external magnet at different locations. The external magnet was moved clockwise around the droplet. (a–d) The movement of the entrapped MNP in the membrane wall due to the attraction from the magnet. The diameter of the vesicle is 12 μm, imaged with a 100× objective. The imaged area is 20 × 25 μm2. | ||
Conclusion
In conclusion, we developed a novel and facile approach of liposome-based biosensing. The functional vesicles can be readily immobilized by external magnets and applied to detections of analytes. Unlike other immobilizing methods using chemically derivatized lipids, MNP-assisted immobilization preserves the integrity of lipid bilayer since MNP were encapsulated in, not intercalated on, lipid membrane and did not undermine important properties, including encapsulation of sensing reagents, transbilayer mechanism of aHL and enzymatic reactions. This developed platform has many potential applications. Simply by enclosing different enzymatic systems in liposomes, many other analytes can be determined. Furthermore, this means can be also applied to simultaneous detection of multianalyte and/or high-throughput screening in complicated matrices.Acknowledgements
This work was supported by University of Massachusetts Lowell.Reference
- A. Moscho, O. Orwar, D. T. Chiu, B. P. Modi and R. N. Zare, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 11443–11447 CrossRef CAS.
- V. Noireaux and A. Libchaber, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17669–17674 CrossRef CAS.
- D. T. Chiu, C. Wilson, F. Ryttsen, A. Stromberg, C. Farre, A. Karlsson, S. Nordholm, A. Gaggar, B. P. Modi, A. Moscho, R. A. Garza-Lopez, O. Orwar and R. N. Zare, Science, 1999, 283, 1892–1895 CrossRef CAS.
- T.-M. Hsin and E. S. Yeung, Angew. Chem., Int. Ed., 2007, 46, 8032–8035 CrossRef CAS.
- A. Karlsson, K. Scott, M. Markstrom, M. Davidson, Z. Konkoli and O. Orwar, J. Phys. Chem. B, 2005, 109, 1609–1617 CrossRef CAS.
- A. Wijaya and K. Hamad-Schifferli, Langmuir, 2007, 23, 9546–9550 CrossRef CAS.
- M.-S. Martina, J.-P. Fortin, C. Ménager, O. Clément, G. Barratt, C. Grabielle-Madelmont, F. Gazeau, V. Cabuil and S. Lesieur, J. Am. Chem. Soc., 2005, 127, 10676–10685 CrossRef CAS.
- G. Beaune, C. Ménager and V. Cabuil, J. Phys. Chem. B, 2008, 112, 7424–7429 CrossRef CAS.
- C. Ménager, D. Guemghar, R. Perzynski, S. Lesieur and V. Cabuil, Langmuir, 2008, 24, 4968–4974 CrossRef.
- C. Ménager, D. Guemghar, V. Cabuil and S. Lesieur, Langmuir, 2010, 26, 15453–15463 CrossRef.
- G. Beaune, B. Dubertret, O. Clément, C. Vayssettes, V. Cabuil and C. Ménager, Angew. Chem., Int. Ed., 2007, 46, 5421–5424 CrossRef CAS.
- I. Cisse, B. Okumus, C. Joo and T. Ha, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12646–12650 CrossRef CAS.
- B. Okumus, S. Arslan, S. M. Fengler, S. Myong and T. Ha, J. Am. Chem. Soc., 2009, 131, 14844–14849 CrossRef CAS.
- G. Favero, L. Campanella, S. Cavallo, A. D'Annibale, M. Perrella, E. Mattei and T. Ferri, J. Am. Chem. Soc., 2005, 127, 8103–8111 CrossRef CAS.
- C. J. Moreau, J. P. Dupuisi, J. Revilloud, K. Arumugam and M. Vivaudou, Nat. Nanotechnol., 2008, 3, 620–625 CrossRef CAS.
- S. R. Reiken, B. J. VanWie, H. Sutisna, D. F. Moffett, A. R. Koch, M. Silber and W. C. Davis, Biosens. Bioelectron., 1996, 11, 91–102 CrossRef CAS.
- L.-Q. Gu, O. Braha, S. Conlan, S. Cheley and H. Bayley, Nature, 1999, 398, 686–690 CrossRef CAS.
- L. Movileanu, S. Howorka, O. Braha and H. Bayley, Nat. Biotechnol., 2000, 18, 1091–1095 CrossRef CAS.
- O. Braha, L.-Q. Gu, L. Zhou, X. Lu, S. Cheley and H. Bayley, Nat. Biotechnol., 2000, 18, 1005–1007 CrossRef CAS.
- S. Howorka, J. Nam, H. Bayley and D. Kahne, Angew. Chem., Int. Ed., 2004, 43, 842–846 CrossRef CAS.
- X. Kang, S. Cheley, X. Guan and H. Bayley, J. Am. Chem. Soc., 2006, 128, 10684–10685 CrossRef CAS.
- J. Clarke, H.-C. Wu, L. Jayasinghe, A. Patel, S. Reid and H. Bayley, Nat. Nanotechnol., 2009, 4, 265–270 CrossRef CAS.
- M. Chen, S. Khalid, M. S. P. Sansom and H. Bayley, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 6272–6277 CrossRef CAS.
- S. Majd, E. C. Yusko, Y. N. Billeh, M. X. Macrae, J. Yang and M. Mayer, Curr. Opin. Biotechnol., 2010, 21, 439–476 CrossRef CAS.
- E. Boukobza, A. Sonnenfeld and G. Haran, J. Phys. Chem. B, 2001, 105, 12165–12170 CrossRef CAS.
- C. Yoshina-Ishii and S. G. Boxer, J. Am. Chem. Soc., 2003, 125, 3696–3697 CrossRef CAS.
- H. Jung, J. Kim, J. Park, S. Lee, H. Lee, R. Kuboi and T. Kawai, J. Biosci. Bioeng., 2006, 102, 28–33 CrossRef CAS.
- L. Zhang, L. Hong, Y. Yu, S. C. Bae and S. Granick, J. Am. Chem. Soc., 2006, 128, 9026–9027 CrossRef CAS.
- M. Bally, K. Bailey, K. Sugihara, D. Grieshaber, J. Vörös and B. Städler, Small, 2010, 6, 2481–2497 CrossRef CAS.
- K. A. Edwards and A. J. Baeumner, Talanta, 2006, 8, 1421–1431 CrossRef.
- K. Sott, M. Karlsson, J. Pihl, J. Hurtig, T. Lobovkina and O. Orwar, Langmuir, 2003, 19, 3904–3910 CrossRef CAS.
- K. P. Liem, G. T. Noble, S. L. Flitsch and S. J. Webb, Faraday Discuss., 2010, 145, 219–233 RSC.
- C. P. Wilson, C. Boglio, L. Ma, S. L. Cockroft and S. J. Webb, Chem.–Eur. J., 2011, 17, 3465–3473 CrossRef CAS.
- S. Giri, B. G. Trewyn, M. P. Stellmaker and V. S.-Y. Lin, Angew. Chem., Int. Ed., 2005, 44, 5038–5044 CrossRef CAS.
- L. E. Euliss, S. G. Grancharov, S. O'Brien, T. J. Deming, G. D. Stucky, C. B. Murray and G. A. Held, Nano Lett., 2003, 3, 1489–1493 CrossRef CAS.
- A. Bee, R. Massart and S. Neveu, J. Magn. Magn. Mater., 1995, 149, 6–9 CrossRef CAS.
- A. P. Heuck, R. K. Tweten and A. E. Johnson, J. Biol. Chem., 2003, 278, 31218–31225 CrossRef CAS.
- A. Kleefen, D. Pedone, C. Grunwald, R. Wei, M. Firnkes, G. Abstreiter, U. Rant and R. Tampe, Nano Lett., 2010, 10, 5080–5087 CrossRef CAS.
- K. A. Edwards and A. J. Baeumner, Talanta, 2006, 68, 1432–1441 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1an15565b |
| This journal is © The Royal Society of Chemistry 2012 |