A hybrid biocathode: surface display of O2-reducing enzymes for microbial fuel cell applications†
Alon
Szczupak‡
,
Dan
Kol-Kalman‡
and
Lital
Alfonta
*
Avram and Stella Goldstein-Goren Department of Biotechnology Engineering and the Ilse Katz Institute for Nanoscale Science and Technology, P.O. Box 653, Beer-Sheva 84105, Israel. E-mail: alfontal@bgu.ac.il; Fax: +972 8 6479576; Tel: +972 8 6479066
First published on 31st October 2011
Abstract
Laccase and bilirubin oxidase were successfully displayed on the surface of yeast cells. Subsequently, these modified yeast cells were used in the cathode compartment of a microbial fuel cell. The performance of the fuel cells is compared.
Biofuel cells are electrochemical devices that convert chemical energy to electricity using biochemical pathways and redox enzymes. In enzymatic fuel cells, purified enzymes are used to catalyze the oxidation of fuel in the anode and the reduction of the electron acceptor in the cathode.1 In microbial fuel cells (MFCs), the entire metabolism of microorganisms is exploited to realize the oxidation of fuels or reduction of electron acceptors.2,3 This arrangement allows for full oxidation of different fuels, unlike what occurs with enzymatic fuel cells, where reactions are limited by the enzymes used. On the other hand, when using whole microorganisms, one encounters mass transport problems due to difficulties in translocating redox mediators across the cellular membrane, due to challenges in fuel delivery as well as due to long electron transfer pathways. Together, the obstacles decrease the power output of the cell.4
In previous work performed in our group, a hybrid anode containing glucose oxidase (GOx) from Aspergillus niger displayed on the yeast cell surface was described.5 The surface-displayed GOx catalyzes the oxidation of β-D-glucose to glucono-δ-lactone on the yeast surface, thereby bypassing membrane transfer-related hindrance, while offering the possibility of regenerating the active enzyme by the living organism in situ.
While most efforts to improve the performance of biofuel cells have focused on fuel oxidation in the anode compartment, the cathode, in which the electrons are transferred to the electron acceptor, remains a bottleneck, preventing further development and rendering it the limiting electrode.6 To date, the cathode used in many biofuel cells is abiotic, using inert metals, such as platinum, to catalyze electron transfer to either a soluble electron acceptor in a cathode solution or to molecular oxygen in the air when using an air cathode. In such cases, the reduction of oxygen also results in an oxygen to hydrogen peroxide two-electron reduction process, as well as the reduction of four electrons upon converting hydrogen to water,7,8 resulting in decreased fuel cell performance. Other fuel cells use enzymatic cathodes containing a purified enzyme, such as laccase, which specifically catalyzes the reduction of oxygen to water,9,10 thereby reducing the amount of hydrogen peroxide formed and thus achieving better performance. This system, however, requires the use of purified enzymes.
A few biocathodes using microorganisms have been described. These include cells in which biofilms originating from sea water11 or denitrifying microorganisms12 were grown on the cathode. Here, cellular metabolism was exploited to catalyze electron transfer from the cathode to the electron acceptor, resulting in better performance than obtained with inorganic cathodes, although limitations of mass transfer through the microorganismal membranes were encountered.
Here, we present a method to overcome these limitations using yeast surface display of bilirubin oxidase (BOD) or laccase. The biofuel cell described here contains yeast displaying redox enzymes on their surface in both compartments (Fig. 1). BOD from Myrothecium verrucaria and laccase from Trametes versicolor were surface-displayed in the cathode compartment, while at the anode, YSD of GOx was used. Both enzymes were displayed on the surface of S. cerevisiae via the a-agglutinin YSD system.13 Using YSD in both compartments is a novel concept which enables regeneration of both fuel cell compartments and offers the advantages of over-expression of the enzyme catalyzing the reaction of a compartment, yet eliminating the need to purify the over expressed enzyme. Hence, for the first time, laccase and BOD are displayed on the surface of yeast, and a cathode containing genetically modified microorganisms is presented.
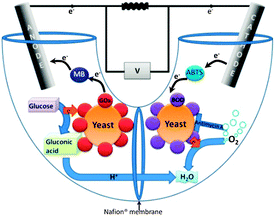 | ||
| Fig. 1 Schematic presentation of the hybrid biofuel cell. Yeast at the cathode express BOD on their surface, while those at the anode express GOx. | ||
Laccases are blue multi-copper enzymes that catalyze the oxidation of many aromatic compounds in reactions coupled to the four electron reduction of molecular oxygen to water with pH optima between 4 to 5, depending on the source organism.11 The ability of laccases to catalyze the full reduction of oxygen at a high redox potential of up to 0.79 V, relative to a normal hydrogen electrode (NHE), has made them useful in many biocathodes.14BOD also catalyzes oxygen to water, although at a potential of 0.67 V relative to a NHE,15 and has a pH optimum of 7, making this enzyme ideal for cathodes working at neutral pH, as well as in membrane-less biofuel cells. Both enzymes contain four copper ions as co-factors.
Surface display of the various enzymes was realized by cloning the corresponding genes into the YSD vector. The resulting pLCHOOP and pC-BOD vectors include a myc epitope tag fused to the C-terminus of each enzyme, enabling subsequent detection of the surface-displayed enzymes using anti-myc-fluorescein isothiocyanate (FITC)-labeled antibodies, followed by flow cytometry analysis. The signals measured in this manner were compared to those obtained from yeast not transformed to express the surface display system and to yeast containing the surface display system but without induction, as negative controls. When displayed on the yeast surface, both enzymes demonstrated high fluorescence (ESI†, Fig. S1).
Biochemical and electrochemical activity assays were conducted to verify that surface-displayed enzymes were active. Based on a colorimetric biochemical assay of the free enzymes a calibration curve was first plotted. The specific activity of displayed laccase was estimated to be 2.5 mU mL−1, a value which can be translated to ca. 2 × 103 enzymes per yeast cell. The specific activity of displayed BOD was estimated to be 43 mU mL−1, corresponding to approx. 4 × 104 enzymes per cell. The addition of copper ions to the laccase- or BOD-displaying yeast induction medium led to an increase in displayed enzyme activity (ESI†, Fig. S2). In the electrochemical assay, the biocatalytic reduction of oxygen to water using ABTS as an electron transfer mediator was measured.
The cyclic voltammograms (CVs) shown in Fig. 2A (BOD) and Fig. 2B (laccase) present biocatalytic currents that evolve when using modified yeast or purified enzymes in the presence of ABTS, oxygen and, for the BOD-expressing yeast, antimycin A.
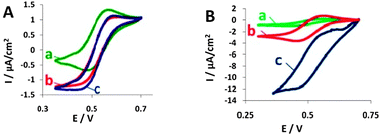 | ||
| Fig. 2 Cyclic voltammograms (CVs) of the electro-reduction of O2 by BOD (A) and by laccase (B) in the presence of ABTS and antimycin A. (a) ABTS (CVs shown are similar to those of yeast not expressing the enzyme); (b) ABTS with free enzyme; (c) ABTS with yeast displaying each enzyme, respectively. Reference electrode: Ag/AgCl, scan rate: 1 mV s−1. | ||
It can be clearly seen that the cathodic current measured in the presence of the enzymes (either free or expressed on yeast) is higher than the background current. The voltammograms show that laccase and BOD expressing yeast are able to catalyze the reduction of O2, serving as the final electron acceptor in the hybrid biocathodes, using ABTS as a mediator. As can be seen from the cyclic voltammograms shown in Fig. 2A(c) and B(c), the biocatalytic current of yeast displaying laccase is ca. 1 order of magnitude higher than that of yeast displaying BOD. We attribute this large difference to the fact that laccase achieves better communication with the electrode than does BOD, possibly due to optimal orientation relative to the electrode upon surface display.
Next, we assembled the hybrid biofuel cells and studied their performance in comparison with biofuel cells containing a hybrid anode and a cathode containing purified enzyme only, unmodified yeast, modified yeast without antimycin A or unmodified yeast together with purified enzyme. The concentration of purified enzyme was similar to that calculated from the activity assay conducted for the displayed enzyme, prior to fuel cell assembly. The MFC contains yeast displaying either laccase or BOD on their surface in the cathode and 2,2′-azino-bis-(3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS) as a redox mediator. In the anode, yeast displaying GOx were used. Glucose was used as fuel and methylene blue (MB) was used as redox mediator. Antimycin A, an antibiotic that inhibits cytochrome c oxidoreductase activity in yeast mitochondria and, therefore, inhibits aerobic respiration,16 was added to the cathode to eliminate competition over oxygen consumption by those yeast cells presenting the reducing surface-displayed enzymes. In the absence of antimycin A, significant losses of power output resulted. As can be seen from Fig. 3A(c), the open circuit voltage (OCV) and maximum power output of the BOD-expressing yeast biofuel cell are 607 mV and 1.38 μW cm−2, respectively, similar to the performances of free BOD-based biofuel cells. Biofuel cells containing BOD in their cathode possess approx. 2.5-fold higher OCV than do biofuel cells without the enzyme and 50-fold higher power output (Fig. 3A(b)). The biofuel cell that contained BOD-expressing yeast without addition of antimycin A had an OCV of 365 mV and a maximum power output of only 0.053 μW cm−2 (Fig. 3A(d)), values approx. 2 and 26 times lower than obtained with antimycin A-containing biofuel cells. In the long-term experiments, BOD-expressing yeast were able to regenerate the enzyme even after 3 weeks in the biofuel cell (ESI†, Fig. S6). This characteristic is one of the advantages MFCs offer over enzyme-based fuel cells. Moreover, BOD can be active for at least 2 months,17 as we could observe hybrid biofuel cells able to maintain their OCV for more than 60 days (Fig. S6, ESI†) even without regeneration, although regeneration may afford a longer cell lifetime.
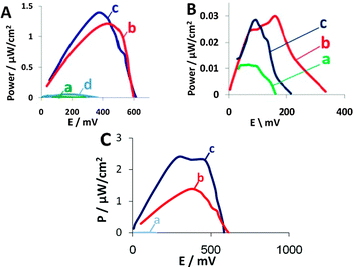 | ||
| Fig. 3 Power output curves for (A) BOD and (B) laccase. (a) Background solution, (b) free enzyme at the cathode, (c) enzyme-displaying yeast and (d) in the absence of antimycin A. (C) An improved fuel cell containing (a) wild type yeast in the cathode, (b) BOD-displaying yeast in the cathode of the fuel cell depicted in Fig. 1 or (c) BOD-displaying yeast in the cathode of the improved design fuel cell. | ||
The biocatalytic current measured with laccase-displaying yeast is much higher than that of the purified enzyme, indicating good communication with the electrode (Fig. 2B(c)). Still, the biofuel cells containing laccase-displaying yeast in the cathode present an open circuit voltage of 215 mV (Fig. 3B(c)), whereas the purified enzyme cathode had a higher OCV of 335 mV (Fig. 3B(b)). The power outputs of the hybrid cathodes were lower than those containing purified enzymes (Fig. 3B(c) and (b)). These results can be related to the low number of active enzymes displayed on the yeast surface, a situation resulting in lower activity than measured with the BOD-displaying yeast, as well as lower power outputs. The difference between the enzyme-displaying yeast and purified enzyme is much larger with the laccase-surface display system, as the laccase CV measurements were performed in the absence of antimycin A, indicating better communication with the electrode, even though the higher hydrophobicity of the 5% Nafion-coated carbon cloth electrode that was used for the measurements may have also contributed to the difference, as yeast cell adhesion to this surface is larger than that of the free enzyme.
The hybrid cells also show relatively high internal resistance, originating from a fuel cell design that reduced the power outputs significantly. Better design of the fuel cell will result in lower internal resistances and higher power outputs. To demonstrate this, we assembled a cell with a different architecture, namely one that afforded a shorter distance between the electrodes, more porous electrode material and a different separating membrane. As predicted, this biofuel cell demonstrated superior performance and much lower internal resistances, as depicted in Fig. 3C. In this cell, we used yeast displaying BOD at the cathode and yeast displaying GOx at the anode. The power output for YSD with BOD was 2.5 μW cm−2 (Fig. 3C(c)) at the maxima of the power output curve, a value that corresponds to an internal resistance of only 1 kΩ. The power output was 20-fold higher than that obtained with wild type yeast at the cathode (Fig. 3C(a)) and 2 times higher (Fig. 3C(b)) than that noted with the U-shaped fuel cell depicted in Fig. 1.
The stability of the hybrid fuel cells and their regeneration ability were checked in a long-term assay, conducted over 30 days. During this interval, the OCV of the hybrid fuel cells remained stable, and the inoculated population of yeast survived, enabling re-induction of the fuel cells and maintaining the OCV unchanged when a decrease in cell performance was observed (ESI†, Fig. S5 and S6).
We have observed that the BOD surface display system allows for better fuel cell performance than does the laccase-based system, probably due to higher surface expression of the former enzyme. Expression parameters can be optimized in future, rendering laccase a better performing cathodic enzyme than BOD, due to the superior cathodic potential of the former. YSD of enzymes bearing high oxygen-reducing activity for biofuel cells production offers the advantages of both MFCs and enzyme-based fuel cells. A hybrid biofuel cell can achieve similar power outputs and OCVs as with a cell relying on free enzyme alone, yet with the added advantage of catalyst regeneration abilities. The addition of antimycin A to the cathode chamber in the hybrid is necessary since the intact yeast compete with the expressed enzymes for dissolved oxygen. Unlike other microorganisms, yeast cannot exploit their metabolism to improve cathode performance. While the addition of antimycin A has solved this problem, it could also be overcome by engineering a biofuel cell cathode designed to work in the face of a continuous flow of oxygen-saturated medium. We also observed that one of the most performance-limiting factors is fuel cell architecture. When this is improved, fuel cell performances improve by orders of magnitude. We believe that further technical improvement will further augment performance.
The helpful assistance of Ms Liron Amir is gratefully acknowledged. This research was supported by the Converging Technologies program of the Israel Science Foundation (grant no. 1693/07) and by the Edmond J. Safra Center for the Design and Engineering of Functional Biopolymers, as well as by the F.I.R.S.T program of the Israel Science Foundation (grant no. 763/07) (L. A.). L. A. is an Elaine S. and Alvin W. Wene Career Development Chair in Biotechnology Engineering.
Notes and references
- J. A. Cracknell, K. A. Vincent and F. A. Armstrong, Chem. Rev., 2008, 108, 2439–2461 CrossRef CAS.
- D. R. Lovley, Nat. Rev. Microbiol., 2006, 4, 497–508 Search PubMed.
- B. E. Logan, Microbial Fuel Cells, Wiley-Interscience, Hoboken, NJ, 2008 Search PubMed.
- Z. Du, H. Li and T. Gu, Biotechnol. Adv., 2007, 25, 464–482 CrossRef CAS.
- S. Fishilevich, L. Amir, Y. Fridman, A. Aharoni and L. Alfonta, J. Am. Chem. Soc., 2009, 131, 12052–12053 CrossRef CAS.
- P. Clauwaert, D. Van der Ha, N. Boon, K. Verbeken, M. Verhaege, K. Rabaey and W. Verstraete, Environ. Sci. Technol., 2007, 41, 7564–7569 CrossRef CAS.
- Z. He and L. T. Angenent, Electroanalysis, 2006, 18, 2009–2015 CrossRef CAS.
- E. Katz, I. Willner and A. B. Kotlyar, J. Electroanal. Chem., 1999, 479, 64–68 CrossRef CAS.
- S. Riva, Trends Biotechnol., 2006, 24, 219–226 CrossRef CAS.
- P. Giardina, V. Faraco, C. Pezzella, A. Piscitelli, S. Vanhulle and G. Sannia, Cell. Mol. Life Sci., 2010, 67, 369–385 CrossRef CAS.
- A. Bergel, D. Feron and A. Mollica, Electrochem. Commun., 2005, 7, 900–904 CrossRef CAS.
- P. Clauwaert, K. Rabaey, P. Aelterman, L. De Schamphelaire, T. H. Pham, P. Boeckx, N. Boon and W. Verstraete, Environ. Sci. Technol., 2007, 41, 3354–3360 CrossRef CAS.
- T. Boder and K. D. Wittrup, Nat. Biotechnol., 1997, 15, 553–557 CAS.
- L. Amir, T. K. Tam, M. Pita, M. M. Meijler, L. Alfonta and E. Katz, J. Am. Chem. Soc., 2009, 131, 826–832 CrossRef CAS.
- A. Christenson, S. Shleev, N. Mano, A. Heller and L. Gorton, Biochim. Biophys. Acta, 2006, 1757, 1634–1641 Search PubMed.
- R. W. Estabrook, J. Biol. Chem., 1957, 227, 1093–1108 Search PubMed.
- X. Li and Z. Rosenzweig, Anal. Chim. Acta, 1997, 353, 263–273 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental information, control experiments and long term performance characterization. See DOI: 10.1039/c1cc16207a |
| ‡ These authors contributed equally to the research. |
| This journal is © The Royal Society of Chemistry 2012 |
