Jet-cooled laser-induced fluorescence spectroscopy of methylcyclohexoxy radicals†
Jiali
Lin
,
Qijun
Wu
,
Gaiting
Liang
,
Lily
Zu
* and
Weihai
Fang
Department of Chemistry, Beijing Normal University, Beijing, 100875, P. R. China. E-mail: zull@bnu.edu.cn; Fax: +86-10-58802075
First published on 14th November 2011
Abstract
Cyclohexoxy and its methyl substituted variants play an important role in atmospheric chemistry. Direct spectral observation of these radicals can provide information about their structures and serve as convenient diagnostics for their monitoring. In this work, we report the first time observation of the LIF spectra of 2-, 3-, and 4-methylcyclohexoxy radicals in supersonic jet-cooled conditions. All spectra are consistent with a single conformer, and thermodynamic analysis of the conformers suggests that the conformer has the diequatorial (e, e) chair structure. Vibrational frequency analysis was performed using CASSCF/6-31+G(d) method on the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) state of radicals and the vibronic bands were assigned. The origin band of the methyl substituted cyclohexoxy shifted to red with the decrease of the separation distance between the methyl group and the oxygen atom on the ring. Different spectral character of 2-methylcyclohexoxy radical suggested that the neighboring methyl group affected the behavior of the radical on the
state of radicals and the vibronic bands were assigned. The origin band of the methyl substituted cyclohexoxy shifted to red with the decrease of the separation distance between the methyl group and the oxygen atom on the ring. Different spectral character of 2-methylcyclohexoxy radical suggested that the neighboring methyl group affected the behavior of the radical on the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) excited state.
excited state.
Introduction
Cyclic-alkoxy radicals, the oxidation intermediates of cycloaliphatic hydrocarbons which form a major fraction in modern fuels, play an important role in atmospheric chemistry.1–3 In cyclic-alkoxy radicals, the cyclohexoxy radical and its substituted variants are of special interest due to the particular stability of the six-membered ring.4,5 Several techniques including Fourier transform infrared spectroscopy,6 air-sampling atmospheric pressure ionization tandem mass spectrometry,2 and UV absorption spectroscopy1 were used to detect the precursors or products in the atmospheric reaction of cyclohexoxy for monitoring the changes in cyclohexoxy concentration indirectly.Direct spectral observation of cyclohexoxy and its substituted variants would provide information about their structures and serve as convenient diagnostics for their monitoring. Zhang et al. demonstrated these advantages by observing the laser-induced fluorescence (LIF) excitation spectrum of cyclohexoxy,7trans-4-methylcyclohexoxy, and d11-cyclohexoxy radicals8 in near ambient temperature conditions and used the LIF intensity as a probe in the kinetic study of the radicals' reaction with O2. However, little is known about the changes in spectroscopy and chemistry of the cyclohexoxy derivatives induced by the substitution on the six-membered ring. High resolution spectra under jet-cooled conditions hold the potential for a better understanding of these matters.9 The conformational and spectroscopic studies of substituted cyclohexoxy will help understand the dependence of the energetics and reactivity of cyclic-alkoxy species on the substitution location of the ring. It will also contribute to the fundamental chemical and structure investigation of ring compounds.
In this work, we obtained the LIF spectra of 2-, 3-, and 4-methylcyclohexoxy radicals for the first time in supersonic jet-cooled conditions. Vibrationally resolved spectroscopic bands were assigned in assistance with quantum chemical calculations using B3LYP/6-31+G(d) and CASSCF/6-31+G(d) methods. Different spectral characters of 2-, 3-, and 4-methylcyclohexoxy radicals were observed and the spectra were assigned to specific conformers. The effect of substitution of a methyl group on different locations of the cyclohexoxy ring to the spectral property of the radicals was discussed.
Experimental
The experimental setup was described previously in ref. 10. Briefly, it consists of a dye laser (Narrowscan, Radiant Dyes) pumped by the second harmonic (532 nm) of a Nd:YAG laser (Surelite III, Continuum). The laser output was frequency-doubled to generate the UV radiation required to probe the![[X with combining tilde]](https://www.rsc.org/images/entities/i_char_0058_0303.gif) −
− ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) transition of substituted cyclohexoxy radicals. Photolysis of the precursor molecules was achieved using the third harmonic (355 nm) of another Nd:YAG laser (Surelite II, Continuum), with power of typically 30 mJ per pulse. The methylcyclohexanyl nitrite precursors were synthesized by dropwise addition of sulfuric acid to a mixture of an appropriate methylcyclohexanol and sodium nitrite.11 Single isomer samples cis-3-methylcyclohexanol (95%), trans-4-methylcyclohexanol (98%), and cis-4-methylcyclohexanol (98%) were purchased from Alfa Aesar (UK). The cis-trans mixtures of 2-methylcyclohexanol (97%), 3-methylcyclohexanol (99%), and 4-methylcyclohexanol (99%) came from Alfa Aesar (UK) and Aladdin Company (China). A backing pressure of ∼0.1 atm argon passed over the nitrite sample and expanded into a vacuum chamber using a standard pulse valve (General Valve) with a 0.5 mm orifice. The photolysis beam was focused just above the throat of the nozzle, and the radicals produced were excited about 10 mm downstream by the counter-propagating probe beam. The total fluorescence was collected perpendicular with an f = 80 mm lens and imaged onto a photomultiplier tube (Hamamasu, CR110). The valve was operated in a continuous mode. The chamber vacuum was maintained using a molecular pump (600 L s−1) backed with a mechanical pump (8 L s−1). The initial vacuum was ∼3 × 10−3 Pa and increased to ∼7 Pa when injecting the sample.
transition of substituted cyclohexoxy radicals. Photolysis of the precursor molecules was achieved using the third harmonic (355 nm) of another Nd:YAG laser (Surelite II, Continuum), with power of typically 30 mJ per pulse. The methylcyclohexanyl nitrite precursors were synthesized by dropwise addition of sulfuric acid to a mixture of an appropriate methylcyclohexanol and sodium nitrite.11 Single isomer samples cis-3-methylcyclohexanol (95%), trans-4-methylcyclohexanol (98%), and cis-4-methylcyclohexanol (98%) were purchased from Alfa Aesar (UK). The cis-trans mixtures of 2-methylcyclohexanol (97%), 3-methylcyclohexanol (99%), and 4-methylcyclohexanol (99%) came from Alfa Aesar (UK) and Aladdin Company (China). A backing pressure of ∼0.1 atm argon passed over the nitrite sample and expanded into a vacuum chamber using a standard pulse valve (General Valve) with a 0.5 mm orifice. The photolysis beam was focused just above the throat of the nozzle, and the radicals produced were excited about 10 mm downstream by the counter-propagating probe beam. The total fluorescence was collected perpendicular with an f = 80 mm lens and imaged onto a photomultiplier tube (Hamamasu, CR110). The valve was operated in a continuous mode. The chamber vacuum was maintained using a molecular pump (600 L s−1) backed with a mechanical pump (8 L s−1). The initial vacuum was ∼3 × 10−3 Pa and increased to ∼7 Pa when injecting the sample.
Two dyes, Styryl8 and Pyridine2 (Exciton, USA), were used to provide the tunable excitation laser source for the methylcyclohexoxy LIF spectra. Lasers were operated at 10 Hz rate and sequentially controlled by a Digital Pulse Generator (DG535, SRS). The output of the photomultiplier was digitized on an oscilloscope (Tektronics, TBS3032B) and the gated signal was integrated by a LabView program. LIF spectra were obtained by recording the integrated fluorescence signal intensity while continuously scanning the excitation laser wavelength. While measuring the spectra, the dye laser wavelength was scanned at 0.01 nm per step and all the spectral intensities were corrected by subtracting the background obtained with the photolysis laser off.
Computational
The conformational analysis of cyclohexane and cyclohexoxy was well addressed in the literature.9,12,13 It is known that there are two types of conformer for cyclohexoxy, chair form and twisted-boat form. The twisted-boat conformers are ∼6 kcal mol−1 higher in energy than chair conformers.9 Hence, only chair conformers are likely present under our experimental conditions.7–10 Therefore, we will concentrate our studies on the chair conformers. For methylcyclohexoxy radicals, oxygen and methyl group can be treated as the two substitute groups of the cyclohexane chair. In this view, there are two isomers (cis and trans) in each methylcyclohexoxy radical. For every isomer, two different conformers can exist according to the axial or equatorial position that the oxygen and the methyl group take (Table 1). The geometry optimization of all chair conformers of methylcyclohexoxy radicals was performed using B3LYP/6-31+G(d) and CASSCF/6-31+G(d) for ground state and excited state, respectively. Vibrational frequency analysis was conducted for the ground and excited states to assist the assignment of experimental spectra. The adiabatic excitation energy between the![[X with combining tilde]](https://www.rsc.org/images/entities/i_char_0058_0303.gif) and
and ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) states was obtained by the CASSCF/6-31+G(d) method with a scaled factor of 0.89. All calculations were performed using Gaussian 03 program.14
states was obtained by the CASSCF/6-31+G(d) method with a scaled factor of 0.89. All calculations were performed using Gaussian 03 program.14
| Substitutes | Isomer | Chair conformera | E(rel)+ ΔZPE b/kcal mol−1 |
|---|---|---|---|
| a The notation of substitution position is in the order of (oxygen, methyl group). b The energies are relative energy to the (e, e) conformer of each methylcyclohexoxy radical. | |||
| 2-Methylcyclohexoxy | cis | (e, a) | 2.29 |
| (a, e) | 0.83 | ||
| trans | (e, e) | 0 | |
| (a, a) | 2.68 | ||
| 3-Methylcyclohexoxy | cis | (e, e) | 0 |
| (a, a) | 2.61 | ||
| trans | (e, a) | 2.44 | |
| (a, e) | 0.58 | ||
| 4-Methylcyclohexoxy | cis | (e, a) | 2.32 |
| (a, e) | 0.66 | ||
| trans | (e, e) | 0 | |
| (a, a) | 2.98 |
Results and discussion
The LIF spectra of 2-, 3-, and 4-methylcyclohexoxy taken in supersonic jet conditions are shown in Fig. 1. Like cyclohexoxy,9 the spectra of 3- and 4-methylcyclohexoxy have rich spectral content with great complexity. For 2-methylcyclohexoxy, the spectrum appears considerably simplified with no significant bands observed at ∼1000 cm−1 above the origin band although its origin band has comparable intensity as the origin bands of the other two methylcyclohexoxy radicals.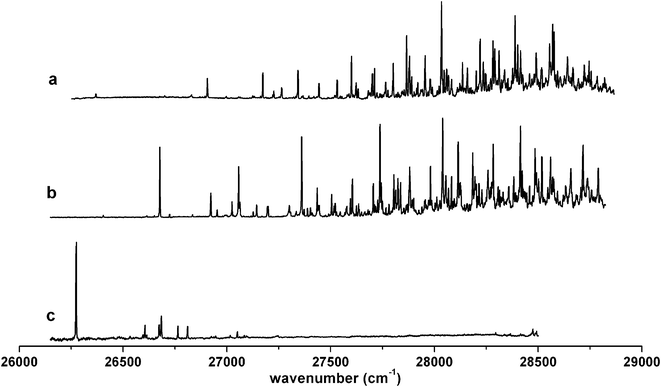 | ||
| Fig. 1 Laser-induced fluorescence spectra of methylcyclohexoxy radicals in supersonic jet conditions: (a) 4-methylcyclohexoxy, (b) 3-methylcyclohexoxy, (c) 2-methylcyclohexoxy. | ||
The jet-cooled LIF spectra of cis-4-methylcyclohexoxy and trans-4-methylcyclohexoxy were also obtained separately. The trans isomer yielded a spectrum (Fig. 2a) which basically resembled the spectrum of the cis-trans mixture (Fig. 1a), while some very weak signals were not accountable in the mixture spectrum. The LIF spectrum obtained from the cis-4-methylcyclohexoxy (Fig. 2b) proved that the weak features were from the cis isomer. Similar isomer behavior of 4-methylcyclohexoxy in the near-ambient-temperature cell was reported by Zhang, et al.8 However, our high resolution spectrum taken under low-temperature supersonic jet conditions revealed that hot bands and low resolution complicated the assignment of the LIF spectrum in near ambient temperature conditions. It is necessary to reassign the LIF spectrum with high resolution. Considering of the spectral significance, we will focus our analysis on the spectrum of trans-4-methylcyclohexoxy.
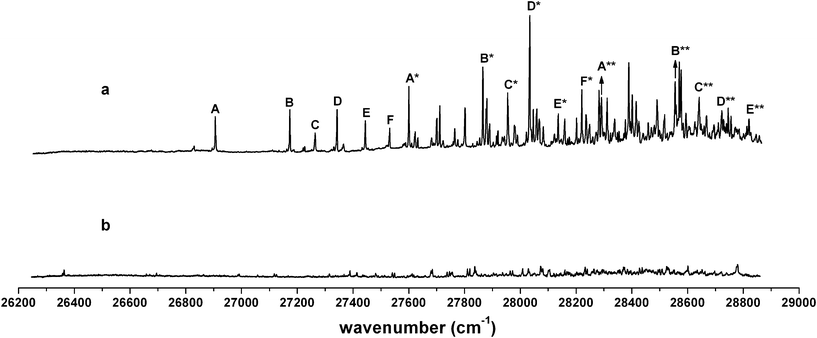 | ||
| Fig. 2 The LIF spectra of individual isomers of 4-methylcyclohexoxy: (a) trans-4-methylcyclohexoxy, (b) cis-4-methylcyclohexoxy. | ||
For the trans-4-methylcyclohexoxy isomer, there are two possible chair conformers, diequatorial (e, e) and diaxial (a, a). Theoretical calculation predicted that the (e, e) conformer was 2.98 kcal mol−1 lower in energy than the (a, a) conformer (Table 1). This is consistent with the stability studies of cyclohexane and its substitutes in the literature.12,13 On the other hand, analysis of the rotationally resolved LIF spectra of chain alkoxy radicals revealed that the conformer which was calculated to be lowest in energy had always been observed although multiple conformers could co-exist in supersonic jet non-equilibrium conditions.15–18 As for the cyclohexoxy radical, the lowest energy conformer (chair-equatorial) was identified as the spectral carrier in the jet-cooled LIF experiment.9 Hence, we will analyze the spectrum of the trans-4-methylcyclohexoxy radical using the calculated parameters of the diequatorial (e, e) conformer.
The lowest three electronic states ![[X with combining tilde]](https://www.rsc.org/images/entities/i_char_0058_0303.gif) , Ã , and
, Ã , and ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) of alkoxy radicals were well defined in the literature.19–22 The observed LIF spectra were the transition between the
of alkoxy radicals were well defined in the literature.19–22 The observed LIF spectra were the transition between the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) and
and ![[X with combining tilde]](https://www.rsc.org/images/entities/i_char_0058_0303.gif) states, which corresponds to the promotion of one electron from the σ orbital of the C–O bond (HOMO-2) to the half-filled non-bonding orbital (HOMO) that mainly localized on O atom. In the CASSCF/6-31+G(d) calculation, we chose an active space of 9 electrons distributed in 7 orbitals, involving the non-bonding orbitals of the O atom and the σ orbital of the C–O bond. The other active orbitals and electrons were chosen by the program automatically. The experimental band origin of trans-4-methylcyclohexoxy radical was at 26
states, which corresponds to the promotion of one electron from the σ orbital of the C–O bond (HOMO-2) to the half-filled non-bonding orbital (HOMO) that mainly localized on O atom. In the CASSCF/6-31+G(d) calculation, we chose an active space of 9 electrons distributed in 7 orbitals, involving the non-bonding orbitals of the O atom and the σ orbital of the C–O bond. The other active orbitals and electrons were chosen by the program automatically. The experimental band origin of trans-4-methylcyclohexoxy radical was at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906 cm−1. The calculated adiabatic excitation energy between the
906 cm−1. The calculated adiabatic excitation energy between the ![[X with combining tilde]](https://www.rsc.org/images/entities/i_char_0058_0303.gif) and
and ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) states of the chair (e, e) conformer was 26
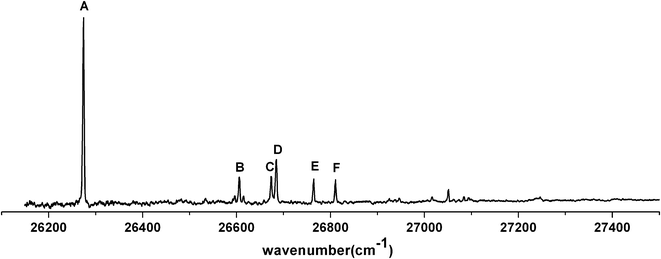
states of the chair (e, e) conformer was 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 cm−1. Six vibrational progressions were observed in the LIF spectrum of trans-4-methylcyclohexoxy (Fig. 2a). The intervals of the six progressions are all ∼690 cm−1. The origin bands of progressions B, C, D, E, F are shifted to 268 cm−1, 358 cm−1, 437 cm−1, 538 cm−1, 626 cm−1 higher frequency with respect to the origin in progression A. The chair (e, e) conformer of trans-4-methylcyclohexoxy has a Cs symmetry and the calculated lowest 20 harmonic vibrational frequencies (in Mulliken notation23) are given in Table 2. The same number (0.95) is used to scale the CASSCF(9,7)/6-31+G(d) harmonic frequencies in the
610 cm−1. Six vibrational progressions were observed in the LIF spectrum of trans-4-methylcyclohexoxy (Fig. 2a). The intervals of the six progressions are all ∼690 cm−1. The origin bands of progressions B, C, D, E, F are shifted to 268 cm−1, 358 cm−1, 437 cm−1, 538 cm−1, 626 cm−1 higher frequency with respect to the origin in progression A. The chair (e, e) conformer of trans-4-methylcyclohexoxy has a Cs symmetry and the calculated lowest 20 harmonic vibrational frequencies (in Mulliken notation23) are given in Table 2. The same number (0.95) is used to scale the CASSCF(9,7)/6-31+G(d) harmonic frequencies in the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) state and ground state, while the scale factor of 0.98 is used for B3LYP/6-31+G(d) calculated ground state frequencies. The assignments of the vibronic bands in the low frequency region of the experimental spectrum and bands in progressions are summarized in Table 3. Band A*, which is at 694 cm−1 above the origin band (band A), has typical C–O stretch motion in the calculation. Therefore, progression A (band A, A*, A**) can be assigned as C–O stretch progression. Band B, which is 268 cm−1 higher in frequency with respect to band A, is the swing motion (ν55) of the C–CH3 and C–O bonds. But progression B possesses a same interval like progression A, which is the frequency of C–O stretch. Hence, progression B is a combination progression of ν55+νCO. Band C, D, E are ring-stretch (ν30), ring-scissors (ν28), and ring-breathing (ν27) modes respectively. Band F is the combination band of ν55+ν30. Correspondingly, progression C, D, E, and F are the combination progressions of ν30+νCO, ν28+νCO, ν27+νCO, and ν55+ν30+νCO. The domination of the progressions in C–O stretch is not surprising as a large change in C–O bond length (
state and ground state, while the scale factor of 0.98 is used for B3LYP/6-31+G(d) calculated ground state frequencies. The assignments of the vibronic bands in the low frequency region of the experimental spectrum and bands in progressions are summarized in Table 3. Band A*, which is at 694 cm−1 above the origin band (band A), has typical C–O stretch motion in the calculation. Therefore, progression A (band A, A*, A**) can be assigned as C–O stretch progression. Band B, which is 268 cm−1 higher in frequency with respect to band A, is the swing motion (ν55) of the C–CH3 and C–O bonds. But progression B possesses a same interval like progression A, which is the frequency of C–O stretch. Hence, progression B is a combination progression of ν55+νCO. Band C, D, E are ring-stretch (ν30), ring-scissors (ν28), and ring-breathing (ν27) modes respectively. Band F is the combination band of ν55+ν30. Correspondingly, progression C, D, E, and F are the combination progressions of ν30+νCO, ν28+νCO, ν27+νCO, and ν55+ν30+νCO. The domination of the progressions in C–O stretch is not surprising as a large change in C–O bond length (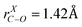 ,
, 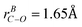 , CASSCF(9,7)/6-31+G(d)) was seen in the calculation of the
, CASSCF(9,7)/6-31+G(d)) was seen in the calculation of the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) −
− ![[X with combining tilde]](https://www.rsc.org/images/entities/i_char_0058_0303.gif) excitation of methylcyclohexoxy. The intensity distribution in the progressions was mainly determined by Franck–Condon principle. However, quantitative results cannot be drawn from the band intensities as the fluctuation of laser power at different laser frequencies was not normalized although efforts were taken to stabilize the laser energy at a certain value (typically 3 mJ).
excitation of methylcyclohexoxy. The intensity distribution in the progressions was mainly determined by Franck–Condon principle. However, quantitative results cannot be drawn from the band intensities as the fluctuation of laser power at different laser frequencies was not normalized although efforts were taken to stabilize the laser energy at a certain value (typically 3 mJ).
![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) excited statea
excited statea
| Assignment | Symmetry | CASSCF(9,7)b![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) |
CASSCF(9,7)b![[X with combining tilde]](https://www.rsc.org/images/entities/i_char_0058_0303.gif) |
B3LYPc![[X with combining tilde]](https://www.rsc.org/images/entities/i_char_0058_0303.gif) |
|---|---|---|---|---|
| a All calculations employed the 6-31+G(d) basis set. b A uniform scale factor of 0.95 was used. c The calculated B3LYP frequencies are scaled by 0.98. | ||||
| ν 32 | a′ | 108 | 109 | 106 |
| ν 57 | a′′ | 214 | 219 | 211 |
| ν 56 | a′′ | 240 | 241 | 228 |
| ν 31 | a′ | 245 | 250 | 244 |
| ν 55 | a′′ | 267 | 256 | 252 |
| ν 54 | a′′ | 331 | 336 | 328 |
| ν 30 | a′ | 355 | 377 | 364 |
| ν 29 | a′ | 426 | 426 | 411 |
| ν 28 | a′ | 456 | 455 | 431 |
| ν 53 | a′′ | 463 | 465 | 450 |
| ν 27 | a′ | 536 | 596 | 579 |
| ν 26 | a′ | 694 | 781 | 749 |
| ν 25 | a′ | 797 | 814 | 769 |
| ν 52 | a′′ | 817 | 851 | 795 |
| ν 24 | a′ | 884 | 914 | 808 |
| ν 51 | a′′ | 908 | 951 | 882 |
| ν 50 | a′′ | 975 | 960 | 933 |
| ν 23 | a′ | 989 | 991 | 948 |
| ν 22 | a′ | 999 | 1024 | 951 |
| ν 21 | a′ | 1009 | 1032 | 1001 |
| trans-4-Methylcyclohexoxy chair (e, e) conformer |
|
||
|---|---|---|---|
| Band | Experimentala | Predictedb | Assignment |
a Observed band maxima (cm−1) relative to 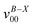 at 26 at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906.3 cm−1.
b Scaled CASSCF(9, 7)/6-31+G(d) frequencies (scale factor 0.95) for the fundamentals, and sum of the fundamental frequencies (cm−1) for combination and overtone bands. 906.3 cm−1.
b Scaled CASSCF(9, 7)/6-31+G(d) frequencies (scale factor 0.95) for the fundamentals, and sum of the fundamental frequencies (cm−1) for combination and overtone bands.
|
|||
| A | 0 |
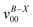
|
|
| B | 268 | 267 | ν 55 |
| C | 358 | 355 | ν 30 |
| D | 437 | 456 | ν 28 |
| E | 538 | 536 | ν 27 |
| F | 626 | 622 | ν 55+ν30 |
| A* | 694 | 694 | ν 26 (νCO) |
| 717 | 723 | ν 55+ν28 | |
| 795 | 797 | ν 25 | |
| 805 | 811 | ν 30+ν28 | |
| 860 | |||
| 895 | 884 | ν 24 | |
| B* | 960 | 961 | ν 55+νCO |
| 974 | 975 | ν 50 | |
| 984 | 999 | ν 22 | |
| C* | 1049 | 1049 | ν 30+νCO |
| D* | 1128 | 1150 | ν 28+νCO |
| E* | 1230 | 1230 | ν 27+νCO |
| F* | 1315 | 1316 | ν 55+ν30+νCO |
| A** | 1385 | 1388 | 2νCO |
| B** | 1649 | 1655 | ν 55+2νCO |
| C** | 1736 | 1743 | ν 30+2νCO |
| D** | 1816 | 1844 | ν 28+2νCO |
| E** | 1914 | 1924 | ν 27+2νCO |
The cis isomer of 3-methylcyclohexoxy radical yielded the same spectrum (Fig. 3) as that obtained from the cis-trans 3-methylcyclohexoxy mixture (Fig. 1b). Comparing the two chair conformers of cis-3-methylcyclohexoxy, the (e, e) conformer is 2.61 kcal mol−1 lower in energy than the (a, a) conformer. Therefore, the diequatorial conformer is assigned as the spectrum carrier. For cis-3-methylcyclohexoxy, three vibrational progressions stand out in the LIF spectrum. The intervals of the three progressions are all ∼680 cm−1. Progression A consists of four peaks (bands A, A*, A** and A***). The bands of other progressions are displaced to higher frequency with respect to each peak in progression A: ∼246 cm−1 higher for progression B, 379 cm−1 higher for progression C. The chair (e, e) conformer of cis-3-methylcyclohexoxy has a C1 symmetry and the assignments of the vibronic bands are listed in Table 4. Band A is at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 677 cm−1, which is consistent with the calculated adiabatic excitation energy (26
677 cm−1, which is consistent with the calculated adiabatic excitation energy (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 664 cm−1) of the chair (e, e) conformer of cis-3-methylcyclohexoxy. The band (band A*) at 683 cm−1 above the origin band has typical C–O stretch motion. The vibronic bands at 1363 cm−1 (band A**) and 2039 cm−1 (band A***) above are assigned as the first and second overtones of the C–O stretch mode. Therefore, progression A can be assigned as C–O stretch progression. Band B, which is 246 cm−1 higher in frequency with respect to band A, is the swing motion (ν54) of the C–O and C–CH3 bonds. Progression B possesses a same interval like progression A, which is the frequency of C–O stretch. Hence, progression B is a combination progression of ν54+νCO. Band C is the ring-stretch mode (ν51), indicating that progression C is a combination progression of ν51+νCO.
664 cm−1) of the chair (e, e) conformer of cis-3-methylcyclohexoxy. The band (band A*) at 683 cm−1 above the origin band has typical C–O stretch motion. The vibronic bands at 1363 cm−1 (band A**) and 2039 cm−1 (band A***) above are assigned as the first and second overtones of the C–O stretch mode. Therefore, progression A can be assigned as C–O stretch progression. Band B, which is 246 cm−1 higher in frequency with respect to band A, is the swing motion (ν54) of the C–O and C–CH3 bonds. Progression B possesses a same interval like progression A, which is the frequency of C–O stretch. Hence, progression B is a combination progression of ν54+νCO. Band C is the ring-stretch mode (ν51), indicating that progression C is a combination progression of ν51+νCO.
 | ||
| Fig. 3 Vibrational progressions in the LIF spectrum of cis-3-methylcyclohexoxy. | ||
| cis-3-Methylcyclohexoxy chair (e, e) conformer |
|
||
|---|---|---|---|
| Band | Experimentala | Predictedb | Assignment |
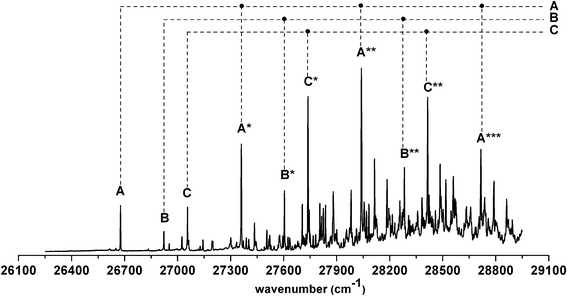
a Observed band maxima (cm−1) relative to 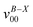 at 26 at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 677.4 cm−1.
b Scaled CASSCF(9, 7)/6-31+G(d) frequencies (scale factor 0.95) for the fundamentals, and sum of the fundamental frequencies (cm−1) for combination and overtone bands. 677.4 cm−1.
b Scaled CASSCF(9, 7)/6-31+G(d) frequencies (scale factor 0.95) for the fundamentals, and sum of the fundamental frequencies (cm−1) for combination and overtone bands.
|
|||
| A | 0 |
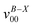
|
|
| B | 246 | 244 | ν 54 |
| 276 | 278 | ν 53 | |
| 348 | 349 | ν 52 | |
| C | 379 | 380 | ν 51 |
| 450 | 454 | ν 49 | |
| 466 | 463 | ν 48 | |
| 522 | 525 | ν 47 | |
| 624 | 624 | ν 54+ν51 | |
| A* | 683 | 680 | ν 46 (νCO) |
| B* | 928 | 924 | ν 54+νCO |
| C* | 1061 | 1060 | ν 51+νCO |
| A** | 1363 | 1360 | 2νCO |
| B** | 1606 | 1604 | ν 54+2νCO |
| C** | 1738 | 1740 | ν 51+2νCO |
| A*** | 2039 | 2040 | 3νCO |
As for 2-methylcyclohexoxy, no vibrational progression was observed. The assignment of 2-methylcyclohexoxy LIF spectrum was based on the calculation results of the chair (e, e) conformer of trans-2-methylcyclohexoxy radical (Table 5). The chair (e, e) conformer of the trans-2-methylcyclohexoxy also has a C1 symmetry. The origin band is at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 cm−1 compared to the calculated adiabatic excitation energy 26
275 cm−1 compared to the calculated adiabatic excitation energy 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198 cm−1. Band B, which is 332 cm−1 above the origin band has the ring-twist motion. The adjacent band C and band D, which are 400 cm−1 and 410 cm−1 higher in frequency with respect to band A, are assigned as the C–CH3 swing mode and ring-stretch mode, respectively. Band E and F are ring-breathing mode and ring-scissors mode. The calculated strongest vibration motion, C–O stretch (676 cm−1 in frequency) was not observed in the spectrum. The strong intensity of the origin band in the LIF spectrum of 2-methylcyclohexoxy indicates that the amount of the radicals generated in the jet was comparable with other substituted methylcyclohexoxy radicals. The fact that no C–O stretch vibrational band was observed from 2-methylcyclohexoxy radical, and the silence of its LIF spectrum 1000 cm−1 beyond the origin band, suggest that nonradiative relaxation channels might set in at low excitation energies above the vibrationless level of the
198 cm−1. Band B, which is 332 cm−1 above the origin band has the ring-twist motion. The adjacent band C and band D, which are 400 cm−1 and 410 cm−1 higher in frequency with respect to band A, are assigned as the C–CH3 swing mode and ring-stretch mode, respectively. Band E and F are ring-breathing mode and ring-scissors mode. The calculated strongest vibration motion, C–O stretch (676 cm−1 in frequency) was not observed in the spectrum. The strong intensity of the origin band in the LIF spectrum of 2-methylcyclohexoxy indicates that the amount of the radicals generated in the jet was comparable with other substituted methylcyclohexoxy radicals. The fact that no C–O stretch vibrational band was observed from 2-methylcyclohexoxy radical, and the silence of its LIF spectrum 1000 cm−1 beyond the origin band, suggest that nonradiative relaxation channels might set in at low excitation energies above the vibrationless level of the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) state.
state.
| trans-2-Methylcyclohexoxy chair (e, e) conformer |
|
||
|---|---|---|---|
| Band | Experimentala | Predictedb | Assignment |
a Observed band maxima (cm−1) relative to 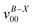 at 26 at 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274.6 cm−1.
b Scaled CASSCF(9, 7)/6-31+G(d) frequencies (scale factor 0.95) for the fundamentals, and sum of the fundamental frequencies (cm−1) for combination and overtone bands. 274.6 cm−1.
b Scaled CASSCF(9, 7)/6-31+G(d) frequencies (scale factor 0.95) for the fundamentals, and sum of the fundamental frequencies (cm−1) for combination and overtone bands.
|
|||
| A | 0 |
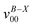
|
|
| B | 332 | 323 | ν 53 |
| C | 400 | 399 | ν 50 |
| D | 410 | 424 | ν 49 |
| E | 490 | 481 | ν 48 |
| F | 536 | 549 | ν 47 |
One hypothesis used in the assignment of 2-methylcyclohexoxy has to be noted here. Because the individual isomer of cis or trans2-methylcyclohexanol was not available, we presumably assigned the spectrum to the trans-2-methylcyclohexoxy while the spectrum was obtained with the cis-trans mixture precursor generated from 2-methylcyclohexanol. However, our calculation results (Table 1) show that the diequatorial chair conformer always has the lowest energy regardless of the different substitution location of the methyl group on the cyclohexoxy ring. In addition, the isomer with the chair (e, e) conformer, which is cis isomer for 3-methylcyclohexoxy and trans isomer for 4-methylcyclohexoxy, was proven to be the LIF spectrum carrier by the experiment. Therefore, we consider the spectrum assignment as the chair (e, e) conformer of trans-2-methylcyclohexoxy is reasonable. Further confirmation might be possible if pure trans-2-methylcyclohexanol could be obtained in the future.
In comparing of the LIF spectra of the three methylcyclohexoxy radicals in the supersonic jet (Fig. 1), the origin band is red shifted from 4-methylcyclohexoxy to 3-methylcyclohexoxy, and red shifted further in 2-methylcyclohexoxy. The red shift of the origin band might be explained by the inductive effect of the methyl group as the interaction between the methyl group and the oxygen atom increased when the two groups became closer on the ring. The spectra of 3- and 4-methylcyclohexoxy have same spectral pattern, i.e. the spectra extend over 2000 cm−1 above the origin and have rich content and C–O stretch dominated vibrational progressions. This rich and long expansion spectral pattern of 3- and 4-methylcyclohexoxy radicals is consistent with the LIF spectrum of cyclohexoxy itself,9 implying that the effect of methyl substitution on the meta- and para-position of the ring did not change the relaxation mechanism of the radical. In contrast, the excitation LIF spectrum of 2-methylcyclohexoxy is much quieter with no significant bands observed beyond 1000 cm−1 of the origin band. This suggests that other relaxation paths4,22,24 in addition to the fluorescence route might exist on the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) excited state of 2-methylcyclohexoxy. A strong fluorescent band with short lifetime (∼0.18 μs compared with 1.0–1.2 μs of methylcyclohexoxy bands) was observed 2510 cm−1 above its origin band. It appeared after a long silence of the 2-methylcyclohexoxy LIF spectrum and no corresponding vibration motion was found in the calculation. No such bands were observed in the same region of 3- and 4-methylcyclohexoxy spectra. We suspect that it might come from a dissociation product of 2-methylcyclohexoxy induced by the increased interaction between the ortho-methyl group and the oxygen atom on the cyclohexane ring. But no unambiguous explanation can be made at this stage as we have not yet identified the carrier of this extra band. Experimental and theoretical studies are being processed to investigate the isomerization and dissociation of the 2-methylcyclohexoxy.
excited state of 2-methylcyclohexoxy. A strong fluorescent band with short lifetime (∼0.18 μs compared with 1.0–1.2 μs of methylcyclohexoxy bands) was observed 2510 cm−1 above its origin band. It appeared after a long silence of the 2-methylcyclohexoxy LIF spectrum and no corresponding vibration motion was found in the calculation. No such bands were observed in the same region of 3- and 4-methylcyclohexoxy spectra. We suspect that it might come from a dissociation product of 2-methylcyclohexoxy induced by the increased interaction between the ortho-methyl group and the oxygen atom on the cyclohexane ring. But no unambiguous explanation can be made at this stage as we have not yet identified the carrier of this extra band. Experimental and theoretical studies are being processed to investigate the isomerization and dissociation of the 2-methylcyclohexoxy.
Conclusion
We have reported the first observation of the jet-cooled LIF excitation spectra of 2-, 3-, and 4-methylcyclohexoxy radicals. All spectra are consistent with a single conformer, and thermodynamic analysis of the conformers suggests that the conformer has the diequatorial chair structure. Vibrational frequency analysis was performed using the CASSCF/6-31+G(d) method on the![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) state of radicals and the vibronic bands were assigned. Three vibrational progressions and six vibrational progressions were identified and assigned for 3-methylcyclohexoxy and 4-methylcyclohexoxy, respectively. The origin band of the methyl substituted cyclohexoxy shifted to red with the decrease of the separation between the methyl group and the oxygen atom on the ring. When the methyl group and the CO group are in the neighboring position, a dissociation path might coexist with the fluorescence route for the
state of radicals and the vibronic bands were assigned. Three vibrational progressions and six vibrational progressions were identified and assigned for 3-methylcyclohexoxy and 4-methylcyclohexoxy, respectively. The origin band of the methyl substituted cyclohexoxy shifted to red with the decrease of the separation between the methyl group and the oxygen atom on the ring. When the methyl group and the CO group are in the neighboring position, a dissociation path might coexist with the fluorescence route for the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) state of 2-methylcyclohexoxy. However, because of the complexity of the spectra and conformations, further experimental and theoretical studies are required to understand the relaxation mechanism on the
state of 2-methylcyclohexoxy. However, because of the complexity of the spectra and conformations, further experimental and theoretical studies are required to understand the relaxation mechanism on the ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) state of methylcyclohexoxy radicals, and rotationally resolved spectra are desired to confirm the conformer identification.
state of methylcyclohexoxy radicals, and rotationally resolved spectra are desired to confirm the conformer identification.
Acknowledgements
The authors are pleased to acknowledge the financial support of this research by National Natural Science Foundation of China (Grant No. 20673013) and support from Beijing Municipal Commission of Education. We also acknowledge the grant from the Major State Basic Research Development Program (Grant No. 2007CB815206).References
- J. Platz, J. Sehested and O. J. Nielsen, J. Phys. Chem. A, 1999, 103, 2688–2695 CrossRef CAS.
- S. M. Aschmann, A. A. Chew, J. Arey and R. Atkinson, J. Phys. Chem. A, 1997, 101, 8042–8048 CrossRef CAS.
- O. Welz, F. Striebel and M. Olzmann, Phys. Chem. Chem. Phys., 2008, 10, 320–329 RSC.
- L. S. Alconcel and R. E. Continetti, Chem. Phys. Lett., 2002, 366, 642–649 CrossRef CAS.
- S. Wilsey, P. Dowd and K. N. Houk, J. Org. Chem., 1999, 64, 8801–8811 CrossRef CAS.
- J. J. Orlando, L. T. Iraci and G. S. Tyndall, J. Phys. Chem. A, 2000, 104, 5072–5079 CrossRef CAS.
- L. Zhang, K. A. Kitney, M. A. Ferenac, W. Deng and T. S. Dibble, J. Phys. Chem. A, 2004, 108, 447–454 CrossRef CAS.
- L. Zhang, K. M. Callahan, D. Derbyshire and T. S. Dibble, J. Phys. Chem. A, 2005, 109, 9232–9240 CrossRef CAS.
- L. Zu, J. Liu, G. Tarczay, P. Dupré and T. A. Miller, J. Chem. Phys., 2004, 120, 10579–10593 CrossRef CAS.
- J. Lin, L. Zu and W. Fang, J. Phys. Chem. A, 2011, 115, 274–279 CrossRef CAS.
- A. H. Blatt, Organic Syntheses, Wiley Press, New York, 1966, pp. 108–109 Search PubMed.
- E. L. Eliel, S. H. Wilen and L. N. Mander, Stereochemistry of Organic Compounds, Wiley Press, New York, 1997 Search PubMed.
- D. Nasipuri, Stereochemistry in Organic Compounds, Principles and Applications, New Age International (P) Ltd. Press, New Delhi, 1994 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D.Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision D.02, Gaussian, Inc., Wallingford, CT, 2004 Search PubMed.
- S. Gopalakrishnan, C. C. Carter, L. Zu, V. Stakhursky, G. Tarczay and T. A. Miller, J. Chem. Phys., 2003, 118, 4954–4969 CrossRef CAS.
- S. Gopalakrishnan, L. Zu and T. A. Miller, J. Phys. Chem. A, 2003, 107, 5189–5201 CrossRef CAS.
- G. Tarczay, S. Gopalakrishnan and T. A. Miller, J. Mol. Spectrosc., 2003, 220, 276–290 CrossRef CAS.
- T. A. Miller, Mol. Phys., 2006, 104, 2581–2593 CrossRef CAS.
- D. L. Osborn, D. J. Leahy and D. M. Neumark, J. Phys. Chem. A, 1997, 101, 6583–6592 CrossRef CAS.
- S. C. Foster, P. Misra, T. Y. D. Lin, C. P. Damo, C. C. Carter and T. A. Miller, J. Phys. Chem., 1988, 92, 5914–5921 CrossRef CAS.
- C. C. Carter, S. Gopalakrishnan, J. R. Atwell and T. A. Miller, J. Phys. Chem. A, 2001, 105, 2925–2928 CrossRef CAS.
- L. Lin, L. Zu, W. Fang, J. Yu and R. Liu, Chin. J. Chem., 2007, 25, 1467–1473 CrossRef CAS.
- R. S. Mulliken, J. Chem. Phys., 1955, 23, 1997–2011 CrossRef.
- S. Gopalakrishnan, L. Zu and T. A. Miller, Chem. Phys. Lett., 2003, 380, 749–757 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: Calculated vibrational frequencies of the diequatorial chair conformers of 2-, 3-, and 4-methylcyclohexoxy radicals in its ground state and ![[B with combining tilde]](https://www.rsc.org/images/entities/i_char_0042_0303.gif) excited state are provided. See DOI: 10.1039/c1ra00216c excited state are provided. See DOI: 10.1039/c1ra00216c |
| This journal is © The Royal Society of Chemistry 2012 |