Molecular beacon-based half-adder and half-subtractor†
Chia-Ning
Yang
*,
Chun-Yu
Hsu
and
Yu-Chung
Chuang
Institute of Biotechnology, National University of Kaohsiung, Kaohsiung, Taiwan. E-mail: cnyang@nuk.edu.tw; Fax: +886-75919404; Tel: +886-75919717
First published on 7th November 2011
Abstract
This work demonstrates two DNA-based logic circuits that behave as a half-adder and a half-subtractor. A half-adder is composed of an AND gate and an XOR gate, whereas a half-subtractor consists of an INH gate and an XOR gate. The proposed designs are inspired by molecular beacons.
As nanotechnology has become a principal research interest, nano-scale devices that can be built by either top-down or bottom-up approach are widely studied.1 After Adleman introduced DNA computing to solve travelling salesman problems in 1994,2 numerous works using a similar concept have been proposed theoretically and/or proved by experiments to demonstrate the possibility that designed DNA sequences can serve as elementary computing devices.3–9
Boolean algebra circuits that process computations of binary digits according to the composition of logic gates are a key requirement in constructing computing devices. For nearly a decade, delicately designed enzyme-inhibitors,10 small supermolecules,11–16 and DNA sequences17–24 mimicking Boolean circuits have demonstrated the power of molecular-scale computation. A. Prasanna de Silva and Seiichi Uchiyama, recently wrote a review on the topic of molecular logic and computing. They stated:25 “computation with molecules is no less realistic than with well-established semiconductor materials that dominates in today's computers”.
The present study demonstrates two DNA-based logic circuits capable of performing addition and subtraction. A logic half-adder circuit adds two binary digits and outputs two binary digits: sum-bit (standing for 20 digit) and carry-bit (standing for 21 digit), generated by an XOR (eXclusive OR) and an AND logic gate, respectively. Likewise, a logic circuit of a half-subtractor takes two binary digits for subtraction and outputs two binary digits, including borrow-bit and difference-bit by an INH (inhibit) and an XOR logic gate, respectively.
The constructed systems in this study are based on the concept of molecular beacon, which commonly serves as a DNA probe.26 In short, a molecular beacon is a DNA sequence labeled by a fluorophore at the 5′ end and a corresponding quencher at the 3′ end. Without recognizing any substrate, the DNA sequence is in a hairpin conformation (with a loop in the single-strand and a stem in the double-strand by hybridizing its two ends) and the fluorescence is quenched by the nearby quencher. Upon hybridization with a substrate whose sequence is partially or entirely complementary to the hairpin, the molecular beacon opens up and this conformational change causes the fluorophore to stay away from the quencher and shine. In the close and open forms of hairpin, the fluorescence signal is off and on and this phenomenon is perfect for “0” and “1” readings as in Boolean logic operations. The present design uses molecular beacon-like DNA strands as gate molecules whereas the substrates are used as inputs to control the on/off states of the fluorescence signal. More sophisticated arrangements allow one input strand labelled with a fluorophore to be switched between on and off states by the interplay between the gate molecule and the other input strand.
Design of half-adder: a half-adder is composed of an AND gate and an XOR gate. All the DNA sequence assignment is given in Fig. S of ESI.† The AND logic gate is a 29 nt DNA hairpin containing a loop and a 5 nt stem where the 3′ end is labeled with BHQ-1 (a quencher with an absorption range between 480 and 580 nm). The XOR gate molecule is similar to the AND gate molecule, except that it is additionally labeled with FAM (a green dye, λem = 520 nm) at its 5′ end. The XOR gate molecule per se is a molecular beacon probe, whose open and close forms respond to generate a fluorescence signal or not. Two input molecules, IA and IB, work with both AND and XOR Boolean logic operations in the constructed half-adder circuit. IA is a 46 nt DNA sequence and is labeled with CAL Fluoro Orange 560 (a red dye, λem = 560 nm) at the 5′ end; IB is simply a 46 nt DNA sequence. The 27 nt sequences of the IB 5′ end and of the IA 3′ end are complementary. Besides the sequence complementarity between IA and IB, the 19 nt in the IA 5′ end (marked in grey, in Fig. S of ESI†) is complementary to that in the 3′ ends of the AND gate and the XOR gate, whereas the 19 nt in the IB 3′ end (marked in grey, in Fig. S of ESI†) is complementary to that in the 5′ ends of the AND gate and the XOR gate. Accordingly, in the presence of IA or IB, the AND gate and the XOR gate no longer maintain the hairpin shape. In four states of inputs of (IA, IB), i.e., (0, 0), (0, 1), (1, 0), and (1, 1), the red fluorescence signal released by IA follows the truth table for AND operation, and the green fluorescence signal released by the XOR gate follows the truth table of XOR operation. Because two distinct fluorophores are used to label the XOR gate and IA, the reading channel is set to 560 nm for the emission of CAL Fluoro Orange 560 in the designed AND operation, whereas the reading channel is set to 520 nm for the emission of FAM in XOR operation. The choice of BHQ-1 is owing to its high quenching efficiency to the two selected fluorophores.27
Fig. 1(A) illustrates the AND gate operations. The output reading depends on the CAL Fluoro Orange 560 of an IA strand.
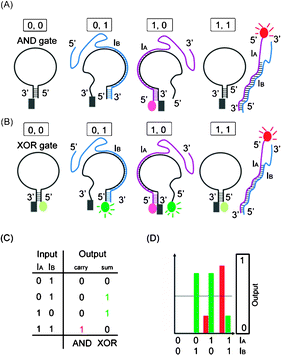 | ||
Fig. 1 Schematic representation of the half-adder. (A) AND operation with the reading channel set to red fluorophore (on IB) emission. (B) XOR operation with the reading channel set to the green fluorophore (on XOR gate) emission. (C) Truth table for AND and XOR operations. (D) The observed fluorescence output of AND (in red bars) and XOR (in green bars) operations with every combination of inputs.  : CAL Fluoro Orange 560, : CAL Fluoro Orange 560,  : FAM, : FAM,  : BHQ1. : BHQ1. | ||
- In the (0, 0) state where neither IA nor IB is present, no fluorescent signal is shown. Accordingly, the output reads 0.
- In the (0, 1) state, only IB is added to the AND gate. Because there is no fluorophore-carrying molecule, no fluorescence signal is shown and the output reads 0.
- In the (1, 0) state, IA is added and recognized by the AND gate molecule. The fluorescence emitted from CAL Fluoro Orange 560 of the IA 5′ end is quenched by BHQ-1 of the AND gate molecule. The output reads 0.
- In the (1, 1) state, both IA and IB are present. Because of the higher affinity between IA and IB, most input strands are not associated with the AND gate molecule, and therefore the fluorescence signal from IA is observed. The output reads 1.
Fig. 1(B) demonstrates the XOR gate operation. The output reading depends on the FAM on the XOR gate molecule.
- In the (0, 0) state where neither IA nor IB is added, the XOR logic gate molecule is in hairpin shape and the fluorescent signal of FAM is quenched by BHQ-1. The output reads 0.
- In the (0, 1) state, IB is added and recognized by the 5′ end of the XOR gate molecule. The XOR gate molecule is in open form, which separates BHQ-1 and FAM. The fluorescence signal of FAM generates output reading 1.
- In the (1, 0) state, IA is added and recognized by the 3′ end of the XOR gate molecule. The XOR gate molecule is in open form and the fluorescence signal of FAM sets output as 1.
- In the (1, 1) state, both IB and IA are added. Because of higher affinity between IB and IA, IA and IB are hybridized to each other and the XOR logic gate is in hairpin conformation. The fluorescence signal of FAM from the XOR gate is quenched, and the output reads 0. In this state, the fluorescence from IA is inescapably emitted but does not affect the overall output pattern of XOR operation, because the reading channel is set to read the FAM signal only.
To summarize, the on/off signal patterns of the designed AND and XOR gate operations follow the truth table in Fig. 1(C) and are proved by the observation of the relative fluorescence intensity of the CAL Fluoro Orange 560 (the red bars) in AND operation and of the FAM (the green bars) in the XOR operation in Fig. 1(D).
Design of half-subtractor: a half-subtractor consists of an INH gate and an XOR gate. The sequences of two input strands, 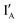 in 37 nt and
in 37 nt and 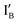 in 46 nt, are given in Fig. S in ESI.†IB and
in 46 nt, are given in Fig. S in ESI.†IB and 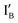 share exactly the same sequence, whereas
share exactly the same sequence, whereas 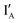 is 9 nt shorter than IA at the 3′ end. The
is 9 nt shorter than IA at the 3′ end. The 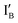 is labeled with a fluorophore, CAL Fluoro Orange 560, at its 5′ end;
is labeled with a fluorophore, CAL Fluoro Orange 560, at its 5′ end; 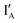 is labeled with a quencher group, BHQ-1, at its 3′ end that quenches the fluorescence emitted from
is labeled with a quencher group, BHQ-1, at its 3′ end that quenches the fluorescence emitted from 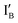 when these two input strands are hybridized, as seen in IA and IB.
when these two input strands are hybridized, as seen in IA and IB. 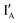 and
and 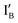 work with both the INH and XOR Boolean logic operations. Interestingly, in our designed INH operation, there is no gate molecule and the input
work with both the INH and XOR Boolean logic operations. Interestingly, in our designed INH operation, there is no gate molecule and the input 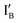 strand in four input (
strand in four input (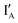 ,
,  ) states produces a fluorescence on/off pattern according to the truth table of the INH operation. As to the XOR operation, the XOR gate molecule in the previously demonstrated half-adder also works in the half-subtractor. In short, the output readings in INH and XOR operations are based on CAL Fluoro Orange 560 (λem = 560 nm) of the input
) states produces a fluorescence on/off pattern according to the truth table of the INH operation. As to the XOR operation, the XOR gate molecule in the previously demonstrated half-adder also works in the half-subtractor. In short, the output readings in INH and XOR operations are based on CAL Fluoro Orange 560 (λem = 560 nm) of the input 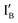 strand and on FAM of the XOR gate (λem = 520 nm), respectively.
strand and on FAM of the XOR gate (λem = 520 nm), respectively.
The INH gate operation is given in Fig. 2(A), in which no INH gate molecule is needed and the 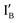 strand carrying CAL Fluoro Orange 560 is responsible for the output reading.
strand carrying CAL Fluoro Orange 560 is responsible for the output reading.
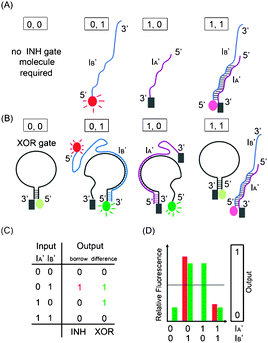 | ||
Fig. 2 Schematic representation of the half-subtractor. (A) INH operation with the reading channel set to red fluorophore (on IB’) emission. (B) XOR operation with the reading channel set to green fluorophore (on XOR gate) emission. (C) Truth table for INH and XOR operations. (D) The observed fluorescence output of INH (in red bars) and XOR (in green bars) operations with every combination of inputs.  : CAL Fluoro Orange 560, : CAL Fluoro Orange 560,  : FAM, : FAM,  : BHQ1. : BHQ1. | ||
- In the (0, 0) state that discusses 0 − 0 = 0, neither 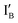 nor
nor 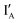 is present and the output reading is 0.
is present and the output reading is 0.
- In the (0, 1) state that discusses 0 − 1 = −1, only 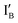 is present and the fluorescence signal of CAL Fluoro Orange 560 is observed. The output reading is 1.
is present and the fluorescence signal of CAL Fluoro Orange 560 is observed. The output reading is 1.
- In the (1, 0) state that discusses 1 − 0 = 1, only 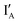 is present and there is no fluorescence signal. The output reading is 0.
is present and there is no fluorescence signal. The output reading is 0.
- In the (1, 1) state that discusses 1 − 1 = 0, both 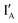 and
and 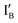 are present and the hybridization brings CAL Fluoro Orange 560 of
are present and the hybridization brings CAL Fluoro Orange 560 of 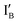 and BHQ-1 of
and BHQ-1 of 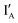 into close contact with the quenched fluorescence. The output reading is 0.
into close contact with the quenched fluorescence. The output reading is 0.
Fig. 2(B) illustrates the XOR operation in the designed half-subtractor where the output reading relies on the FAM group attached in the 5′ end of the XOR gate molecule.
- In the (0, 0) state, neither 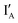 nor
nor 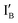 is present and the XOR gate molecule is in hairpin shape. The fluorescence is quenched and the output reads 0.
is present and the XOR gate molecule is in hairpin shape. The fluorescence is quenched and the output reads 0.
- In the (0, 1) state, input 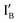 is added to the XOR gate. Recognition of
is added to the XOR gate. Recognition of 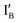 opens the XOR gate molecule and the fluorescence emitted from FAM sets the output reading as 1.
opens the XOR gate molecule and the fluorescence emitted from FAM sets the output reading as 1.
- In the (1, 0) state, input 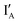 is added. Recognition of
is added. Recognition of 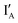 opens up the XOR gate molecule and the fluorescence emitted from FAM sets the output reading as 1.
opens up the XOR gate molecule and the fluorescence emitted from FAM sets the output reading as 1.
- In the (1, 1) state, both 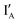 and
and 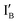 are present and are mostly hybridized instead of interacting with the XOR gate molecule. The fluorescence from FAM is quenched by BHQ-1 due to the hairpin-shaped XOR gate molecule. The output reading is 0.
are present and are mostly hybridized instead of interacting with the XOR gate molecule. The fluorescence from FAM is quenched by BHQ-1 due to the hairpin-shaped XOR gate molecule. The output reading is 0.
To summarize, the on/off signal patterns of the designed INH and XOR gate operations follow the truth table in Fig. 2(C) and are proved by the observation of the relative fluorescence intensity of the CAL Fluoro Orange 560 (the red bars) in INH operation and of the FAM (the green bars) in the XOR operation in Fig. 2(D).
This work demonstrated two DNA-based logic circuits for arithmetic operations, including addition and subtraction of two binary digits. Each logic circuit comprises two logic gates that output two different fluorescence signals at λem = 520 nm and λem = 560 nm in response to two input strands. That is, IA and IB are operated by the present AND and XOR gates in the half-adder; 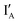 and
and 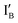 are operated by the INH and XOR operations in the present half-subtractor. The two resulted output signals are encoded in the IB or
are operated by the INH and XOR operations in the present half-subtractor. The two resulted output signals are encoded in the IB or 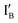 strand and in the XOR gate molecule where the interplay among the input strand(s) and gate molecule regulates the output signal on/off switch.
strand and in the XOR gate molecule where the interplay among the input strand(s) and gate molecule regulates the output signal on/off switch.
Compared to DNA-based half-adders established by Stojanović and Stefanović17 and Voelcker et al.,24 our half-adder is relatively compact. In the half-adder reported by Stojanović team, the XOR and AND gate operations recognize the same set of two input strands, but there are two gate molecules to achieve their designed XOR operation and additionally an output molecule carrying the fluorescence signal is needed after an operation. In the half-adder of Voelcker work, the XOR and AND gates work with different sets of input strands, although these four strands are later integrated to two strands. Herein, the delicate design of the proposed AND and XOR logic operations is achieved by three components, and the proposed INH logic operation is achieved by two input DNA strands. The simplicity in the proposed systems, where molecular beacons switching between open and closed conformations serve as a mechanistic basis, may bring some ease when the design is integrated to a complicated application in the future.
This work demonstrated prototypical arithmetic operations in solution phase. Our next step is to immobilize gate molecules onto solid supports and process the experiment in solid phase. It is possible to conduct AND and XOR operations of a half-adder simultaneously on one single substrate, because (i) different gate molecules on the substrate will not interfere with each other, (ii) the two logic operations take the same set of input molecules, and (iii) the combined output signals still obey the truth table. Likewise, the proposed subtraction can also be done on one substrate.
Notes and references
- J.-S. Shin and N. A. Pierce, Nano Lett., 2004, 4, 905 CrossRef CAS.
- L. M. Adleman, Science, 1994, 266, 1021 CrossRef CAS.
- C. N. Yang and C. B. Yang, BioSystems, 2005, 81, 1 CrossRef CAS.
- C. N. Yang, K. S. Huang, C. B. Yang and C. Y. Hsu, Int. J. Innovative Comput. Inf. Control, 2009, 5, 3045 Search PubMed.
- K. Sakamoto, H. Gouzu, K. Komiya, D. Kiga, S. Yokoyama, T. Yokomori and M. Hagiya, Science, 2000, 288, 1223 CrossRef CAS.
- D. Faulhammer, A. R. Cukras, R. J. Lipton and L. F. Landweber, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 1385 CrossRef CAS.
- R. S. Braich, N. Chelyapov, C. Johnson, P. W. Rothemund and L. Adleman, Science, 2002, 296, 499 CrossRef CAS.
- C. H. Lin, H. P. Cheng, C. B. Yang and C. N. Yang, BioSystems, 2007, 90, 242 CrossRef CAS.
- R. Pei, E. Matamoros, M. Liu, D. Stefanovic and M. N. Stojanovic, Nat. Nanotechnol., 2010, 5, 773 CrossRef CAS.
- S. Sivan, S. Tuchman and N. Lotan, BioSystems, 2003, 70, 21 CrossRef CAS.
- G. Ashkenasy and M. R. B. Ghadiri, J. Am. Chem. Soc., 2004, 126, 11140 CrossRef CAS.
- D. H. Qu, Q. C. Wang and H. Tian, Angew. Chem., Int. Ed., 2005, 44, 5296 CrossRef CAS.
- S. Ami, M. Hliwa and C. Joachim, Chem. Phys. Lett., 2003, 367, 662 CrossRef CAS.
- J. A. Andréasson, G. Kodis, Y. Terazono, P. A. Liddell, S. Bandyopadhyay, R. H. Mitchell, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2004, 126, 15926 CrossRef CAS.
- O. Hod, R. Baer and E. Rabani, J. Am. Chem. Soc., 2005, 127, 1648 CrossRef CAS.
- A. P. de Silva and N. D. McClenaghan, J. Am. Chem. Soc., 2000, 122, 3965 CrossRef CAS.
- M. N. Stojanović and D. Stefanović, J. Am. Chem. Soc., 2003, 125, 6673 CrossRef CAS.
- H. Lederman, J. Macdonald, D. Stefanović and M. N. Stojanović, Biochemistry, 2006, 45, 1194 CrossRef CAS.
- A. Okamoto, K. Tanaka and I. Saito, J. Am. Chem. Soc., 2004, 126, 9458 CrossRef CAS.
- A. Saghatelian, N. H. Volcker, K. M. Guckian, V. S. Lin and M. R. Ghadiri, J. Am. Chem. Soc., 2003, 125, 346 CrossRef CAS.
- B. M. Frezza, S. L. Cockroft and M. R. Ghadiri, J. Am. Chem. Soc., 2007, 129, 14875 CrossRef CAS.
- X. F. Guo, D. Q. Zhang, G. X. Zhang and D. B. Zhu, J. Phys. Chem. B, 2004, 108, 11942 CrossRef CAS.
- H. Yan, L. Feng, T. H. LaBean and J. H. Reif, J. Am. Chem. Soc., 2003, 125, 14246 CrossRef CAS.
- N. H. Voelcker, K. M. Guckian, A. Saghatelian and M. R. Ghadiri, Small, 2008, 4, 427 CrossRef CAS.
- A. P. de Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399 CrossRef CAS.
- S. Tyagi and F. R. Kramer, Nat. Biotechnol., 1996, 14, 303 CrossRef CAS.
- http://www.biosearchtech.com/ .
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and sequence assignment. See DOI: 10.1039/c1cc14518e |
| This journal is © The Royal Society of Chemistry 2012 |
