Enhancement of static incubation time in microfluidic cell culture platforms exploiting extended air–liquid interface†
Nilanjana
Bose
a,
Tamal
Das
a,
Debapriya
Chakraborty
b,
Tapas K.
Maiti
a and
Suman
Chakraborty
*b
aDepartment of Biotechnology, Indian Institute of Technology, Kharagpur, India 721302
bDepartment of Mechanical Engineering, Indian Institute of Technology, Kharagpur, India. 721302. E-mail: suman@mech.iitkgp.ernet.in
First published on 10th November 2011
Abstract
Microfluidics based cell culture applications have facilitated the study of cellular dynamics at the single entity level. Yet, long term versions of such applications in a static framework suffer from the fast exhaustion of available oxygen, dissolved in the limited media volume available per cell, within the microconfined environment. In order to circumvent such drawbacks, we have improvised a microfluidic cell culture platform for prolonged sustenance of adherent mammalian cells by formation of an air–liquid interface through functionalizing inner surfaces of a polydimethylsiloxane (PDMS) based microdevice. We have demonstrated an augmented static incubation time for different cell lines using this approach.
Microfluidics has created advanced cell culture platforms with integrated features and ways to characterize important physical and biochemical aspects of the cellular micro-environment. It has also enabled precise cell manipulation, integration of numerous analytical elements and means to achieve high-throughput devices for several cell-based assays.1–5 Attributed to intrinsic high surface-area to volume ratios, microfluidic systems offer a number of advantages including low consumption of reagents, high sensitivity, high spatio-temporal resolution, and cost effectiveness.4,5 Moreover, due to the commensurate length-scales and confined chemical milieu similar to physiological tissue-matrices and capillary systems, they provide the most appropriate micro-environment mimicking the in vivo niche of a living cell.2,3,6–8 As it has been demonstrated recently, such confined environment can promote enrichment of secreted growth factors, due to restricted diffusion, and significantly influence the stress responsive characteristics of mammalian cells.8 Although these advancements delineate characteristic superiority of microfluidic devices over conventional flask-based cell culture systems, there remain some major limitations of microconfined platforms. Unlike macro-scale cell culture flasks, confined microchannels are devoid of any air–liquid interface and therefore, do not permit continuous exchanges of gaseous elements (O2, CO2etc.) between air and the cell culture medium. Consequently, reduction of the dissolved oxygen (O2) content in cell culture medium influences cell signaling, cell proliferation, growth, differentiation and death in the long run.9 In such conditions, permeation of gases through the solid channel walls remains the only feasible means of exchange, which nevertheless is controlled by the bulk and surface properties of the substrate material.
Most of the microfluidic devices are fabricated by soft lithography method using a polymer named polydimethylsiloxane (PDMS) as the substrate.10 As a material, PDMS has some inherent qualities like its elastic nature, optical transparency, biocompatibility and importantly, partial permeability to gases.11 These last two attributes facilitate its applications in cell-based studies. There are several works which have exploited different properties of PDMS. For example, the permeability of PDMS has been used to initiate multiple processes in droplets,12 influence transport characteristics of PDMS in the gas phase,13–15 and perform quantitative analysis on the oxygen mass transfer through PDMS.16 However, it is also known that diffusivity and oxygen permeability of PDMS vary with its surface conditions which, in turn, depend on targeted surface modification (e.g., plasma surface oxidization) or spontaneous protein adsorption (e.g.cell culture). In this respect, it has been found that coating of the PDMS with silane, use of excess of cross linking reagents as well as plasma treatments alter and reduce PDMS oxygen permeability by 40–80%.17–19 Additionally, the diffusion and rapid partitioning of uncured PDMS oligomers into the culture media,6,8 absorption of small hydrophobic molecules, like serum proteins (bovine serum albumin) or hormones, into the polymer bulk20 reduce the supply of gases through PDMS during long term cell culture to great extent. Although these effects are not manifested in a short-term culture period, they impose unique challenges for maintaining long-term cell culture conditions that need to be addressed. The most common solution to the aforementioned problem is to use perfusion21–24 based culture in the microchannel either incessantly or at pre-designated time intervals. Perfusion culture is useful in studying cell and tissue biology, and turns out to be more challenging in design and operation than static culture. However, in such situations, cells may get exposed to long lasting mechanical shear stress23,25,26 in order to augment the mass transport of oxygen and nutrients in microfluidic devices.
In the background of the aforementioned limitation of microfluidic systems, designed towards long term cell maintenance in a static framework, we report here the development of a PDMS based microfluidic device where a continuous air–liquid interface can form along the whole channel length. We have taken advantage of side-by-side wettability patterning, for the formation of the air–liquid interface known as a “virtual wall” (as there is no physical wall to confine the liquid) within the microfluidic channels.27,28 With this method, aqueous media and cells can be selectively restricted to the hydrophilic side of the channel while gaseous medium (connected to ambience through an inlet) expand over the hydrophobic regime conferring a repertoire of oxygen. It is important to note that the formation and sustenance of the virtual wall (interface) in the PDMS-based platform is potentially threatened (while it is kept for prolonged cell culture) because of the inherent nature of hydrophobic recovery of PDMS. This threat has been overcome by treatment of PDMS with serial solvent extraction.29 Subsequently, the inner surfaces of the microchannel were first patterned through two stream laminar flow to develop side-by-side hydrophobic and hydrophilic regions across the whole channel length.27,28 The medium containing mammalian cells was seeded from a hydrophilic inlet and the liquid remained confined to the hydrophilic side only, should the infusion flow rate be kept below a critical level. Thus, mammalian cells were confined and grown in a restricted media-filled hydrophilic domain. The liquid-devoid hydrophobic portion served as the source for the constant supply of oxygen by diffusion and therefore, has enabled the cellular oxygen uptake throughout the cell culture period to be maintained. Moreover, the proper exchange of O2–CO2 gases through the interface was able to sustain the cells proliferating for prolonged periods of time in a static condition.
The PDMS based microfluidic system has been fabricated as a Y-shaped microfluidic channel with dimensions of length (L) = 25 mm, width (W) = 2 mm and height (H) = 40 μm, using a standard soft lithographic technique,30 without performing the bonding step (for details see ESI†). Subsequently, before bonding the PDMS stamp containing the microchannels to the glass slide, the stamp was treated by a serial solvent extraction method. This step is critical to postpone the hydrophobic recovery of the PDMS from 30 min to 1 week by extracting the uncrosslinked PDMS oligomers from the bulk.29 The dissolution of PDMS oligomers in different solvents was performed according to the decreasing order swelling ratios.31 At first, molded PDMS stamps were immersed in 200 ml of triethylamine solution at 25 °C and were allowed to stir for 2 h. Next the stamps were removed from triethylamine solution and placed in 200 ml of ethyl acetate at 25 °C for 2 h with stirring followed by treating in 200 ml of acetone for 2 h. After the treatment, the stamps were dried completely at 70 °C for about 6 h. Holes were punched for inlet and outlet in the PDMS stamps and then the oligomer-free PDMS stamps were oxidized in oxygen plasma, generating a stable hydrophilic surface and were plasma-bonded to piranha-cleaned glass slides. The bonded microdevice was heated at 70 °C for 10–15 min after placing needles and reservoirs in the inlets and outlets respectively. The silicone tubings were connected to the open end of the needles, and the other ends were connected to a syringe pump (Harvard Apparatus PHD 2000 Programmable, USA). A pure stream of hexadecane and octadecyltrichlorosilane (OTS) solution (C% v/v in hexadecane, 0.5–20%) were passed through each inlets of Y-shaped microchannel at a flow rate (QH) of 50–1000 μl min−1) for a time (t) 0.5–5 min. The flow of the solvents were followed by sequential flushing with hexane and isopropanol and then dried with a flow of nitrogen gas. The parts of the top and bottom surfaces of the microchannel which came in contact with the OTS solution (Fig. 1), became hydrophobic through formation of self-assembled monolayers (SAM) of OTS, while the other region of the channel remained hydrophilic. Then, an aqueous solution (either of deionized water or phosphate buffered saline PBS) was infused at a constant flow rate (Q μl min−1) through the inlet at hydrophilic side. This fluid was sustained by the “virtual wall” (air–liquid interface) (Fig. 1) and remained confined within the hydrophilic zone, provided that the infusion rate was maintained below a threshold magnitude (Q < QC). The hydrophilicity of the treated PDMS surface was further sustained by keeping deionised water inside the hydrophilic pathway before use for cell culture. Importantly, the serial solvent extraction of PDMS helped in prolonged sustenance of the virtual wall, which then made the microfluidic system better suited for the long term cell culture under confined environment.
 | ||
| Fig. 1 An image of a patterned microchannel with an air–liquid interface. Two inlets (A and B) and the outlet reservoir (at the exit) are shown. The hydrophilic region contains an aqueous rhodamine solution for visualization and the hydrophobic region is white in color containing atmospheric air confined inside the microdevice. | ||
For culturing cells inside the patterned microchannel and in control microchannels, cells were seeded at a density of 105cells ml−1. For this study, three well-known representative mammalian cell lines, namely, NIH 3T3 (murine fibroblast, mentioned 3T3 henceforth), L929 (murine fibroblast) and MG63 (human osteosarcoma) were selected. Before cell seeding, however, the hydrophilic pathway of the patterned microdevice was treated with 10 μg ml−1 of fibronectin, an extracellular matrix protein for 2 h to promote cell-surface adhesion (for details see ESI†). During seeding, the flow rate was maintained (at <1 μl min−1 for 15 min) far below the critical value as higher flow rates might damage the interface and also might impart hydrodynamic stress large enough to rupture the cells.25,26,32 This flow rate for cell seeding gave repetitive good results. After seeding of the cells, both the microdevice and control sets (non-patterned microchannels) were incubated in a CO2 incubator (Heraeus, Germany) at 37 °C. Phase-contrast images were captured at regular time intervals to study the cell adhesion, cell proliferation and growth kinetics of different cell types. All the imaging studies (cell proliferation study, cell culture and cell viability) have been performed in a CO2 microscope stage incubator (Olympus, MIU-IBC-IF, Germany) with appropriate conditions (37 °C and 5% CO2) for cell cultures right on the microscope stage, thus allowing prolonged observations of cell events in a controlled environment. This eliminates the influences of the external or ambient atmosphere (non-physiological environmental conditions) on the performance of the cell culture in the microdevice as well as in control microchannels.
In order to optimize the stability of virtual wall in the parametric space of OTS concentration (C), input flow rates of OTS and hexadecane (QH) and the time of laminar flow (t), we have evaluated the critical flow rate of the liquid through the hydrophilic side (QC) after patterning, beyond which the fluid spills over the hydrophilic–hydrophobic interface and fills the whole channel width. It is pertinent to mention that the virtual wall is destroyed when the hydrostatic pressure (because of the axial infusion of aqueous solution to evaluate the strength) exceeds the Laplace pressure due to surface tension. For a shallow microchannel (w ≫ H), the liquid pressure may be estimated as 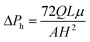 where Q is the flow rate of the fluid, A is the area of cross-section of the hydrophilic region, L is the axial length of the microchannel from the inlet and H is the height of the microchannel. The pressure responsible for stopping the liquid at the virtual wall is given by the Laplace pressure as
where Q is the flow rate of the fluid, A is the area of cross-section of the hydrophilic region, L is the axial length of the microchannel from the inlet and H is the height of the microchannel. The pressure responsible for stopping the liquid at the virtual wall is given by the Laplace pressure as 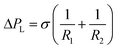 where σ is the surface tension co-efficient, R1 and R2 are the radii of the curvature in two perpendicular planes respectively. Hence, the ratio of these two pressures ΔPh and ΔPL are used to obtain a critical parameter –
where σ is the surface tension co-efficient, R1 and R2 are the radii of the curvature in two perpendicular planes respectively. Hence, the ratio of these two pressures ΔPh and ΔPL are used to obtain a critical parameter – 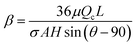 (for details see ESI†), where the flow rate Q is replaced by the critical flow rate Qc and θ is the contact angle at the interface. In order for the virtual wall to break, the value of the parameter should be of the order 1, below which the surface tension dominates and supports the virtual wall, while beyond which the hydrostatic pressure dominates resulting in damage of the interface. Hence, this parameter is an indicator of the strength of the virtual wall in dynamic conditions in contrast to the earlier studies where the stability is determined by a static pressure head.28 The non-dimensional form of the input flow rate for patterning (QH) of the hexadecane and OTS is expressed by Reynolds number
(for details see ESI†), where the flow rate Q is replaced by the critical flow rate Qc and θ is the contact angle at the interface. In order for the virtual wall to break, the value of the parameter should be of the order 1, below which the surface tension dominates and supports the virtual wall, while beyond which the hydrostatic pressure dominates resulting in damage of the interface. Hence, this parameter is an indicator of the strength of the virtual wall in dynamic conditions in contrast to the earlier studies where the stability is determined by a static pressure head.28 The non-dimensional form of the input flow rate for patterning (QH) of the hexadecane and OTS is expressed by Reynolds number 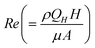 and the non-dimensional form of time of flow (t) is expressed by a reference diffusion time scale,
and the non-dimensional form of time of flow (t) is expressed by a reference diffusion time scale, 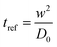 , where D0 is the diffusion co-efficient of OTS in hexadecane. The contact angle (both static and dynamic) over the substrate was measured using goniometer to characterize the patterned substrate (see ESI†).
, where D0 is the diffusion co-efficient of OTS in hexadecane. The contact angle (both static and dynamic) over the substrate was measured using goniometer to characterize the patterned substrate (see ESI†).
It is important to mention in this context that diffusion of OTS at low flow rates creates unwanted deposition of OTS on the two-phase patterned microdevice walls. This necessitates the use of high flow rates of the streams of hexadecane and OTS in hexadecane solution. Again, it has also been observed that PDMS swells31 in the presence of hexadecane. Further, with the flow rate increased to a very high value, there are chances of damaging the bonding between PDMS and glass substrate. Hence, it is important to find the appropriate Re so that a straight virtual wall is maintained along the microchannel. Concentration of OTS also plays a crucial role in determining the strength of the virtual wall. In order to characterize this strength, we have experimentally obtained the critical flow rate QC of aqueous solution (beyond which the liquid no longer remains confined to the hydrophilic region) and reported the mean critical flow rate with standard deviation (using error bars) using the non-dimensional parameter β in Fig. 2a, as a function of Re for different concentrations of OTS. It has been observed that β increases with an increase in both C and Re but remains nearly constant for Re > 16 for a given concentration. We have restricted Re to a value of 16.7, beyond which the interface was obtained straight but the improvement in β is not substantial (less than 5%). We have also varied the time of flow (t) of the hexadecane and OTS to observe its effects on the parameter β in Fig. 2b. For low time of flow the monolayers of OTS are not able to form on the inner surfaces of the microchannel. Interestingly, the critical flow rate of liquid (and hence β) first increases with an increase in laminar flow time, then decreases beyond t/tref ∼ 80. A longer time of flow of OTS solution results in a thicker film formation (greater than monolayer thickness) with a loss of hydrophobicity, although the exact reason behind this is unclear.33 This is consistent with our observation of drastic reduction in β with duration of flow t/tref > 80.
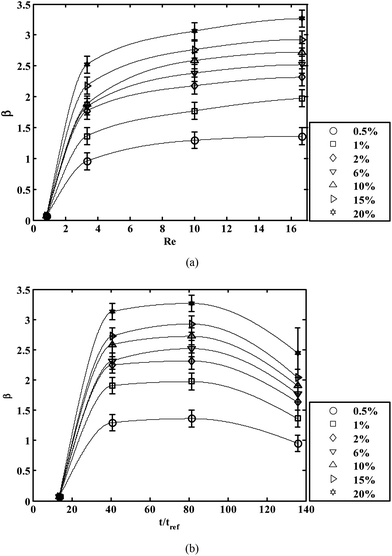 | ||
| Fig. 2 Characterizing the strength of the interface using the parameter β (a) Effect of varying Reynolds number (Re) of the flow of hexadecane and OTS in hexadecane with varying concentration (C%) of OTS (b) Effect of time of flow of these solutions for different concentrations (C%) of OTS. The time of flow is non-dimensionalised by tref. The solid lines are used to connect each data point to aid visualization. | ||
Although the strength of the virtual wall is increased by increasing concentrations of OTS, it does not necessarily imply that the prepared microdevice is suitable for cell culture. For prolonged static cell culture, higher concentrations of OTS over the substrate may result in toxic effects to the cell which may not be manifested during initial growth periods. In order to observe this effect, we have studied the proliferation of 3T3 fibroblast cells in the hydrophilic pathways of the microdevice with different concentrations of OTS in hexadecane (C%) for patterning and have compared with a control set of microchannel (see ESI†). As the cell population grows, the essential nutrients in the media content available per cell decreases which is a significant drawback in microfluidic static culture systems. This deficiency has been taken care of in the present setup by placing a reservoir at the outlet30,34,35 fluidic port (see Fig. 1) which contains 100 μl of media which diffuses into the setup as the time progresses, to replenish the consumed nutrients by the cells. Both the microdevice and control microchannels were supplemented with this outlet reservoir, however, the outlet reservoir was isolated from any kind of perfusion of the cell media to maintain a static culture. The cells were monitored at regular intervals and their growth kinetics was studied for about 96 h (or 4 days) in a static condition (without external media replenishment). Here, all cell proliferation data have been normalized with respect to the initial adhered cell number present after 12 h of seeding (N0), i.e., allowing sufficient time for cell adhesion yet not long enough for cells to undergo a cell division cycle. The comparative study of the maximum growth rate of cells in microchannels patterned with different OTS concentration has been shown in Fig. 3. It may be observed from the Figure that the cells in the microchannel, modified with 2% OTS concentration, have shown a statistically significant augmented growth in comparison to other patterned and control microchannels. Considering the rationale behind the proposition of patterned microchannel, this enhancement may be attributed to the depletion of essential gaseous nutrients (O2 supply) from the culture medium and also for accumulation of inhibitory gaseous metabolites (CO2) in control microchannel which is prevented in the patterned system by virtue of the air–liquid interface. For a lower concentration (<2%) of OTS patterned microdevice, the interface gets damaged (also evident from the β value in Fig. 2a) and eventually resembles the cell growth as that of the control microchannel, while higher concentrations (>2%) proved to be toxic for long-term culture36 (demonstrated in ESI†). In this relevance, the patterned microdevice with 2% OTS concentration provides a more homeostatic micro-environment than that of the conventional microfluidic channels.
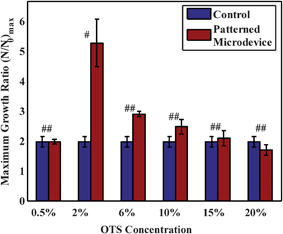 | ||
| Fig. 3 Maximum growth ratio (N/N0)max of the 3T3 fibroblast cells grown in the microdevice (patterned with different concentrations of OTS) and control (non-patterned microchannels) where N is the number of cells at different time intervals and N0 is the initial adhered cell number. # and ## represents significance with respect to control. # refers to p < 0.05 while ## refers to p > 0.05 with respect to control. Error bars represent standard deviation with number of experiments n = 5. | ||
Further, to test the cell-type variability of the device performance, cell culture and proliferation of three different representative cell lines namely 3T3, L929 and MG63 in the patterned microdevice have been monitored against proliferation of respective cell types in control microchannel (Fig. 4). Subsequently, though each of these cell lines has shown an augmented growth rate in the system with an air–liquid interface than in the control microchannel, 3T3 cells have shown the highest device selective growth among the three cell types (see Fig. 4).
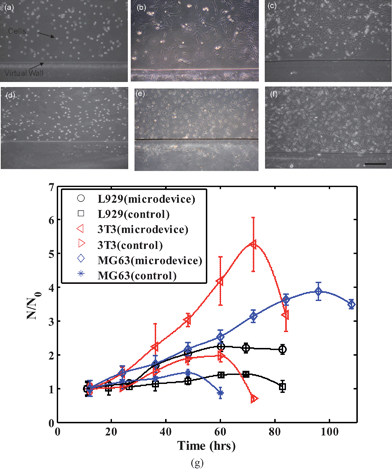 | ||
| Fig. 4 Representative images of culture of fibroblast cells - 3T3 (a–c) and L929 (d–f) in the microdevice with two-phase (air–liquid) interface at different time intervals – 0 h, 36 h, 72 h are shown (scale bar represents 200 μm). Optical micrograph of the cells after seeding inside the microdevice with a virtual wall (air–liquid interface) are shown in (a) and (d). (g) Different cell lines - 3T3, L929 and MG63 were cultured in the patterned microdevice and the ratio of the number of cells at different time intervals (N) to the initial adhered cell number (N0) is shown with respect to the control (non-patterned microchannel). The error bars show the standard deviation for each data set. | ||
To summarize, inspired from macroscale cell culture, we have translated the concept of an air–liquid interface for proper gaseous exchange to a microscale environment. This has been achieved by selectively patterning the surface hydrophobicity of the microchannel, which yielded in co-existing stripes of hydrophilic and hydrophobic domains along the channel length. While the hydrophilic zone is selectively filled with aqueous cell culture medium, it is prevented to spread over the hydrophobic regime by virtue of the interfacial tension. This mechanism, therefore, conferred a large air–liquid interfacial contact area which facilitates the exchange of gaseous medium between the cell culture confined within the microchannel and the ambience. Cell culture in the patterned microdevice neither subjects the cells to experience a large convective environment (like flask/Petri dish), nor washes away and dilutes the soluble factor signals from their targets through perfusion, which may retard cellular responses and finally inhibit cell growth. By integration of a large air–liquid interface into microchannels, we have demonstrated a hybrid system which could provide in situ culture of mammalian cells and could monitor the growth kinetics of different cells by controlling the cellular micro-environment with minimum perturbation in a static condition. In this context, there prevails a huge scope where the proposed microdevice model could enable cell culture for longer timescales and could be used in applications such as development of artificial tissue or organs (mimic of alveoli of the lungs) with micrometre control of the neighboring environment and effective mass transport and in long term cell based studies.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the Department of Biotechnology (DBT), Govt. of India (Grant no. BT/PR8130/GBD/27/8/2006, Dated 06/03/07), for executing this work.References
- I. Meyvantsson and D. J. Beebe, Annu. Rev. Anal. Chem., 2008, 1, 423–49 CrossRef CAS.
- E. M. Lucchetta, M. S. Munson and R. F. Ismagilov, Lab Chip, 2006, 6, 185–190 RSC.
- C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, Science, 1997, 276, 1425–1428 CrossRef CAS.
- G. M. Walker, H. C. Zeringue and D. J. Beebe, Lab Chip, 2004, 4, 91–97 RSC.
- S. K. W. Dertinger, D. T. Chiu, N. L. Jeon and G. M. Whitesides, Anal. Chem., 2001, 73, 1240–1246 CrossRef CAS.
- A. L. Paguirigan and D. J. Beebe, Integr. Biol., 2009, 1, 182–195 RSC.
- B. Zhang, M. C. Kim, T. Thorsen and Z. Wang, Biomed. Microdevices, 2009, 11, 1233–1237 CrossRef CAS.
- T. Das, T. K. Maiti and S. Chakraborty, Integr. Biol., 2011, 3, 684–695 RSC; H. M. Yu, C. M. Alexander and D. J. Beebe, Lab Chip, 2007, 7, 726–730 RSC.
- A. P. Vollmer, R. F. Probstein, R. Gilbert and T. Thorsen, Lab Chip, 2005, 5, 1059–1066 RSC.
- K. J. Regehr, M. Domenech, J. T. Koepsel, K. C. Carver, S. J. Ellison-Zelski, W. L. Murphy, L. A. Schuler, E. T. Alarid and D. J. Beebe, Lab Chip, 2009, 9, 2132–2139 RSC.
- S. K. Sia and G. M. Whitesides, Electrophoresis, 2003, 24, 3563–3576 CrossRef CAS.
- J. U. Shim, S. N. Patil, J. T. Hodgkinson, S. D. Bowden, D. R. Spring, M. Welch, W. T. S. Huck, F. Hollfelder and C. Abell, Lab Chip, 2011, 11, 1132–1137 RSC.
- E. Baltussen, F. David, P. Sandra, H. G. Janssen and C. Cramers, J. Microcolumn Sep., 1999, 11, 471–474 CrossRef CAS.
- T. Mohammadi, A. Aroujalian and A. Bakhshi, Chem. Eng. Sci., 2005, 60, 1875–1880 CrossRef CAS.
- E. Ostuni, C. S. Chen, D. E. Ingber and G. M. Whitesides, Langmuir, 2001, 17, 2828–2834 CrossRef CAS.
- G. Mehta, K. Mehta, D. Sud, J. W. Song, T. Bersano-Begey, N. Futai, Y. S. Heo, M. A. Mycek, J. J. Linderman and S. Takayama, Biomed. Microdevices, 2007, 9, 123–134 CrossRef CAS.
- K. S. Houston, D. H. Weinkauf and F. F. Stewart, J. Membr. Sci., 2002, 205, 103–112 CrossRef CAS.
- S. Jon, J. Seong, A. Khademhosseini, T. N. T. Tran, P. E. Laibinis and R. Langer, Langmuir, 2003, 19, 9989–9993 CrossRef CAS.
- W. J. Chang, D. Akin, M. Sedlak, M. R. Ladisch and R. Bashir, Biomed. Microdevices, 2003, 5, 281–290 CrossRef CAS.
- K. J. Regehr, M. Domenech, J. T. Koepsel, K. C. Carver, S. J. Ellison-Zelski, W. L. Murphy, L. A. Schuler, E. T. Alarid and D. J. Beebe, Lab Chip, 2009, 9, 2132–2139 RSC.
- Y. Wen, X. Zhang and S.-T. Yang, Biotechnol. Prog., 2010, 26, 1135–1144 CAS.
- Y.-C. Toh, C. Zhang, J. Zhang, Y. M. Khong, S. Chang, V. D. Samper, D. van Noort, D. W. Hutmacher and H. Yu, Lab Chip, 2007, 7, 302–309 RSC.
- L. Kim, Y. C. Toh, J. Voldman and H. Yu, Lab Chip, 2007, 7, 681–694 RSC.
- A. Tourovskaia, X. F. Masot and A. Folch, Lab Chip, 2005, 5, 14–19 RSC.
- Y. Chisti, Crit. Rev. Biotechnol., 2001, 21, 67–110 CrossRef CAS; H. Lu, L. Y. Koo, W. M. Wang, D. A. Lauffenburger, L. G. Griffith and K. F. Jensen, Anal. Chem., 2004, 76, 5257–5264 CrossRef.
- D. D. Carlo, L. Y. Wu and L. P. Lee, Lab Chip, 2006, 6, 1445–1449 RSC.
- B. Zhao, J. S. Moore and D. J. Beebe, Science, 2001, 291, 1023–1026 CrossRef CAS.
- B. Zhao, J. S. Moore and D. J. Beebe, Anal. Chem., 2002, 74, 4259–4268 CrossRef CAS.
- J. A. Vickers, M. M. Caulum and C. S. Henry, Anal. Chem., 2006, 78, 7446–7452 CrossRef CAS.
- T. Das, T. K. Maiti and S. Chakraborty, Lab Chip, 2008, 8, 1308–1318 RSC.
- J. N. Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75, 6544–6554 CrossRef CAS.
- J. C. Merchuk, Adv. Biochem. Eng./Biotechnol., 1991, 44, 66–95 Search PubMed.
- N. Tillman, A. Ulman, J. S. Schildkraut and T. L. Penner, J. Am. Chem. Soc., 1988, 110, 6136–6144 CrossRef CAS.
- M. Domenech, H. Yu, J. Warrick, N. M. Badders, I. Meyvantsson, C. M. Alexander and D. J. Beebe, Integr. Biol., 2009, 1, 267–274 RSC.
- I. Meyvantsson, J. W. Warrick, S. Hayes, A. Skoien and D. J. Beebe, Lab Chip, 2008, 8, 717–724 RSC.
- A. M. P. Dupraz, S. A. T. v.d. Meer, J. R. Dewijn and J. H. Goedemoed, J. Mater. Sci.: Mater. Med., 1996, 7, 731–738 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1lc20888h |
| This journal is © The Royal Society of Chemistry 2012 |
