A thin-layer chromatography plate prepared from BODIPY-based receptor immobilized SiO2 nanoparticles as a portable chemosensor for Pb2+†
Hyunjong
Son
,
Gyusik
Kang
and
Jong Hwa
Jung
*
Department of Chemistry, Research Institute of Natural Science, Gyeongsang National University, Jinju, 660-701, Korea. E-mail: jonghwa@gnu.ac.kr
First published on 14th November 2011
Abstract
A new fluorescence receptor based on BODIPY-immobilized silica nanoparticles (BODIPY-SiO22) exhibits a high affinity and selectivity for Pb2+ over competing metal ions in water. An overall emission change of ca. 100-fold at the emission maximum was observed for Pb2+. The fluorescence receptor BODIPY-SiO22 can remove 97% and 95% of the initial 100 ppb Pb2+ from human blood and waste solution, respectively. Experiments show the fluorescence receptor BODIPY-SiO22 can be a potentially useful and effective agent for the selective separation and rapid removal of Pb2+in vivo. We also prepared a portable chemosensor kit by coating a 4 μm thick film of BODIPY-SiO22 onto a glass substrate. We found that this BODIPY-SiO22 film detects Pb2+ ions at pH 7.4 with a sensitivity of 3.2 nM. Finally, we tested the effect of pH on BODIPY-SiO22 with Pb2+ ions between pH 3.0 and 11.0. The fluorescence changes of BODIPY-SiO22 were almost constant between pH 3 and 11. The results imply that the BODIPY-SiO22 film is applicable as a portable chemosensor for detection of Pb2+ ions in the environmental field.
Introduction
Metal ions can pose severe risks to human health and the environment, and methods for the facile preparation of fluorogenic and chromogenic receptors with high selectivity and sensitivity for heavy metal ions have continued to receive much interest.1 Among the known heavy metal ions, Pb2+ is one of the most dangerous, causing adverse health effects from lead exposure, particularly in children.2 A variety of symptoms have been attributed to lead poisoning. Once introduced into the body, Pb2+ can cause abdominal pain and vomiting. The long accumulation of Pb2+ in the body leads to many serious human afflictions, including muscle paralysis, mental confusion, memory loss, and anemia, suggesting that Pb2+ affects multiple targets in vivo.3 Plausible molecular targets of lead include calcium- and zinc-binding proteins that control cell signalling and gene expression, respectively.4 Thus, it is important to develop a safe and effective procedure to detect and remove lead from the body following toxic-lead contamination.5Silica nanoparticles are of great interest for biomedical and environmental research applications such as bioseparation, drug targeting, cell isolation, enzyme immobilization and protein purification, because of their biocompatibility and stability against degradation.6 In particular, silica nanoparticles can be easily modified with a wide range of functional groups. It is clear that the receptor-immobilized nanoparticles have some important advantages as a solid chemosensor and adsorbent in heterogeneous solid–liquid phases.7 First, such nanoparticles are readily synthesized by sol–gel condensation, a versatile technique that allows the introduction of chemical functionalities. Second, immobilized receptors on an inorganic support can remove the guest molecules (toxic metal ions and anions) from the pollutant solution. Third, the nanoparticles can be easily isolated from pollutants by filtration, allowing the nanoparticles to be repeatedly utilized after suitable treatment. In particular, the nanoparticles can also provide efficient binding of the guest molecules because their high surface-to-volume ratio simply offers more contact area.
In the above context, the design of fluorescent probes for the detection of lead ions is of special interest due to the selective, sensitive, nondestructive, and rapid nature of fluorescence emission signals.8 However, most of the probes so far described do not have high selectivity for Pb2+ over other metal ions and, in general, they cannot be used for aqueous systems.9 Chen and Huang have described a Pb2+ ion sensor whose fluorescence intensity enhancement was about 18-fold in the presence of Cu2+ (100 equiv.) in acetonitrile.5d Yoon and co-workers reported a selective sensor for Pb2+ ions employing a rhodamine B-based fluorescent receptor for use in acetonitrile.9 We have also reported a Pb2+ sensor based on a 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (hereafter abbreviated as BODIPY) receptor whose fluorescence intensity enhancement was only ca. 25-fold.9a Very recently, the use of nanoparticles as solid supports in combination with supramolecular concepts has led to the development of new hybrid nanomaterials with improved functionalities that offer much promise for use in the development of simple and efficient sensors of heavy metal ions in biological, toxicological, and environmental applications.7b,10–12 Although a number of signalling systems for the detection of heavy metal ions are now known,7b,10,13–15 there are no precedents for the use of hybrid nanoparticles to detect Pb2+ that employ a dual signal fluoro-chromophore that operates via a “turn-on” approach.
In order to obtain a highly sensitive and selective OFF–ON sensor for Pb2+ in aqueous solution for practical application, a BODIPY moiety was employed as the fluorophore/chromophore because of its characteristic strong absorption and fluorescence behavior (including high fluorescence quantum yields) and its high chemical stability. Mesoporous SiO2 nanoparticles were employed as the inorganic support because of their biocompatibility with in vivo systems. We now report the construction of a BODIPY-based fluorescence immobilized mesoporous silica nanoparticle (BODIPY-SiO22) as a new OFF–ON fluorescent Pb2+ solid sensor that operates via a PET (photoinduced electron transfer) mechanism. The solid BODIPY-SiO22 was shown to be highly sensitive and selective for Pb2+ in aqueous solution. The application of the new adsorbent for the removal of lead ions in human blood is also described.
Experimental
General
All reagents were purchased from Aldrich and Tokyo Kasei Chemicals and used without further purification.Photospectroscopy
Fluorescence emission spectra were recorded with a Shimadzu RF-5301-PC instrument. Stock solutions (0.01 M) of the hydrated metal perchlorate salts were prepared in H2O at pH 7. Stock solutions of BODIPY-SiO22 were prepared in H2O. For all measurements, excitation was at 326 nm, with excitation and emission slit widths of 1.5 nm. The pH value was adjusted by using HEPES (20 mM, pH 7). Fluorescence quantum yields were determined by reference to rhodamine 6G (Φ = 0.76).16,17Characterization
1H and 13C NMR spectra were measured with a Bruker ARX 300 MHz spectrometer. IR spectra were obtained for KBr pellets, in the range 400–4000 cm−1, with a Shimadzu FTIR 8400S instrument, and the MS spectrum was obtained with a JEOL JMS-700 mass spectrometer. Transmission electron microscopy (TEM) images were taken with a JEOL JEM-2100 F instrument operated at 150 keV.Preparation of compound 2
Compound 2 was synthesized as described previously.9a,18Preparation of compound 1
A solution of 2 (0.1 g, 0.194 mmol) containing triethylamine (0.1 mL, 0.388 mmol) at 80 °C was treated with 3-(triethoxysilyl) propyl-isocyanate (0.2 mL, 0.388 mmol). The reaction mixture was stirred overnight at 80 °C, and then cooled to room temperature. After removal of solvent, the crude product was purified by flash column chromatography on silica gel, by elution with ethyl acetate/n-hexane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to afford a blue solid (53.8 mg, yield 27.5%). 1H NMR (300 MHz, CDCl3): δ 7.6 (m, 9H), 6.80 (d, 3H, J = 8.7 Hz), 6.07 (s, 1H), 5.2 (t, 1H, J = 5.4 Hz), 4.25 (t, 4H, J = 5.7 Hz), 3.83 (m, 12H), 3.65 (t, 4H, J = 5.4 Hz), 3.17 (m, 6H), 2.53 (s, 3H), 1.42 (s, 3H), 1.39 (s, 3H), 1.23 (m, 18H). 13C NMR (300 MHz, CDCl3): 156.3, 154.5, 153.1, 148.4, 142.8, 141.0, 139.0, 137.1, 135.3, 133.0, 131.2, 129.4, 128.9, 128.3, 125.2, 120.5, 117.6, 114.8, 111.9, 61.5, 58.4, 50.4, 43.4, 23.2, 18.2, 14.6, 14.2, 7.6; MS (FAB): [M + H]+m/z = 1009.5665 (calcd Mw = 1009.5035). Anal. Calcd for C50H74BF2N5O10Si2: C, 59.45; H, 7.38; N, 6.93. Found: C, 59.55; H, 7.48; N, 6.97%.
1 v/v) to afford a blue solid (53.8 mg, yield 27.5%). 1H NMR (300 MHz, CDCl3): δ 7.6 (m, 9H), 6.80 (d, 3H, J = 8.7 Hz), 6.07 (s, 1H), 5.2 (t, 1H, J = 5.4 Hz), 4.25 (t, 4H, J = 5.7 Hz), 3.83 (m, 12H), 3.65 (t, 4H, J = 5.4 Hz), 3.17 (m, 6H), 2.53 (s, 3H), 1.42 (s, 3H), 1.39 (s, 3H), 1.23 (m, 18H). 13C NMR (300 MHz, CDCl3): 156.3, 154.5, 153.1, 148.4, 142.8, 141.0, 139.0, 137.1, 135.3, 133.0, 131.2, 129.4, 128.9, 128.3, 125.2, 120.5, 117.6, 114.8, 111.9, 61.5, 58.4, 50.4, 43.4, 23.2, 18.2, 14.6, 14.2, 7.6; MS (FAB): [M + H]+m/z = 1009.5665 (calcd Mw = 1009.5035). Anal. Calcd for C50H74BF2N5O10Si2: C, 59.45; H, 7.38; N, 6.93. Found: C, 59.55; H, 7.48; N, 6.97%.
Immobilization of receptor 1 onto silica nanoparticles
Compound 1 (100 mg) was dissolved in toluene (10 mL). The silica nanoparticles (100 mg) were added as a solid. The suspension of silica and compound 1 were mixed and then stirred under reflux conditions for 24 h in toluene. Then, the collected solid was washed copiously with toluene (50 mL) to rinse away any surplus 1 and was dried under vacuum.Preparation of BODIPY-based receptor 1 immobilized SiO2 films
Mesoporous silica was synthesized starting from the preparation of a hydrochloric acid solution of P-123 [(poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer)]. Tetraethyl orthosilicate (TEOS) was then added and the mixture was stirred at 40 °C for 20 h. The molar composition was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9
5.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 193
193![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.017 TEOS
0.017 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl
HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P-123. The solid was aged at 65 °C for 1 day and then was filtered, washed and dried at 90 °C. To cleave the template to generate mesopores, 1.0 g of as-synthesized SBA-15 was mixed with 100 mL of 60 wt% H2SO4 solution and refluxed at 95 °C for 1 day. The product was recovered by washing with water and dried at 90 °C. To generate mesopores, the acid-treated sample was heated to 200 °C in air. To remove cationic surfactants from the resulting dried fiber-like flocculates and particles, the sample was calcined in a box furnace in air at 500 °C for 5 h, with a ramp rate of 1 °C min−1. For the film SiO2 preparation, 3 mL of the solution detailed above were spread on glass slides with a glass rod and using adhesive tape as spacers. After the films were dried in air, they were sintered at 450 °C for 20 min. All samples used in this work were films of 4 μm thickness. Functionalization of the SiO2 films was achieved by immersing the films in a 1 mM solution of receptor 1 in 1
P-123. The solid was aged at 65 °C for 1 day and then was filtered, washed and dried at 90 °C. To cleave the template to generate mesopores, 1.0 g of as-synthesized SBA-15 was mixed with 100 mL of 60 wt% H2SO4 solution and refluxed at 95 °C for 1 day. The product was recovered by washing with water and dried at 90 °C. To generate mesopores, the acid-treated sample was heated to 200 °C in air. To remove cationic surfactants from the resulting dried fiber-like flocculates and particles, the sample was calcined in a box furnace in air at 500 °C for 5 h, with a ramp rate of 1 °C min−1. For the film SiO2 preparation, 3 mL of the solution detailed above were spread on glass slides with a glass rod and using adhesive tape as spacers. After the films were dried in air, they were sintered at 450 °C for 20 min. All samples used in this work were films of 4 μm thickness. Functionalization of the SiO2 films was achieved by immersing the films in a 1 mM solution of receptor 1 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile/tert-butyl alcohol mixture overnight, followed by rinsing in ethanol to remove unadsorbed receptor 1.
1 acetonitrile/tert-butyl alcohol mixture overnight, followed by rinsing in ethanol to remove unadsorbed receptor 1.
Results and discussion
We targeted the styryl-BODIPY derivative 1 (Scheme 1). In designing this compound, we kept in mind that the meso-substituent is more likely to interfere with the excited state processes only, partly because of the orthogonal arrangement of the meso-(8)-phenyl moiety under the steric influence of the neighboring (1,7) methyl groups, and partly due to the fact that, in the HOMO of the BODIPY chromophore, the C8 position is a nodal point. Therefore, the substituents at the meso position are expected to alter the efficiency of the photoinduced electron transfer (PET) process, but not the intramolecular charge transfer (ICT). The synthesis of the target compound started with the known BODIPY dye, 4,4-difluoro-8-phenyl-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene. For example, BODIPY dye 2 was reacted with 4-N,N-bis-(2-hydroxyethyl)aminobenzaldehyde under the usual condition for BODIPY synthesis. The BODIPY dye 2 was then reacted with aminopropyltriethoxysilane as a precursor for the sol–gel reaction to afford the target compound 1 as a blue powder. Immobilization of the chromogenic receptor 1 to mesoporous silica nanoparticles was conducted under reflux for 24 h in toluene. In this process, the triethoxysilyl group of 1 attached onto mesoporous silica underwent hydrolysis and attached covalently to the surface of the mesoporous silica. After cooling to room temperature, the blue solid product was filtered, washed with THF, and dried. The BODIPY-based receptor 1 immobilized mesoporous silica (BODIPY-SiO22) was characterized by transmission electron microscopy (TEM), UV-vis spectroscopy, thermogravimetric analysis (TGA) and FT-IR.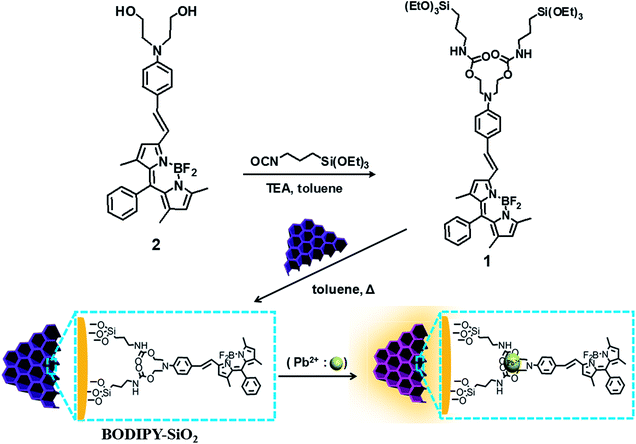 | ||
| Scheme 1 Preparation of BODIPY derivative-functionalized mesoporous silica (BODIPY-SiO22). | ||
The TEM image of the BODIPY-based receptor 1 immobilized mesoporous silica nanoparticle (BODIPY-SiO22) is shown in Fig. 1. The BODIPY-SiO22 and mesoporous silica nanoparticles clearly showed the mesopore structures before and after attachment of receptor 1 (Fig. 1A). We measured the surface area and pore volumes of both mesoporous silica nanoparticle and BODIPY-SiO22 using nitrogen adsorption–desorption isotherms (Fig. 1B). The mesoporous silica has a Brunauer–Emmett–Teller (BET) surface area of 1125.35 m2 g−1 and a pore volume of 0.47 cm3 g−1. On the other hand, we observed that the BODIPY-SiO22 has a BET surface area of 670.05 m2 g−1 and a pore volume of 0.25 cm3 g−1. The mesoporous silica and the BODIPY-SiO22 have Barrett–Joyner–Halenda (BJH) pore diameters of 2.22 and 2.14 nm, respectively (Fig. S1†). The decreased surface area and pore diameter in BODIPY-SiO22 are attributable to the attachment of the BODIPY-based receptor 1 to the mesoporous silica. Furthermore, from the results of the TGA measurement (Fig. S2†), we determined that the BODIPY-SiO22 consists of only 49.0 wt% of 1.
 | ||
| Fig. 1 (A) TEM image of BODIPY-SiO22. (B) Nitrogen adsorption–desorption isotherms of (a) mesoporous silica and (b) BODIPY-SiO22. | ||
For further structural proof of the BODIPY-SiO22, we carried out IR spectroscopy of both mesoporous silica and BODIPY-SiO22. For the mesoporous silica, IR peaks appear at 3450, 1658 and 1084 cm−1. For BODIPY-SiO22 (Fig. S3†), peaks appear at 3405, 1879, 1636, 1598, 1517, 1363, 1093, 949 and 811 cm−1. These new peaks originate from the BODIPY-based receptor 1, providing solid evidence that 1 is indeed attached to the surface of the mesoporous silica.
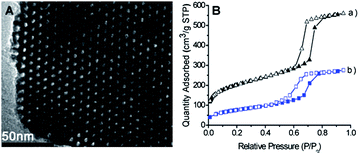
Spectroscopic measurements for BODIPY-SiO22 were performed in 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer, pH 7.4. The solid UV/vis absorption spectrum of free 1 showed two absorption bands at 324 and 596 nm (ε = 3.20 × 104 and 4.50 × 104 M−1 cm−1, respectively). The absorption maximum at 596 nm for BODIPY-SiO22 was red shifted in its UV/vis absorbance by almost 70 nm relative to the behavior reported previously by our group10a for a BODIPY parent system without the styryl group. When Pb2+ was added gradually to the above spectrophotometric solution, the λabs showed a 45 nm blue shift with an isosbestic point occurring at 570 nm; the color of the solution turned from light blue to pale red (Fig. S4a†). Furthermore, BODIPY-SiO22 is virtually nonfluorescent in its apo state (Φ < 0.0005, λex = 326 nm), which is a consequence of the lone pair electrons of the nitrogen atom in the benzoyl moiety of the fluorophore. Upon addition of increasing Pb2+ concentrations, BODIPY-SiO22 showed a large CHEF (chelation-enhanced fluorescence) effect in the fluorescence emission spectra that resulted from the blocking of the PET process and an overall increase in emission intensity of approximately 100-fold (Φ = 0.054, Fig. 2A) at the emission maximum (λem = 460) was observed.
 | ||
| Fig. 2 (A) Fluorescence spectra of BODIPY-SiO22 (10 μM) upon addition of increasing Pb2+ concentrations (0–250 equiv.) in 20 mM HEPES in pure aqueous solution at pH 7.4 (λex = 326 nm). (B) Fluorescence responses of BODIPY-SiO22 to various metal ions. Black bars represent the addition of selected metal ions (250 equiv.) to a 10 μM solution of BODIPY-SiO22. Red bars represent subsequent addition of Pb2+ (250 equiv.) to the solution. For all measurements, the pH value was adjusted by using 20 mM HEPES in pure aqueous solution, pH 7.4. Excitation was provided at 326 nm, and the emission was monitored at 456 nm. | ||
With few exceptions,19 most reported BODIPY-fluorescent sensors are based on an intramolecular charge transfer (ICT) mechanism.20,21 However, in our case, the noticeable fluorescence intensity enhancement of the receptor 1 attached to SiO2 nanoparticles may be due to the inhibition of the PET process during the binding of Pb2+. Preliminary computational simulations indicated that 1 is twisted at the BODIPY moiety, presumably blocking the ICT. In addition, the direct conjugation of the amine to the styryl group results in a very significant red shift, and high quantum yields for this BODIPY-based receptor.20a These results are in complete accord with our present observations.15a
We also investigated the binding ability of BODIPY-SiO22 as an ion-selective fluoro-chromogenic probe for the other metal ions: Ca2+, Hg2+, Cd2+, Li+, Ag+, Cu2+, Fe2+, Mg2+, Zn2+, K+, Na+, Mn2+, Co2+, Fe3+, Al3+ and Ni2+. However, no significant spectral changes were observed upon addition of any of these metal ions (Fig. S4b†), indicating that BODIPY-SiO22 is a highly selective chemosensor for the detection of Pb2+.
For comparison, we performed spectroscopic measurements using 1 in an acetonitrile solution (1 was insoluble in water). Under these conditions 1 gave two absorption bands at 324 and 596 nm (ε =3.25 × 104 and 5.0 × 104 M−1 cm−1, respectively). In the absence of Pb2+, 1 also exhibited no fluorescence emission when excited at 326 nm. Upon the addition of Pb2+, in the case of chemosensor 1, the emission band was at 456 nm, but the fluorescence emission intensity of 1 increased by approximately 77-fold (Φ = 0.042, Fig. S5†) with an emission maximum at 456 nm. The fluorescence intensity enhancement of BODIPY-SiO22 compared to 1 alone is likely due to its preorganized structure on the surface of the nanoparticles.
The regeneration of BODIPY-SiO22 after exposure to Pb2+ was examined. The Pb2+-bound BODIPY-SiO22 was treated with an aqueous EDTA solution (10 μM). As expected, upon the addition of the solution, the fluorescence intensity of Pb2+-bound BODIPY-SiO22 was enhanced. However, when the washed, stripped nanoparticles BODIPY-SiO22 were re-exposed to Pb2+, the increased fluorescence emission was again observed, with no reduction in response (Fig. S6†). The fluorescence change was reproducible over several cycles of detection/stripping. The Job plot using the fluorescence changes indicated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding for BODIPY-SiO22 with Pb2+ (Fig. S7†). Using the fluorescence titration data, the association constant (K) for Pb2+ coordination with BODIPY-SiO22 was calculated to be 6.5 × 104 M−1.22,23
1 binding for BODIPY-SiO22 with Pb2+ (Fig. S7†). Using the fluorescence titration data, the association constant (K) for Pb2+ coordination with BODIPY-SiO22 was calculated to be 6.5 × 104 M−1.22,23
The spectral changes upon addition of the previously mentioned biologically and environmentally relevant metal ions were also screened by fluorophotometry and UV/vis spectroscopy. The emission and UV/vis profiles of apo or Pb2+-bound BODIPY-SiO22 are unchanged in the presence of 10 μM Ca2+, Hg2+, Cd2+, Li+, Ag+, Cu2+, Fe2+, Mg2+, Zn2+, K+, Na+, Mn2+, Co2+, Fe3+, Al3+ or Ni2+ (Fig. 2B, S8 and S9†), indicating that BODIPY-SiO22 shows great promise as a useful selective chemosensor for the selective detection of Pb2+in vivo.
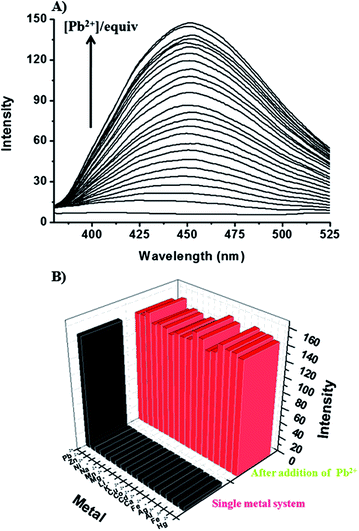
The above results encouraged us to test separation of Pb2+ from human blood (Fig. 3). The overall procedure of using BODIPY-SiO22 to remove Pb2+ from human blood is illustrated in Fig. 3. The test sample was prepared by the addition of BODIPY-SiO22 (10 mg) to 1 mL of human blood containing 100 ppb Pb2+, which is the CDC's level of concern for children's blood lead levels, followed by sonication. Then, the Pb2+-bound BODIPY-SiO22 was removed from the human blood by filtration. To determine the amount of Pb2+ separated by the silica nanoparticles, the amount of Pb2+ left in human blood was determined by inductively coupled plasma mass spectrometry (ICP-MS). The ICP-MS measurements indicated that only 4% Pb2+ remained in human blood, suggesting that the designed silica nanoparticles BODIPY-SiO22 removed 96% of Pb2+. Additionally, we tested the separation of Pb2+ with BODIPY-SiO22 from water. Using the ICP-MS measurements, we found that BODIPY-SiO22 can remove 97% of the initial 15 ppb Pb2+ from water. Thus, receptor BODIPY-SiO22 can be a potentially useful and effective agent for the selective separation and rapid removal of Pb2+in vivo.
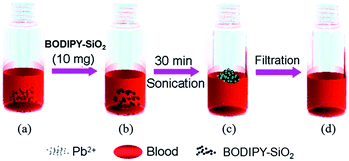 | ||
| Fig. 3 Illustration of the removal of Pb2+ from human blood; (a) preparation of human blood containing Pb2+ (100 ppb), (b) addition of BODIPY-SiO22, (c) sonication for 30 min and (d) filtration. | ||
Furthermore, we examined the addition of BODIPY-SiO22 (10 mg) to 1 mL of wastewater containing 2.0 μM Pb2+. The Pb2+-loaded BODIPY-SiO22 was then removed by filtration from the wastewater. To determine the amount of Pb2+ separated by BODIPY-SiO22, the amount of residual Pb2+ in the waste solution was determined by ICP-MS. Only 7% of the original Pb2+ remained in the waste solution, suggesting that BODIPY-SiO22 removed 93% of the Pb2+.
For preparing BODIPY-SiO22 films on glass slides, 3 mL of the solution detailed above were spread on a glass slide with a glass rod, using adhesive tape as spacers. After the films were dried in air, they were sintered at 450 °C for 20 min. Sensitization of the SiO2 films was achieved by immersing the films in a 1.0 mM solution of receptor 1 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile/tert-butyl alcohol overnight, followed by rinsing in ethanol to remove unadsorbed receptor 1. Finally, a slightly blue colored film of BODIPY-SiO22 was obtained.
1 acetonitrile/tert-butyl alcohol overnight, followed by rinsing in ethanol to remove unadsorbed receptor 1. Finally, a slightly blue colored film of BODIPY-SiO22 was obtained.
We next addressed the metal cation sensing ability of BODIPY-SiO22 films. For the cation sensing experiments, the BODIPY-SiO22 films were dipped into cation solutions of interest, then dried in air, monitoring any fluorescence change by measurement of solid fluorescence spectra. In all cases, changes in film fluorescence were found to be complete within 1–2 s of immersion, with the resulting fluorescence being stable for in excess of one day, both for films immersed continuously in the cation solution and for films dried in air. Typical data are shown in Fig. 4, showing a photographic image of the films after immersion in 10 μM solutions of seven cations (Pb2+, Co2+, Cd2+, Zn2+, Fe3+, Cu2+ and Hg2+). The non-fluorescence of the BODIPY-SiO22 film changed to a strong green fluorescence when the film was dipped in Pb2+ solution (Fig. 4A). On the other hand, no significant fluorescence changes were observed with other metal ions.
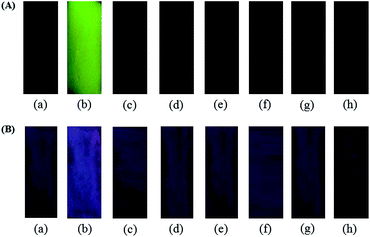 | ||
| Fig. 4 Photographs for (A) emission and (B) color changes of BODIPY-SiO22 after immersion in metal ion (10 μM) solution; (a) non-metal ion, (b) Pb2+, (c) Co2+, (d) Cd2+, (e) Zn2+, (f) Fe3+, (g) Cu2+ and (h) Hg2+. | ||
The visual color changes of BODIPY-SiO22 films were observed after immersion of the plates in solutions of metal ions. As expected, the blue color of the BODIPY-SiO22 film changed to red color when the film was dipped in Pb2+ solution (Fig. 4B). On the other hand, no significant color changes were observed with other metal ions. These results indicate that the BODIPY-SiO22 film selectively detected only Pb2+ ions. Since the preparation of the BODIPY-SiO22 films prepared in this work is much simpler than the sol–gel grafting method, we propose that this BODIPY-SiO22 film is highly useful as a chemosensor to detect Pb2+ ions.
We also observed the solid fluorescence spectra of the BODIPY-SiO22 films after dipping them into the various cation solutions. Fig. 5 shows the corresponding fluorescence spectra for selected films. It is apparent that the film emission is only sensitive to Pb2+ ions. Pb2+ exposure resulted in strong green emission of the film at 450 nm. On the other hand, no significant changes in the fluorescence spectra were observed for any other cations tested (including Co2+, Cd2+, Hg2+, Zn2+, Fe3+ and Cu2+) for concentrations up to 10 μM.
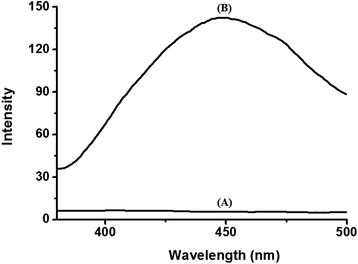 | ||
| Fig. 5 Fluorescence spectra of BODIPY-SiO22 films (A) before and (B) after immersion in Pb2+ solution (10 μM). | ||
The fluorescence change observed following the dipping of BODIPY-SiO22 into Pb2+ solution (10 μM) was found to be fully reversible when BODIPY-SiO22 was rinsed thoroughly with EDTA (10 μM). Reusability was evaluated by repeated dipping/rinsing cycles, with the BODIPY-SiO22fluorescence spectrum being recorded after each step. Typical data are shown in Fig. S10†. The data show that BODIPY-SiO22 exhibited excellent reusability, as almost no loss in BODIPY-SiO22 sensitivity was observed after ten repeated dipping/rinsing cycles. Fig. S11† shows standard calibration data (fluorescence intensity vs. [Pb2+]) for BODIPY-SiO22. A linear response was observed (between 3.2 nM and 1.0 μM) with a sensitivity of ∼3.2 nM (Fig. S11†).
We considered next the buffer action of the BODIPY-SiO22 film. The BODIPY-SiO22 film was immersed in aqueous solutions with pH values ranging between 3.0 and 11.0. Control experiments were conducted using 10 μM of Pb2+ solution controlling the pH range (Fig. 6 and S12†). The resulting film fluorescence is shown in Fig. S12†. The fluorescence intensities of the BODIPY-SiO22 film in the presence of Pb2+ ions were almost constant, with pH values ranging between 3 and 11. We conclude that BODIPY-SiO22 can reliably detect Pb2+ ions over the wide range of pH values from pH 3–11.
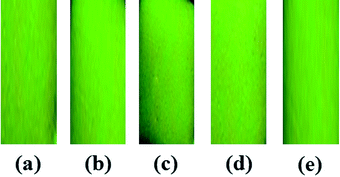 | ||
| Fig. 6 Photograph of fluorescence changes of BODIPY-SiO22 after immersion of Pb2+ (10 μM) at different pH values: (a) pH 3, (b) pH 5, (c) pH 7, (d) pH 9 and (e) pH 11. | ||
Conclusions
We have fabricated BODIPY-SiO22 using a functional BODIPY-based receptor. The BODIPY-SiO22 recognized and separated Pb2+ with a high degree of selectivity among other heavy metal ions in aqueous solution. Beyond its immediate applications in environmental cleanup, BODIPY-SiO22 provides a unique opportunity to introduce molecular binding sites and to rationally design the surface properties of inorganic nanomaterials. Furthermore, BODIPY-SiO22 films with 4 μm thickness on glass substrates were prepared as portable chemosensors for Pb2+ ions in aqueous solution. The BODIPY-SiO22 films show excellent sensitivity and selectivity for Pb2+ ions. The BODIPY-SiO22 film detected Pb2+ at pH = 7.4 with a sensitivity of 3.2 nM. The Pb2+ detection ability was unaffected by the presence of other cations or anions. The results imply that the BODIPY-SiO22 film has strong potential as a portable colorimetric sensor for detection of Pb2+ in the environmental field. We believe the combination of well-defined inorganic nanomaterials and functionalized organic receptors can play a pivotal role in the development of a new generation of hierarchical structures and functionalized composites.Acknowledgements
This work was supported by World Class University (WCU) Program (R32-2008-000-20003-0) and NRF (2011-0003118) supported by Ministry of Education, Science and Technology, and Environmental-Fusion Project (191-091-004) supported by Ministry of Environment.References
- (a) R. Krämer, Angew. Chem., Int. Ed., 1998, 37, 772–773 CrossRef; (b) L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 1995, 24, 197–202 RSC; (c) Y. Wu, X. Peng, B. Guo, J. Fan, Z. Zhang, J. Wang, A. Cui and Y. Gao, Org. Biomol. Chem., 2005, 3, 1387–1392 RSC; (d) X. Peng, J. Du, J. Fan, J. Wang, Y. Wu, J. Zhao, S. Sun and T. Xu, J. Am. Chem. Soc., 2007, 129, 1500–1501 CrossRef CAS; (e) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- H. L. Needleman, Human Lead Exposure, CRC Press, Boca Raton, FL, 1992 Search PubMed.
- (a) S. Araki, H. Sato, K. Yokoyama and K. Murata, Am. J. Ind. Med., 2000, 37, 193–204 CrossRef CAS; (b) N. Rifai, G. Cohen, M. Wolf, L. Cohen, C. Faser, J. Savory and L. DePalma, Ther. Drug Monit., 1993, 15, 71–74 CrossRef CAS; (c) D. A. Cory-Slechta, Adv. Behav. Pharmacol., 1984, 4, 211–255 CAS; (d) C. Winder, N. G. Carmichael and P. D. Lewis, Trends Neurosci., 1982, 5, 207–209 CrossRef CAS; (e) K. M. Lee, X. Chen, W. Fang, J. M. Kim and J. Yoon, Macromol. Rapid Commun., 2011, 32, 497–500 CAS.
- E. S. Claudio, H. A. Godwin and J. S. Magyar, Prog. Inorg. Chem., 2003, 51, 1–144 CAS , and references therein.
- (a) Q. He, E. W. Miller, A. P. Wong and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9316–9317 CrossRef CAS; (b) M. Sun, D. Shangguan, H. Ma, L. Nie, X. Li, S. Xiong, G. Liu and W. Thiemann, Biopolymers, 2003, 72, 413–420 CrossRef CAS; (c) J. Liu and Y. Lu, J. Am. Chem. Soc., 2003, 125, 6642–6643 CrossRef CAS; (d) C.-T. Chen and W.-P. Huang, J. Am. Chem. Soc., 2002, 124, 6246–6247 CrossRef CAS; (e) J. Li and Y. Lu, J. Am. Chem. Soc., 2000, 122, 10466–10467 CrossRef CAS; (f) S. Deo and H. A. Godwin, J. Am. Chem. Soc., 2000, 122, 174–175 CrossRef CAS.
- (a) Y. H. Zhu, H. Da, X. L. Yang and Y. Hu, Colloids Surf., A, 2003, 231, 123–129 CrossRef CAS; (b) D. K. Yi, S. T. Selvan, S. S. Lee and G. C. Papaefthymiou, J. Am. Chem. Soc., 2005, 127, 4990–4991 CrossRef CAS; (c) N. Insin, J. B. Tracy, H. Lee, J. P. Zimmer, R. M. Westervelt and M. G. Bawendi, ACS Nano, 2008, 2, 197–202 CrossRef CAS.
- (a) A. Decalzo, R. Martínez-Máñez, F. Sancenón, K. Hoffmann and K. Rurack, Angew. Chem., Int. Ed., 2006, 45, 4924–4948 Search PubMed; (b) W. S. Han, H. Y. Lee, S. H. Jung, S. J. Lee and J. H. Jung, Chem. Soc. Rev., 2009, 38, 1904–1915 RSC.
- H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008–2012 CrossRef CAS , and references therein.
- (a) J. Y. Kwon, Y. J. Jang, Y. J. Lee, K. M. Kim, M. S. Seo, W. W. Nam and J. Y. Yoon, J. Am. Chem. Soc., 2005, 127, 10107–10111 CrossRef CAS; (b) P. Chen, B. Greenberg, S. Taghavi, C. Romano, D. Lelie and C. He, Angew. Chem., Int. Ed., 2005, 44, 2715–2719 CrossRef CAS.
- (a) H. Y. Lee, D. R. Bae, J. C. Park, H. Song, W. S. Han and J. H. Jung, Angew. Chem., Int. Ed., 2009, 48, 1239–1243 CrossRef CAS; (b) J. H. Jung, M. Park and S. Shinkai, Chem. Soc. Rev., 2010, 39, 4286–4302 RSC; (c) S. J. Lee, S. S. Lee, J. Y. Lee and J. H. Jung, Chem. Mater., 2006, 18, 4713–4715 CrossRef CAS; (d) S. J. Lee, S. S. Lee, M. S. Lah and J. H. Jung, Chem. Commun., 2006, 4539–4541 RSC; (e) S. J. Lee, D. R. Bae, W. S. Han, S. S. Lee and J. H. Jung, Eur. J. Inorg. Chem., 2008, 1559–1594 CrossRef CAS.
- Y.-Q. Weng, F. Yue, Y.-R. Zhong and B.-H. Ye, Inorg. Chem., 2007, 46, 7749–7755 CrossRef CAS.
- H. J. Kim, S. J. Lee, S. Y. Park, J. H. Jung and J. S. Kim, Adv. Mater., 2008, 20, 3229–3235 CrossRef CAS.
- J. E. Ghadiali and M. M. Stvens, Adv. Mater., 2008, 20, 4359–4363 CrossRef CAS.
- B. J. Melde, B. J. Johnson and P. T. Charles, Sensors, 2008, 8, 5202–5228 CrossRef CAS.
- (a) E. Climent, M. D. Marcos, R. Martínez-Máñez, F. Sancenón, J. Soto, K. Rurack and P. Amorós, Angew. Chem., Int. Ed., 2009, 48, 8519–8522 CrossRef CAS; (b) P. Calelo, E. Aznar, J. M. Lloris, M. D. Marcos, R. Martínez-Máñez, J. V. Ros-Lis, J. Soto and F. Sancenón, Chem. Commun., 2008, 1668–1670 Search PubMed; (c) J. V. Ros-Lis, R. Casauús, M. Comes, C. Coll, M. D. Marcos, R. Martínez-Máñez, F. Sancenón, J. Soto, P. Amorós, J. E. Haskouri, N. Garro and K. Rurack, Chem.–Eur. J., 2008, 14, 8267–8278 CrossRef CAS.
- W. Qin, T. Rohand, M. Baruah, A. Stefan, M. V. der Auweraer, W. Dehaen and N. Boens, Chem. Phys. Lett., 2006, 420, 562–568 CrossRef CAS.
- J. Olmsted, III, J. Phys. Chem., 1979, 83, 2581–2584 CrossRef.
- X. Qi, E. J. Jun, L. Xu, S.-J. Kim, J. S. J. Hong, Y. J. Yoon and J. Yoon, J. Org. Chem., 2006, 71, 2881–2884 CrossRef CAS.
- Z. Ekmekci, M. D. Yilmaz and E. U. Akkaya, Org. Lett., 2008, 10, 461–464 CrossRef CAS.
- (a) A. Loudet and K. D. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS; (b) A. Coskun and E. U. Akkaya, J. Am. Chem. Soc., 2005, 127, 10464–10465 CrossRef CAS; (c) K. Rurack, M. Kollmannsberger and J. Daub, Angew. Chem., Int. Ed., 2001, 40, 385–387 CrossRef CAS.
- (a) S. C. Dodani, Q. He and C. J. Chang, J. Am. Chem. Soc., 2009, 131, 18020–18021 CrossRef CAS; (b) D. W. Damaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194–1195 CrossRef.
- Association constants were obtained using the computer program ENZFITTER, available from Elsevier-BIOSOFT, 68 Hills Road, Cambridge CB2 1LA, UK.
- K. A. Conners, Binding Constants: the Measurement of Molecular Complex Stability, Wiley, New York, 1987 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Measurement data. See DOI: 10.1039/c1an15828g |
| This journal is © The Royal Society of Chemistry 2012 |
