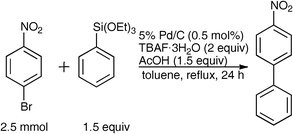
Ligand-free Hiyama cross-coupling reaction catalyzed by palladium on carbon†
Takayoshi
Yanase
,
Yasunari
Monguchi
and
Hironao
Sajiki
*
Laboratory of Organic Chemistry, Gifu Pharmaceutical University, 1-25-4 Daigaku-nishi, Gifu, 501-1196, Japan. E-mail: sajiki@gifu-pu.ac.jp; Fax: (+) 81-58-230-8105
First published on 14th November 2011
Abstract
A ligand-free Pd/C-catalyzed Hiyama cross-coupling reaction has been developed. A variety of aryl bromides were efficiently cross-coupled with aryltriethoxysilanes with only 0.5 mol% of 5% Pd/C. The protocol would be practical for use as an economical synthetic method for the construction of biphenyl derivatives.
Introduction
The Hiyama cross-coupling reaction,1 a palladium-catalyzed carbon–carbon bond formation between organosilanes and organohalides or their equivalents, is drawing increasing attention as one of the useful synthetic methods for the construction of asymmetrical biphenyls or multi-substituted alkenes that are structural components of various functional materials, such as pharmaceuticals, agrochemicals, and natural products. The stability and low toxicity of organosilanes2–4 and the facile conversion of silicon waste generated with reaction which progress to harmless SiO2 by incineration5 have made the Hiyama coupling attractive from environmental and user-friendly points of view.Hiyama coupling has generally been achieved by the combined use of a homogeneous palladium catalyst and a phosphine ligand.6,7 Recently, the phosphine ligand-free cross-coupling reactions have enthusiastically been investigated because of their toxicity and cost burden.8 Only a few ligand-free Hiyama coupling reactions using a homogeneous palladium catalyst, such as palladium(II) chloride (PdCl2),9apalladium(II) acetate [Pd(OAc)2],9b and palladacycles,10 or heterogeneous palladium nanoparticles which are not commercially available, have been reported in the literature.11 Heterogeneous catalysts have recently been recognized as convenient alternatives in organic synthesis due to their stability and ease of handling.12Palladium on carbon (Pd/C), a widely used heterogeneous catalyst for hydrogenation,13 has, in particular, been employed for various kinds of reactions including carbon–carbon, carbon–nitrogen, and carbon–oxygen bond formations14 due to its easy access and low cost. Recently, Novák and co-workers15 and our group16 independently reported the effective use of Pd/C and phosphine ligands for the Hiyama coupling. This paper describes the first ligand-freePd/C-catalyzed Hiyama cross-coupling reaction between a variety of aryl halides and aryltriethoxysilanes.
Results and discussion
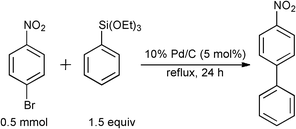
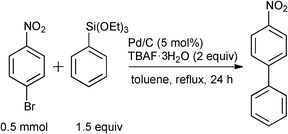
During the course of our previous study on the Pd/C-catalyzed Hiyama cross-coupling using a phosphine ligand [(4-FC6H4)3P],16 it was surprisingly found that the 10% Pd/C-catalyzed17,18 coupling reaction between 4-bromonitrobenzene and phenyltriethoxysilane in toluene proceeded without ligands to give the corresponding 4-nitrobiphenyl in 65% yield (Table 1, Entry 1). The reaction progress under ligand-free conditions was greatly affected by the solvent. The reaction hardly took place in DMF or MeCN (Entries 2 and 3), and the desired 4-nitrobiphenyl was obtained in moderate to good yields in EtOH, THF, 2-butanone, o-xylene, and toluene (Entries 1 and 4–7). TBAF·3H2O was found to be specifically effective as an activator of the organosilane probably due to the high solubility in toluene (Entry 1), while the reaction never proceeded without a fluoride source or with the use of metal fluorides, such as LiF, KF, and CsF (Entries 8–11).|
|
|||
|---|---|---|---|
| Entry | Solvent | F source | Yield (%)b |
| a Unless otherwise noted, reactions were carried out using 10% Pd/C (5.0 mol%, 25 μmol), 4-nitrobromobenzene (0.50 mmol), phenyltriethoxysilane (0.75 mmol), the fluoride source (1.0 mmol), and solvent (1.0 mL) at reflux for 24 h. b Determined by 1H NMR spectroscopy using 1,4-dioxane as an internal standard. c The reaction was carried out at 120 °C. | |||
| 1 | toluene | TBAF·3H2O | 65 |
| 2c | DMF | TBAF·3H2O | 5 |
| 3 | MeCN | TBAF·3H2O | 14 |
| 4 | EtOH | TBAF·3H2O | 44 |
| 5 | THF | TBAF·3H2O | 52 |
| 6 | 2-butanone | TBAF·3H2O | 55 |
| 7c | o-xylene | TBAF·3H2O | 58 |
| 8 | toluene | none | 0 |
| 9 | toluene | LiF | 0 |
| 10 | toluene | KF | 0 |
| 11 | toluene | CsF | 0 |
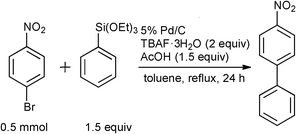
During the reaction indicated in Table 1, Entry 1, the generation of a trace amount of 4-ethoxynitrobenzene by the cross-coupling of the ethoxide anion generated from phenyltriethoxysilane with 4-bromonitrobenzene19,20 was confirmed by 1H NMR spectroscopy and GC-MS, although such an undesirable reaction was effectively suppressed by the addition of acetic acid (1.5 equiv) as a proton source, and the desired biphenyl was obtained in 77% yield (Table 2, Entry 2). On the other hand, the addition of H2O (Entry 3) or benzoic acid (Entry 4) was not effective for the reaction progress. Furthermore, the use of 5% Pd/C together with 1.5 equiv of acetic acid instead of 10% Pd/C significantly enhanced the reaction, and the desired 4-nitrobiphenyl was obtained in 81% yield (Entry 6), but the reaction efficiency was reduced by the decrease (Entry 7) or increase (Entry 8) in the amount of acetic acid.
|
|
||||
|---|---|---|---|---|
| Entry | Pd/Ca | additive | additive quantity | Yield (%)b |
| a 5.0 mol% of Pd/C was used. b Determined by 1H NMR spectroscopy using 1,4-dioxane as an internal standard. | ||||
| 1 | 10% | — | — | 65 |
| 2 | 10% | acetic acid | 1.5 equiv | 77 |
| 3 | 10% | H2O | 5.6 equiv (50 μL) | 60 |
| 4 | 10% | benzoic acid | 1.5 equiv | 62 |
| 5 | 5% | — | — | 68 |
| 6 | 5% | acetic acid | 1.5 equiv | 81 |
| 7 | 5% | acetic acid | 1.0 equiv | 75 |
| 8 | 5% | acetic acid | 2.0 equiv | 70 |
5% Pd/C was indispensable for the reaction progress (Table 3, Entry 1). The usage of 5% Pd/C could markedly be reduced from 5 mol% to only 0.5 mol% with a slight increase in the yield of the desired cross-coupling product presumably due to the suppression of the homo-coupling of the aryl halide (Entries 2–5). Further reduction to 0.1 mol% led to a slight decrease in the reaction efficiency (Entry 6). When the reaction temperature was lowered to 80 °C from reflux conditions (120 °C), the cross-coupling reaction was significantly suppressed and 40% of the starting 4-bromonitrobenzene was recovered (Entry 7).
|
|
||
|---|---|---|
| Entry | 5% Pd/C (mol%) | Yield (%)a |
| a Determined by 1H NMR spectroscopy using 1,4-dioxane as an internal standard. b The reaction was carried out at 80 °C. Unreacted 4-bromonitrobenzene was recovered in 40% yield. | ||
| 1 | 0 | 0 |
| 2 | 5 | 81 |
| 3 | 2 | 80 |
| 4 | 1 | 88 |
| 5 | 0.5 | 88 |
| 6 | 0.1 | 82 |
| 7b | 0.5 | 58 |
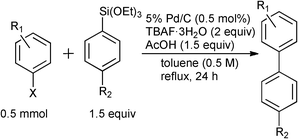
The cross-coupling reaction between a wide range of aryl halide and aryltrialkoxysilane derivatives was next investigated under the optimized reaction conditions (Table 3, Entry 5). 4-Bromoacetophenone was effectively cross-coupled with phenyltrimethoxysilane (Table 4, Entry 1) and phenyl, p-tolyl-, p-chloro, and p-methoxyphenyltriethoxysilanes to give the corresponding biphenyl derivatives in good to excellent yields (Entries 2–5). Aryl bromides possessing either an electron-withdrawing (nitro, acetyl, cyano, formyl,21carboxylic acid, fluoro group) or an electron-donating functionality (methyl or methoxy group), were found to be good substrates for the cross-coupling (Entries 6–14) with rare examples such as 2-bromoanisole (Entry 15). On the other hand, aryl chlorides were poorly reacting substrates under the present reaction conditions (Entries 16 and 17).
|
|
||||
|---|---|---|---|---|
| Entry | X | R1 | R2 | Yield (%)a |
| a Isolated yield. b Phenyltrimethoxysilane was employed. c 1 M solution of 3-bromoanisole in toluene was used. d 48 h. | ||||
| 1 | Br | 4–Ac | Hb | 87 |
| 2 | Br | 4–Ac | H | 81 |
| 3 | Br | 4–Ac | Cl | 85 |
| 4 | Br | 4–Ac | Me | 84 |
| 5 | Br | 4–Ac | OMe | 90 |
| 6 | Br | 4–NO2 | H | 85 |
| 7 | Br | 4–CN | H | 65 |
| 8 | Br | 4–CHO | H | 80 |
| 9 | Br | 2–CHO | H | 80 |
| 10 | Br | 4–COOH | H | 54 |
| 11 | Br | 4–F | H | 77 |
| 12 | Br | 4–Me | H | 65 |
| 13c | Br | 3–OMe | H | 66 |
| 14d | Br | 4–OMe | H | 77 |
| 15 | Br | 2–OMe | H | 12 |
| 16 | Cl | 4–NO2 | H | 7 |
| 17 | Cl | 3–OMe | H | 0 |
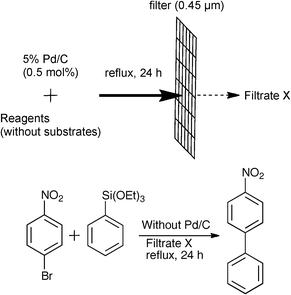
The reuse test of 5% Pd/C using 4-bromonitrobenzene and phenyltriethoxysilane as substrates was examined. 5% Pd/C could be reused until the second run without significant loss of catalyst activity, while the reaction efficiency was drastically reduced in the third run (Table 5).22 The results suggested that the palladium species could be leaching out from the carbon support. The palladium leaching in the reaction media was then measured by an inductively coupled plasma-atomic emission spectrometric analysis (iCAP 6500 Duo, Thermo Scientific). While a very low level of Pd-leaching (0.97% of total Pd amount of the 5% Pd/C) was detected in the filtered reaction media of the 5% Pd/C (5 mol%)-catalyzed cross-coupling reaction between 4-bromonitrobenzene and phenyltriethoxysilane without AcOH (Table 2, Entry 5),23 60 ppm of Pd-leaching was observed in the presence of AcOH (Table 3, Entry 5), suggesting that Pd-leaching was facilitated by AcOH.24 To confirm the catalyst activity of the leached palladium species, we prepared two types [(1) and (2)] of filtrates without substrates (4-bromonitrobenzene and phenyltriethoxysilane).25 (1) 5% Pd/C (0.5 mol%) was stirred with AcOH (1.5 equiv) in refluxing toluene under argon for 24 h and the mixture was filtered with a 0.45 μm membrane filter after cooling to afford Filtrate 1. (2) Filtrate 2 was prepared by the pretreatment of 5% Pd/C with AcOH and TBAF·3H2O (2.0 equiv) in refluxing toluene in a manner similar to Filtrate 1. Although a mixture of 4-bromonitrobenzene and phenyltriethoxysilane in the Filtrates 1 and 2 was heated at reflux for 24 h in the absence of 5% Pd/C, no reaction was confirmed by 1H NMR spectroscopic analysis (Table 6, Entries 1–3).
|
|
||
|---|---|---|
| Entry | Run | Yield (%)a |
| a Determined by 1H NMR spectroscopy using 1,4-dioxane as an internal standard. | ||
| 1 | 1 | 80 |
| 2 | 2 | 88 |
| 3 | 3 | 25 |
|
|
|||
|---|---|---|---|
| Entry | Filtrate X | Reagents | Yield (%)a |
| a Determined by 1H NMR spectroscopy. b 2 equiv of TBAF·3H2O were further added to the cross-coupling reaction mixture in Filtrate 2. | |||
| 1 | Filtrate 1 | AcOH (1.5 equiv) toluene (1.0 mL) | 0 |
| 2 | Filtrate 2 | AcOH (1.5 equiv) TBAF·3H2O (2.0 equiv) toluene (1.0 mL) | 0 |
| 3b | Filtrate 2 | — | 0 |
Next, we confirmed the catalyst activity of the filtrate with substrates. Since Djakovitch et al. have recently reported a so-called hot-filtration method to confirm the catalyst activity of the leached palladium species,26 the catalyst activities of two types of filtrates prepared by the hot-filtration and filtration after cooling were compared. The 5% Pd/C-catalyzed cross-coupling reaction between 4-bromonitrobenzene and phenyltriethoxysilane in the presence of TBAF·3H2O (2 equiv) and AcOH (1.5 equiv) was carried out in boiling toluene. After 30 min, the mixture was filtered without cooling with a glass fiber filter (<1.0 μm). The filtrate containing 14% of the desired cross-coupling product (4-nitrobiphenyl) was further heated at reflux for an additional 5.5 h to increase the formation of 4-nitrobiphenyl to 48% yield (Fig. 1, ■, Hot-filtration), although the reaction efficiency was slightly decreased in comparison to the reaction without filtration (♦, Standard run). Even though the use of the filtrate prepared by the filtration after cooling to rt, the reaction efficiency achieved a level nearly equal (52% yield) to the result of the hot-filtration by refluxing for an additional 5.5 h (▲, Filtration after cooling). These results suggest that (1) the reduced catalyst activity of the reuse test (Table 5) should be attributed to the palladium leaching into the reaction media; (2) coexistence of the substrates, 4-bromonitrobenzene and phenyltriethoxysilane, and acetic acid would enhance the leaching of the palladium species from 5% Pd/C; (3) leached Pd possesses catalyst activity toward the Hiyama cross-coupling reaction; (4) fresh 5% Pd/C should assume a key role as a palladium donor in the present ligand-free Hiyama cross-coupling reaction.
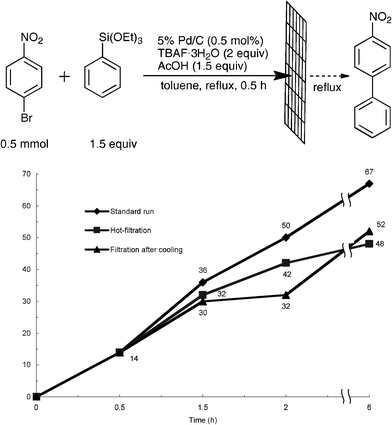 | ||
| Fig. 1 Time-course study on filtrates after the removal of the Pd/C. Standard run (♦): The reaction of 4-bromonitrobenzene and phenyltriethoxysilane was carried out in refluxing toluene for 6 h without filtration. Hot-filtration (■): 5% Pd/C was removed by filtration without cooling after 0.5 h of the reaction at reflux and the resulting filtrate was refluxed again. Filtration after cooling (▲): 5% Pd/C was removed by filtration after cooling at rt after 0.5 h of the reaction at reflux, and the resulting filtrate was refluxed again up to 6 h. | ||
Conclusions
In summary, we have developed a ligand-free Pd/C-catalyzed Hiyama cross-coupling reaction for an effective preparation method of a variety of biphenyl derivatives. To the best of our knowledge, this is the first application of Pd/C as a catalyst in the absence of ligands for the Hiyama cross-coupling reaction. Because only a small amount of 5% Pd/C (0.5 mol%) is required for the efficient reaction progress, an industrial application of the protocol is expected.Experimental section
General method
Dry-type 10% Pd/C (K-type) or dry-type 5% Pd/C (K-type) was supplied by the N.E. Chemicat Corporation (Tokyo, Japan). All reagents and solvents were purchased from commercial sources and used without further purification. Flash column chromatography was performed using Silica Gel 60 N (Kanto Chemical Co., Inc., 63–210 μm spherical, neutral). 1H NMR and 13C NMR spectra were recorded on a JEOL AL 400 (400 MHz for 1H NMR spectroscopy and 100 MHz for 13C NMR spectroscopy) or ECA 500 spectrometer (500 MHz for 1H NMR spectroscopy and 125 MHz for 13C NMR spectroscopy). Chemical shifts (δ) are expressed in ppm and are internally referenced (0.00 ppm for TMS for CDCl3 for 1H NMR spectroscopy and 77.0 ppm for CDCl3 for 13C NMR spectroscopy). Mass spectra were obtained on a JEOL JMS-SX102A or a JEOL T100TD instrument. GC-MS analysis of the side reaction was conducted by a JEOL Jms Q1000GC Mk2.General procedure for the ligand-free hiyama cross-coupling reaction with heterogeneous Pd/C as the catalyst
A mixture of the aryl halide (500 μmol), the aryltriethoxysilane (750 μmol), TBAF·3H2O (316 mg, 1.0 mmol), 5% Pd/C (5.4 mg, 2.5 μmol) and acetic acid (43.0 μL, 750 μmol) in toluene (1.00 mL) was stirred at 120 °C under Ar. After the consumption of the aryl bromide was observed by TLC analyses, the reaction mixture was diluted with EtOAc (10 mL) and water (10 mL) and passed through a Celite pad (3.0 cm) to remove the catalyst. EtOAc (50 mL) and water (50 mL) were added to the filtrate, and the layers were separated. The aqueous layer was extracted with EtOAc (10 mL), and the combined organic layers were washed with brine (20 mL), dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (hexane/EtOAc) to give the corresponding biphenyl (Table 4).Acknowledgements
We thank the N.E. Chemicat Corporation for the gift of the Pd/Cs.References
- (a) S. E. Denmark and M. H. Ober, Aldrichim. Acta, 2003, 36, 76–85 Search PubMed; (b) S. E. Denmark and J. H.-C. Liu, Angew. Chem., Int. Ed., 2010, 49, 2–11; (c) S. E. Denmark and C. S. Regens, Acc. Chem. Res., 2008, 41, 1486–1499 CrossRef CAS.
- (a) R. G. Franzen, Tetrahedron, 2000, 56, 685–691 CrossRef CAS; (b) E. C. Ashby, J. Laemmle and H. M. Neumann, Acc. Chem. Res., 1974, 7, 272–280 CrossRef CAS.
- (a) For reviews of Migita-Kosugi-Stille coupling, see: E. Fouquet and A. Herve, in Handbook of Functionalized Organometallics, Vol. 1 (Ed: P. Knochel), Wiley-VCH, Weinheim, 2005, pp. 203–215 Search PubMed; (b) P. Espinet and A. M. Echavarren, Angew. Chem. Int. Ed., 2004, 43, 4704–4734 CAS; (c) K. Fugami and M. Kosugi, in Topics in Current Chemistry: Cross-coupling Reactions A Practical Guide, Vol. 219, ed. N. Miyaura, Springer-Verlag, Berlin, 2002, pp. 87–130 Search PubMed; (d) J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508–524 CrossRef.
- (a) For reviews of Suzuki-Miyaura coupling, see: N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 Search PubMed; (b) N. Miyaura, in Topics in Current Chemistry: Cross-coupling Reactions A Practical Guide, Vol. 219, ed. N. Miyaura, Springer-Verlag, Berlin, 200211–59 Search PubMed; (c) A. Suzuki, in Handbook of Organopalladium Chemistry for Organic Synthesis, Vol. 1, ed. E. Negishi, A. de Meijere, Wiley-Interscience, New York, 2002249–262 Search PubMed; (d) L. Bai and J. X. Wang, Curr. Org. Chem., 2005, 9, 535–553 CrossRef CAS.
- (a) H. E. Elsayed-Ali and E. Waldbusser, U.S. Pat., 20040076755 2004 Search PubMed; (b) E. R. Boller, U. S. Pat., 3508913 1970 Search PubMed.
- (a) Y. Hatanaka and T. Hiyama, J. Org. Chem., 1988, 53, 918–920 CrossRef CAS; (b) K. Shibata, K. Miyazawa and Y. Goto, Chem. Commun., 1997, 1309–1310 RSC; (c) M. E. Mowery and P. DeShong, J. Org. Chem., 1999, 40, 1673–1676 Search PubMed; (d) T. Hiyama and J. Organomet, Chem., 2002, 653, 58–61 Search PubMed; (e) J. Y. Lee and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 5616–5617 CrossRef CAS; (f) Y. Nakao, H. Imanaka, A. K. Sahoo, A. Yada and T. Hiyama, J. Am. Chem. Soc., 2005, 127, 6952–6953 CrossRef CAS; (g) S. E. Denmark, R. C. Smith, W. T. T. Chang and J. M. Muhuhi, J. Am. Chem. Soc., 2009, 131, 3104–3118 CrossRef CAS; (h) S. E. Denmark and N. S. Werner, J. Am. Chem. Soc., 2010, 132, 3612–3620 CrossRef CAS.
- Combined use of Pd(OAc)2 with DABCO is also reported, see: J.-H. Li, C.-L. Deng, W.-J. Liu and Y.-X. Xie, Synthesis, 2005, 3039–3044 Search PubMed.
- (a) Recent reports, see; Y. Deng, L. Gong, A. Mi, H. Liu and Y. Jiang, Synthesis, 2003, 337–339 Search PubMed; (b) A. Alimardanov, L. Schmieder-van de, A. H. M. de Vries and J. G. de Vries, Adv. Synth. Catal., 2004, 346, 1812–1817 CrossRef CAS; (c) C.-L. Deng, S.-M. Guo, Y.-X. Xie and J.-H. Li, Eur. J. Org. Chem., 2007, 1457–1462 CrossRef CAS; (d) D. Gauthier, S. Beckendorf, T. M. Gøgsig, A. T. Lindhardt and T. Skrydstrup, J. Org. Chem., 2009, 74, 3536–3539 CrossRef CAS; (e) Z. Jiang, J. She and X. Lin, Adv. Synth. Catal., 2009, 351, 2558–2562 CrossRef CAS; (f) S.-R. Guo and Y.-Q. Yuan, J. Chem. Res., 2009, 2009, 745–749 Search PubMed; (g) N. Liu, C. Liu and Z. Jin, Cuihua Xuebao, 2010, 31, 1316–1320 Search PubMed; (h) R. Cano, D. J. Ramón and M. Yus, Tetrahedron, 2011, 67, 5432–5436 Search PubMed.
- (a) C. Pan, M. Liu, L. Zhao, H. Wu, J. Ding and J. Cheng, Catal. Commun., 2008, 9, 1685–1687 CrossRef CAS; (b) Á. Gordillo, E. de Jesús and C. L.-Mardomingo, Chem. Commun., 2007, 4056–4058 RSC.
- E. Alacid and C. Nájara, J. Org. Chem., 2008, 73, 2315–2322 CrossRef CAS.
- (a) L. D. Pachon, M. B. Thathagar, F. Hartl and G. Rothenberg, Phys. Chem. Chem. Phys., 2006, 8, 151–157 RSC; (b) D. Srimani, S. Sawoo and A. Sarkar, Org. Lett., 2007, 9, 3639–3642 CrossRef CAS; (c) B. C. Ranu, R. Dey and K. Chattopadhyay, Tetrahedron Lett., 2008, 49, 3430–3432 CrossRef CAS; (d) R. Dey, K. Chattopadhyay and B. C. Ranu, J. Org. Chem., 2008, 73, 9461–9464 CrossRef CAS.
- For a review, see: L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 Search PubMed.
- S. Nishimura, in Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, Wiley-Interscience, New York, 2001 Search PubMed.
- (a) M. Seki and J. Syn, J. Synth. Org. Chem. Jpn., 2006, 64, 853–866 CAS; (b) M. Seki, Synthesis, 2006, 2975–2992 CrossRef CAS; (c) H. Sajiki, T. Ikawa and K. Hirota, Org. Lett., 2004, 6, 4977–4980 CrossRef CAS; (d) H. Sajiki, T. Kurita, A. Kozaki, G. Zhang, Y. Kitamura, T. Maegawa and K. Hirota, J. Chem. Res., 2004, 593–595 Search PubMed [Erratum: J. Chem. Res., 2005, 344.]; (e) H. Sajiki, T. Kurita, A. Kozaki, G. Zhang, Y. Kitamura, T. Maegawa and K. Hirota, Synthesis, 2005, 537–542 CrossRef [Erratum: Synthesis, 2005, 852.]; (f) H. Sajiki, G. Zhang, Y. Kitamura, T. Maegawa and K. Hirota, Synlett, 2005, 619–622 CrossRef CAS [Erratum: Synlett, 2005, 1046.]; (g) T. Maegawa, Y. Kitamura, S. Sako, T. Udzu, A. Sakurai, A. Tanaka, Y. Kobayashi, K. Endo, U. Bora, T. Kurita, A. Kozaki, Y. Monguchi and H. Sajiki, Chem.–Eur. J., 2007, 13, 5937–5947 CrossRef CAS; (h) Y. Kitamura, A. Sakurai, T. Udzu, T. Maegawa, Y. Monguchi and H. Sajiki, Tetrahedron, 2007, 63, 10596–10602 CrossRef CAS; (i) Y. Kitamura, S. Sako, T. Udzu, A. Tsutsui, T. Maegawa, Y. Monguchi and H. Sajiki, Chem. Commun., 2007, 5069–5071 RSC; (j) S. Mori, T. Yanase, S. Aoyagi, T. Maegawa, Y. Monguchi and H. Sajiki, Chem.–Eur. J., 2008, 14, 6994–6999 CrossRef CAS; (k) T. Kurita, M. Abe, T. Maegawa, Y. Monguchi and H. Sajiki, Synlett, 2007, 2521–2524 CAS; (l) Y. Monguchi, K. Kitamoto, T. Ikawa, T. Maegawa and H. Sajiki, Adv. Synth. Catal., 2008, 350, 2767–2777 CrossRef CAS; (m) Y. Monguchi, T. Takahashi, Y. Iida, Y. Fujiwara, Y. Inagaki, T. Maegawa and H. Sajiki, Synlett, 2008, 2291–2294 CAS; (n) Y. Yabe, T. Maegawa, Y. Monguchi and H. Sajiki, Tetrahedron, 2010, 66, 8654–8660 Search PubMed.
- A. Komáromi, F. Szabó and Z. Novák, Tetrahedron Lett., 2010, 51, 5411–5414 Search PubMed.
- T. Yanase, S. Mori, Y. Monguchi and H. Sajiki, Chem. Lett., 2011, 40, 910–912 Search PubMed.
- The Pd/C used for the present work was commercially available from N.E. Chemcat Corporation in Japan. The Pd particle size and carbon surface area are approximately 5 nm and 1100 m2 g−1, respectively.
- Without 10% Pd/C, 4-bromonitrobenzene never reacted with phenyltriethoxysilane (1.5 equiv) in the presence of TBAF·3H2O.
- Kwong et al. reported that trimethoxyphenylsilane released methoxide ion in the presence of TBAF in the Pd(OAc)2-catalyzed Hiyama coupling of aryl mesylates, see: C. M. So, H. W. Lee, C. P. Rau and F. W. Kwong, Org. Lett., 2009, 11, 317–320 Search PubMed.
- Clarke et al. described that 4-bromoacetophenone was cross-coupled with ethoxide derived from the vinyl triethoxysilane or tetraethoxysilane in the presence of TBAF or NaOH, Pd(OAc)2, and 1-(di-tert-butyl)-1′-(diphenylphosphino)ferrocene, see: E. J. Milton, J. A. Fuentes and M. L. Clarke, Org. Biomol. Chem., 2009, 7, 2645–2648 Search PubMed.
- The cross-coupling of 4-bromobenzaldehyde or 2-bromobenzaldehyde with triethoxyphenylsilane selectively took place with aldehyde moieties intact (Table 4, Entries 8 and 9).
- The recovered 5% Pd/C was contaminated with significant amounts of inseparable colorless silicon residue. The existence of approximately 20 weight % of elemental silicon in the residue was confirmed by scanning electron microscopy-energy dispersion X-ray spectrometry (Roentec QuanTax with XFlash 3001, Roentec), see the ESI. The 5% Pd/C contaminated with silicon residue was used for the next run..
- The cross-coupling of 4-bromonitrobenzene (1.02 g, 5.00 mmol) with phenyltriethoxysilane (1.82 mL, 7.50 mmol) was carried out using 5% Pd/C (532 mg, 250 μmol), and TBAF·3H2O (3.15 g, 10.0 mmol) in distilled toluene (10.0 mL) for the Pd leaching test..
- The cross-coupling of 4-bromonitrobenzene (5.05 g, 25.0 mmol) with phenyltriethoxysilane (9.10 mL, 37.5 mmol) was carried out using 5% Pd/C (266 mg, 125 μmol), AcOH (2.20 mL, 37.5 mmol) and TBAF·3H2O (15.8 g, 50.0 mmol) in distilled toluene (50 mL) for the Pd leaching test..
- (a) I. W. Davies, L. Matty, D. L. Hughes and P. J. Reider, J. Am. Chem. Soc., 2001, 123, 10139–10140 CrossRef CAS; (b) D. E. Bergbreiter, B. Chen and D. Weatherford, J. Mol. Catal., 1992, 74, 409–419 CrossRef CAS; (c) S. Jayasree, A. Seayad and R. V. Chaudhari, Chem. Commun., 1999, 1067–1068 RSC; (d) F. Zhao, B. M. Bhanage, M. Shirai and M. Arai, Chem.–Eur. J., 2000, 6, 843–848 CrossRef CAS; (e) A. Biffis, M. Zecca and M. Basato, Eur. J. Inorg. Chem., 2001, 1131–1133 CrossRef CAS; (f) D. A. Colon, B. Izzo, P. Collins, G.-J. Ho, J. Michael Williams, Y.-J. Shi and Y. Sun, Adv. Synth. Catal., 2003, 345, 931–935 CrossRef CAS; (g) Y. Kitamura, S. Sako, A. Tsutsui, Y. Monguchi, T. Maegawa, Y. Kitade and H. Sajiki, Adv. Synth. Catal., 2010, 352, 718–730 CrossRef CAS.
- L. Joucla, G. Cusati, C. Pinel and L. Djakovitch, Appl. Catal., A, 2009, 360, 145–153 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00776a/ |
| This journal is © The Royal Society of Chemistry 2012 |