A novel efficient cationic flocculant prepared through grafting two monomers onto chitosan induced by Gamma radiation†
Jian-Ping
Wang
ab,
Yong-Zhen
Chen
a,
Yi
Wang
b,
Shi-Jie
Yuan
a,
Guo-Ping
Sheng
a and
Han-Qing
Yu
*a
aDepartment of Chemistry, University of Science & Technology of China, Hefei, 230026, China. E-mail: hqyu@ustc.edu.cn; Fax: +86 551 3601592; Tel: +86 551 3607592
bDepartment of Agricultural and Biological Engineering, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
First published on 14th November 2011
Abstract
Two monomers, acrylamide (AM) and (2-methacryloyloxyethyl) trimethyl ammonium chloride (DMC) were grafted onto chitosan simultaneously in acid-water solution initiated by the highly efficient and environmentally friendly gamma ray radiation at ambient temperatures. The copolymer obtained was analyzed using Fourier-transform infrared, X-ray powder diffraction and thermogravimetric analysis. The cationic degree of the copolymer was determined by the colloid titration method. Its flocculation properties were evaluated in 0.25% (wt) kaolin suspensions and its significant superiority over PAM (polyacrylamide) and chitosan was observed. The results of zeta potential measurement demonstrated that the flocculation mechanism of the copolymer was distinct when it was used as a flocculant under different conditions. The images and the settling rate test of the floccules after treating by the flocculant showed that the capacities of bridging and charge neutralization of the graft copolymer were improved after the grafting of AM and DMC. Jar tests with pulp mill wastewater demonstrated that the flocculation efficiency of the graft copolymer was much better than that of PAM.
Introduction
More than 6300 papermaking mills in China are using straw slurry as the feedstock. The wastewater generated is not easy to treat due to the high level of bases, such as alkali lignin and sodium salts of organic acid, etc. The conventional treatment methods are expensive and waste a great deal of energy. In addition, the large amount of lignin existed in the wastewater could not be reclaimed efficiently and thus increase the burden of the biological wastewater treatment. Therefore, an efficient, convenient and economical method for the treatment of pulp mill wastewater is necessary.Flocculation is an efficient and cost-effective method for wastewater treatment and sludge dewatering.1–5 Among most of the factors influencing the treatment efficiency in the flocculation process, flocculant is crucial. Nowadays, the interests in the natural polyelectrolytes or the modification of natural polyelectrolytes as flocculants have been promoted since the increasing demand for environmentally friendly technologies. Compared with conventional flocculants, the modified natural polyelectrolytes are environmentally friendly, biodegradable and nontoxic.6–8 After the modification of starch, the modification of chitosan as flocculant has attracted increasing interests in recent years. Chitosan, which is antibacterial and renewable, is the second most widespread natural polysaccharide next to cellulose on the earth.9–10 Owing to the inter-molecular and intra-molecular hydrogen bonding, chitosan can only be dissolved in acidic solutions through the interaction between H+ and –NH2,9 which might accelerate its degradation and consequently reduce its flocculation efficiency. On the other hand, however, there are some amino groups and hydroxyl groups in chitosan backbone, which is in favor of the reaction of chitosan with vinyl monomers under mild conditions. Among most of the modification methods, grafting has been proved as a useful and convenient method which is in favor of the improvement of flocculant ability.
The graft copolymers generally combine the main properties of both initial components. On the one hand, the flexible chain grafted onto the rigid or semi-rigid backbone could increase the conformational freedom of flocculant in solution and surface, which is in favor of adsorption and bridging; on the other hand, grafting method could introduce some specific functional groups and increase the molecular weight efficiently, therefore improving its adsorption bridging or charge neutralization ability, thus increasing the flocculation ability of the graft copolymer.11
In general, flocculants exhibit flocculation ability in the flocculation process through adsorption bridging, charge neutralization and sweep-floc mechanisms. Therefore, the modification of chitosan as flocculant should improve one or more aspects of these effects in order to improve its flocculation ability. In the previous works, we have synthesized a novel cationic flocculant by grafting a cationic monomer, (2-methacryloyloxyethyl) trimethyl ammonium chloride (DMC), onto chitosan initiated by gamma radiation12 and potassium persulphate.13 However, due to the homopolymerization of the cationic monomer, and the electrostatic repulsion between chitosan and the cationic monomer, or that among the cationic monomers, the grafting efficiency was unsatisfied, thus a lot of monomers will be wasted in the extraction process. In addition, the cationic monomer was more expensive than anion or non-ionic monomers. These all limited its application in various fields.
Considering all of these factors, in this study, acrylamide (AM) and DMC were selected as the monomers grafted onto chitosan initiated by gamma ray. The addition of AM will introduce functional groups which could improve the grafting percentage and bridging ability efficiently. This will lead to an increase in the molecular weight for the flocculant, and thus increase its flocculation ability. The grafting of DMC could increase the cationic content in the flocculant, which is in favor of increasing the ability of charge neutralization. Moreover, the flexible grafting chain composed by the copolymerization of the two monomers in the rigid chitosan backbone would improve the sweep-floc ability of chitosan in the flocculation process. Besides, it is worthy to mention that the grafting reaction induced by gamma radiation is of high efficiency and does not cause any further contamination associated with chemical initiators. The graft copolymer induced by gamma radiation was characterized by Fourier-transform infrared (FTIR), X-ray powder diffraction (XRD) and thermogravimetric analysis (TGA). The flocculation ability of the graft copolymer was evaluated by jar tests method using kaolin suspension and papermaking wastewater, respectively.
Experimental
The solution which contains chitosan (molecular weight: 520 kDa, degree of deacetylation: 95%), AM, and DMC was prepared with 1.0% (v/v) acetic acid in Pyrex glass vessels. The feeding chitosan concentration was 0.114 M throughout the experiment. DMC and AM concentrations were 0.171 M and 0.597 M, respectively, unless described. Following deoxygenating, the solution was then irradiated by a 60Co–γ–resource under a certain irradiation dose rate for a pre-described time duration at ambient temperatures. Thereafter, the sample solutions were precipitated in acetone and separated by filtration. Homopolymers formed in the reactions were removed by Soxhlet extraction for 24 h using ethanol. Finally, all the samples were dried in a vacuum oven at 50 °C until a constant weight was obtained. The grafting percentage was calculated using the following equation: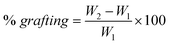 | (1) |
Infrared spectra were recorded with a Fourier-transform infrared (FTIR) spectrometer (Magna-IR 750, Nicolet Instrument Co., USA) using a potassium bromide disc technique. X-ray powder diffraction (XRD) patterns were obtained with an X-ray diffractometer (D/Max–rA, Japan) using graphite monochromatized Cu-Kα radiation (λ = 1.54056 Å). Thermogravimetric analysis (TGA) of chitosan and the grafting copolymers was performed using a thermal analyser (TGA-50, Shimadzu Co., Japan) under nitrogen atmosphere with a heating rate of 10 °C min−1 to 800 °C.
Charge density of the graft copolymer was determined by colloid titration method with potassium poly(vinyl sulfate) (PVSK, esterification degree of 98.6%) using methylene blue as initiator. The charge density (CD) (meqv g−1) was calculated according to the following formula:
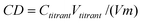 | (2) |
The flocculation ability of chitosan and chitosan-g-PDMC with different grafting percentages was evaluated using the same method as described in a former paper.12
The microscopic photographs of kaolin clay, the floccules of kaolin clay obtained from the suspensions treated with chitosan and chitosan-g-PAM-g-PDMC were taken with a phase contrast microscope (Olympus, U-5RE-2, Japan). Zeta potential of the kaolin suspension was measured using the Nano Zeta Sizer (ZEN3600, Malvern, Inc., UK) at 25 °C.
The settling tests with chitosan and the flocculant respectively were conducted using the same method as described previously.13
Papermaking wastewater was blended black liquor which was taken from the first sedimentation tank in Guoyang paper mill of Anhui, China. Wheat straws were used as the stuffs of papermaking. The initial pH, COD and turbidity of the wastewater were 6.99, 1358 mg L−1 and 1209 NTU, respectively. The average sizes of the colloid particles in the wastewater were 544 nm.
The treatment of the papermaking black liquor was carried out through coagulation-flocculation process using jar tests method. Aluminum chloride was used as coagulant. For comparison, the conventional synthetic polymeric flocculant, PAM, and chitosan-g-PAM-g-PDMC were used as flocculant respectively in the two independent jar tests. Turbidity, removal efficiency of lignin, water recovery and removal efficiency of COD (chemical oxygen demand) were chosen as the dependent output variables. A 23 full factorial central composite design (CCD) was used to evaluate the effect and interactions of the three experimental factors: coagulant dosage, flocculant dosage and pH. The operating conditions were optimized using the response surface methodology (RSM). The experimental design and their respectively levels are listed in Table A (Supplementary online materials)†.
Results and discussion
The occurrence of the graft copolymerization was confirmed by FTIR analysis (Fig. 1). The band at 1597 cm−1, the characteristic peak of primary amine N–H vibration in chitosan, disappeared in the profile of chitosan-g-PAM-g-PDMC, indicating the deformation of the primary amine in the graft copolymer. This implied that the grafting had occurred at –NH2groups. The bands appeared at 1726, 1656 and 1618 cm−1 in the graft copolymer should be ascribed to the vibrating absorption of carbonyl groups in the grafted PDMC, the absorptions of amide I and amide II in the grafted PAM, respectively. The bands at 1475 and 952 cm−1 came from methyl groups of the ammonium14 and the quaternary ammonium salt in PDMC, respectively. These spectra demonstrated that AM and DMC were all successfully grafted onto the chitosan backbone.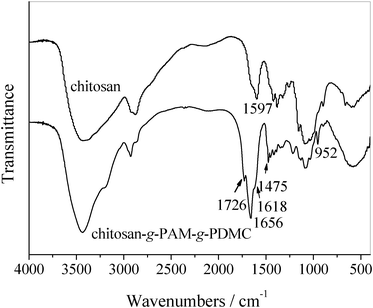 | ||
| Fig. 1 FT-IR spectra of chitosan and chitosan-g-PAM-g-PDMC. | ||
According to the FTIR analysis, the graft copolymerization of chitosan, AM and DMC initiated by gamma radiation in the presence of acid-water resulted in N-substitution. The reaction process is illustrated in Fig. 2.
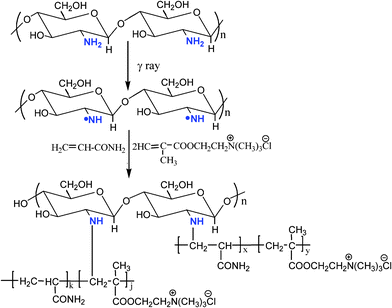 | ||
| Fig. 2 Schematic representation of graft-copolymerization of chitosan. | ||
The X-ray powder diffractions profile of chitosan-g-PAM-g-PDMC presents distinct crystalline peaks compared with that of the raw chitosan (Fig. A in Supplementary online materials)†. The profiles of chitosan showed two peaks around 2θ = 10.4° and 20.1°, which corresponded to 020 and 110 reflections, respectively.15 For chitosan-g-PAM-g-PDMC, the peak at 10.4° disappeared, and the peak at 20.1° decreased drastically, suggesting the decrease in crystallinity, which might be attributed to the branching effects resulting from the grafting of AM and DMC onto chitosan.
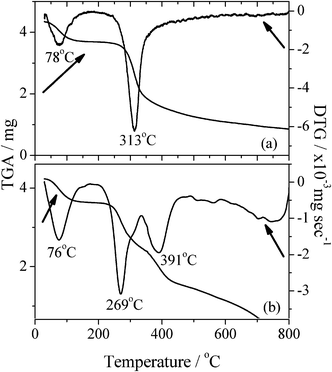
TGA also confirmed the occurrence of the graft copolymerization. The thermal behavior of chitosan-g-PAM-g-PDMC was different from that of chitosan (Fig. 3). Chitosan had weight losses in two stages as shown in its TGA profiles. The first occurred between 30 and 135 °C, which might correspond to the loss of adsorbed and bound water. The second stage of weight loss started at 232 °C and continued up to 800 °C, which was likely to be attributed to the decomposition of chitosan. The DTG curve, the abbreviation of the differential TGA curve, shows that the temperatures for the quickest weight loss in two stages were 78 °C and 313 °C, respectively. However, the two stages of weight losses in the TGA curve of chitosan-g-PAM-g-PDMC were seated between 30 and 128 °C, between 232 and 335 °C, between 350 and 800 °C, respectively. Correspondingly, the three quickest weight losses in the three stages were 76, 269 and 391 °C. Compared with chitosan, chitosan-g-PAM-g-PDMC exhibited two different quickest weight loss temperatures, one was lower and the other was higher than the quickest weight loss temperature of the chitosan, indicating that the grafting of the PAM and PDMC onto the chitosan backbone varied the thermal stability of chitosan greatly.
The flocculation performance of PAM, chitosan and chitosan-g-PAM-g-PDMC for kaolin suspensions (0.25 wt%) at pH 4.0, 7.0 and 10.0 are illustrated in Fig. 4 (a), (b) and (c), respectively. The graft copolymer, chitosan-g-PAM-g-PDMC, with a grafting ratio of 261%, was prepared at a radiation dose rate of 10.0 Gy min−1 for 80 min at room temperatures. The cationic degree of the graft copolymer used in the experiment was 1.02 × 10−3 meqv g−1.
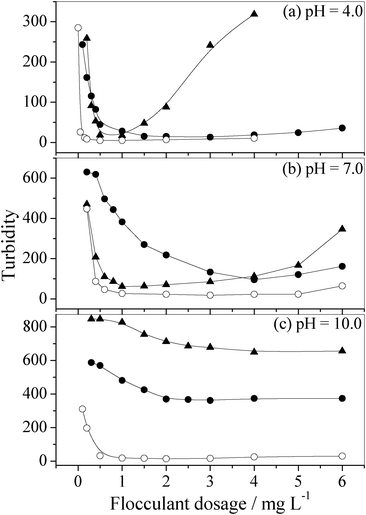 | ||
| Fig. 4 Flocculation performance of PAM, chitosan and chitosan-g-PAM-g-PDMC in kaolin suspension of 0.25% (w/v) at pH 4.0, 7.0 and 10.0. -•-: PAM, -▾-: chitosan; -○-: chitosan-g-PAM-g-PDMC. | ||
Particles can be destabilized through the dose of coagulant/flocculants by three major mechanisms, i.e., adsorption bridging, sweep-floc and charge neutralization.16 When high-molecular-weight polymers are added to water, polymer chains can attach to the particle surface on one side, and the extended end on the other side might attach to the surface of other free particles. In this way, bridges (adsorption bridging) could be formed between particles, resulting in particle destabilization and floc formation; sometimes, when coagulants and flocculants are added to water, big amorphous precipitates (sweeping flocs) can be formed. Particles can be enmeshed in these flocs and then removed by settling or filtration of the flocs; when cationic copolymers (or positively charged metal hydrolysis species) are present in the water, they can attach to the negatively charged particles and thus reduce or eliminate the electric repulsion between particles through charge-neutralization. PAM flocculated the kaolin suspension through adsorption bridging and sweep-floc mechanisms. However, when chitosan and chitosan-g-PAM-g-PDMC used as flocculant, the charge neutralization is important as well as bridging and sweep-floc effect.
Except for chitosan at pH 4.0, the flocculation ability of these three polymers was enhanced with an increase in flocculant dosage (Fig. 4). As chitosan dosage was increased over 1 mg mL−1 at pH 4.0 (Fig. 4 (a)), a gradual increase in the turbidity of kaolin suspension was observed. This is because, at an over-dosage of chitosan, the surface charge of kaolin became positive, causing re-dispersion of the aggregated particles. Under acidic conditions (pH 4.0), chitosan (pKa 6.2∼6.8)17 was positively charged because of the protonation of the amino groups. The positive electric charge density and the molecular weight of chitosan-g-PAM-g-PDMC were higher than those of chitosan. Therefore, the supernatant turbidity of the kaolin suspension treated by the graft copolymer was lower than that treated by chitosan and PAM at identical flocculant dosages, especially, at lower flocculant dosages (e.g., at flocculant dosages of 0.05 and 0.15 mg L−1). At pH around 7.0, the case was similar as that under acidic conditions. The difference was that, to reach the optimal treatment efficiency, the flocculant dosage at pH 7.0 was higher than that at pH 4.0. This is because under neutral conditions (pH 7.0), the cationic characteristic of the graft copolymer was mainly presented by PDMC grafted onto chitosan. As a result, the cationic density of chitosan and the graft copolymer was lower under the neutral condition (pH 7.0) than that under the acidic condition (pH 4.0). That is, the charge neutralization ability was reduced when the pH of the kaolin suspension varied from 4.0 to 7.0. One notable phenomenon was that, under alkaline conditions (for example, at pH 10.0), the graft copolymer had a decent flocculation ability, while the other two, PAM and chitosan, had almost no flocculation ability. Under alkaline conditions, the amino groups in chitosan can't be protonized, and chitosan was indiscerptible. Therefore, chitosan can not present good flocculation efficiency. Nonetheless, the cationic characteristic of the chitosan-g-PAM-g-PDMC was influenced very slightly, because it originated mainly from the grafted quaternary ammonium salts, PDMC. Therefore, significant flocculation efficiency can still be achieved with chitosan-g-PAM-g-PDMC under alkaline conditions, suggesting a great significance for practical application.
An interesting feature, which can be seen from Fig. 4 (a), is that the flocculation window of chitosan-g-PAM-g-PDMC was broader than the natural polymer, chitosan. This was another advantage of the graft copolymer. The grafting chains composed by the copolymer of PAM and PDMC on the semi-rigid chitosan backbone increased the adsorption bridging ability and the flexibility of the flocculant.18 This maybe increase the binding intensity between flocculants and the colloids. As a result, under identical conditions, the restabilization effect induced by charge reversion (from negative to positive) for the graft copolymer was weaker than that for chitosan. The graft copolymer, chitosan-g-PAM-g-PDMC, was superior to chitosan from the application point of view.
Fig. 5 shows the zeta potential and the turbidity of the supernatant treated by the graft copolymer at various dosages. Under acidic (pH 4.0) or neutral conditions (pH 7.0), when the zeta potential approached to zero, the turbidity of the supernatant was minimum, i.e., the flocculation efficiency was maximum at the flocculant dosage point that zeta potential approached to zero. Thus, under these conditions, the charge neutralization ability of the cationic flocculant made the flocculation occurred rapidly. However, under alkaline conditions (for example, at pH 10.0), the zero point of zeta potential was not observed even the flocculant dosage reached 30 mg L−1, although the flocculation efficiency was considerably high. When the flocculant dosage approached to 30 mg L−1, a restabilization phenomenon was observed. Such a behavior was suggested to be a characteristic for bridging flocculation.18 The cationic flocculant neutralized a part of negative charge, so the value of zeta potential (negative) increased with the increase of the flocculant dosage. In addition to charge neutralization, the sweep-floc effects removed the turbidity of the kaolin suspension through adsorption and bridging. This implied that under alkaline conditions, the flocculation was due to the synergetic effects of charge neutralization and sweep-floc mechanism. As the charge neutralization ability being concerned, the graft copolymer was superior to chitosan and PAM under alkaline conditions.
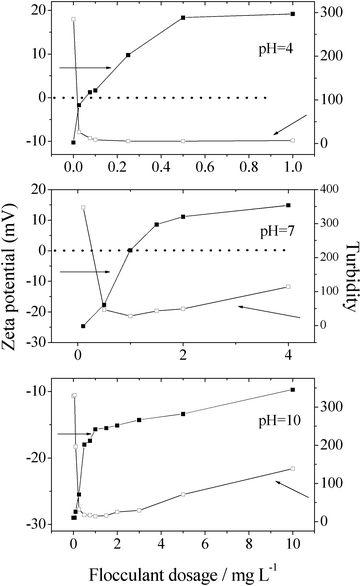 | ||
| Fig. 5 Zeta potential and turbidity of supernate at different chitosan-g-PAM-g-PDMC dosages at pH of 4.0, 7.0 and 10.0. | ||
The settling process of the kaolin floccules treated by chitosan and the graft copolymer is shown in Fig. B (Supplementary online materials)†. Under alkaline conditions, even no clear interface between water and solid was observed when the kaolin suspension was treated by chitosan, because chitosan had negligible flocculation ability in this case. While the kaolin floccules formed by the suspensions treated with the graft copolymer settled quickly. Under the other two conditions, acidic and neutral, the kaolin floccules formed by the suspensions treated with the graft copolymer settled more quickly than that treated by chitosan. This might be because the grafting of DMC was in favor of the compression of the electrical double layer, and the grafting of DMC and AM increased the ability of adsorption bridging. Thus, the floccules formed by the flocculants were denser and larger, and would settle quickly in water because of their larger density and weight.
The images of the floccules obtained from the suspensions treated respectively by no flocculant, chitosan and chitosan-g-PAM-g-PDMC are shown in Fig. C (Supplementary online materials)†. Dense and large floccules from the kaolin suspension treated by chitosan-g-PAM-g-PDMC are displayed in Fig. C(c), compared with those treated by chitosan in Fig. C(b), and with the raw kaolin particles with no flocculation treatment in Fig. C(a). These physical characteristics of the floccules were in well accordance with their corresponding results of settling rate as shown in Fig. B.
The actual design and the results of the experiments for treatment of papermaking black liquor through coagulation-flocculation process are listed in Table 1 for PAM as flocculants and Table 2 for chitosan-g-PAM-g-PDMC as flocculants respectively. The four responses are individual responses, and their optimization was achieved under different optimal conditions. Thus, a compromise among the conditions for the four responses is desirable. This could be achieved using the desirability function approach.19 The regression functions for PAM and chitosan-g-PAM-g-PDMC as flocculants were achieved in Eqn 3 and Eqn 4, respectively.
| Run No. | X1 | X2 | X3 | Turbidity | Phenol removal/% | Water Recovery/% | COD removal/% |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 62.7 | 72.3 | 80.0 | 73.7 |
| 2 | 0 | 0 | 0 | 58.5 | 72.8 | 80.0 | 72.8 |
| 3 | −1 | 1 | −1 | 157.6 | 35.1 | 80.0 | 52.4 |
| 4 | −1 | −1 | −1 | 147.2 | 40.6 | 58.0 | 50.5 |
| 5 | 1 | 1 | −1 | 103.3 | 42.3 | 80.0 | 56.2 |
| 6 | 1 | −1 | −1 | 124.0 | 40.4 | 80.0 | 48.6 |
| 7 | 0 | −2 | 0 | 103.0 | 71.9 | 60.0 | 60.0 |
| 8 | −2 | 0 | 0 | 774.9 | 19.9 | 60.0 | 69.3 |
| 9 | 1 | −1 | 1 | 183.9 | 46.9 | 50.0 | 79.0 |
| 10 | −1 | 1 | 1 | 242.8 | 70.4 | 65.0 | 52.3 |
| 11 | −1 | −1 | 1 | 179.3 | 60.3 | 75.0 | 78.5 |
| 12 | 1 | 1 | 1 | 201.5 | 31.2 | 40.0 | 62.2 |
| 13 | 2 | 0 | 0 | 190.4 | 42.0 | 64.0 | 63.5 |
| 14 | 0 | 2 | 0 | 100.2 | 72.7 | 60.0 | 52.2 |
| 15 | 0 | 0 | 2 | 167.9 | 39.8 | 62.0 | 52.7 |
| 16 | 0 | 0 | −2 | 186.0 | 41.8 | 76.0 | 47.8 |
| 17 | 0 | 0 | 0 | 60.5 | 71.1 | 80.0 | 75.1 |
| 18 | 0 | 0 | 0 | 62.0 | 71.1 | 80.0 | 74.3 |
| 19 | 0 | 0 | 0 | 66.0 | 71.5 | 75.0 | 75.8 |
| 20 | 0 | 0 | 0 | 64.2 | 72.4 | 75.0 | 74.5 |
| Run No. | X1 | X2 | X3 | Turbidity | Phenol removal/% | Water recovery/% | COD removal/% |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 23.2 | 79.6 | 94.0 | 81.2 |
| 2 | 0 | 0 | 0 | 28.4 | 79.6 | 94.0 | 81.8 |
| 3 | −1 | 1 | −1 | 25.8 | 67.0 | 92.0 | 64.2 |
| 4 | −1 | −1 | −1 | 139.5 | 54.8 | 82.0 | 56.1 |
| 5 | 1 | 1 | −1 | 28.4 | 68.6 | 90.0 | 66.9 |
| 6 | 1 | −1 | −1 | 18.1 | 88.0 | 93.0 | 63.8 |
| 7 | 0 | −2 | 0 | 36.2 | 79.6 | 84.0 | 78.4 |
| 8 | −2 | 0 | 0 | 271.2 | 49.5 | 30.0 | 43.2 |
| 9 | 1 | −1 | 1 | 22.1 | 83.8 | 64.0 | 77.1 |
| 10 | −1 | 1 | 1 | 67.2 | 68.2 | 82.0 | 64.9 |
| 11 | −1 | −1 | 1 | 186.0 | 51.5 | 60.0 | 48.0 |
| 12 | 1 | 1 | 1 | 26.4 | 83.6 | 72.0 | 79.9 |
| 13 | 2 | 0 | 0 | 41.3 | 73.4 | 50.0 | 74.6 |
| 14 | 0 | 2 | 0 | 24.1 | 79.6 | 84.0 | 72.8 |
| 15 | 0 | 0 | 2 | 108.5 | 59.6 | 70.0 | 64.5 |
| 16 | 0 | 0 | −2 | 124.0 | 63.9 | 90.0 | 58.9 |
| 17 | 0 | 0 | 0 | 23.4 | 81.6 | 94.0 | 82.2 |
| 18 | 0 | 0 | 0 | 25.2 | 79.9 | 95.0 | 70.9 |
| 19 | 0 | 0 | 0 | 28.3 | 80.6 | 94.0 | 81.2 |
| 20 | 0 | 0 | 0 | 26.7 | 79.6 | 93.0 | 81.8 |
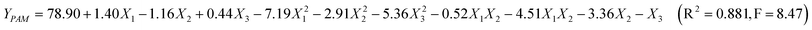
| (3) |
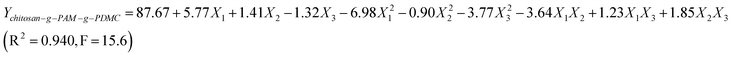
| (4) |
The quadratic regression shows that the two models were significant because the values of Fstatistic (the ratio of mean square due to regression to mean square to real error) of 8.47 and 15.67 were greater than F0.001,9,10 (4.94).
From the regression Eqn (3), the optimal conditions for PAM as flocculants were obtained as follows: coagulant dosage of 915 mg L−1, flocculant dosage of 17.4 mg L−1 and pH 7.2. Under the optimal conditions, the turbidity, the efficiency of lignin removal, water recovery and the efficiency of COD removal were estimated to be 54.9 NTU, 70.6%, 77.2% and 75.0% according to their individual regression equation (Table 3).
| Flocculant | Response | ||||
|---|---|---|---|---|---|
| Turbidity (NTU) | Lignin removal efficiency (%) | Water recovery efficiency (%) | COD removal efficiency (%) | ||
| PAM | Measured | 56.0 ± 1.2 | 70.2 ± 1.5 | 76.5 ± 1.4 | 76.0 ± 1.8 |
| Calculated | 54.9 | 70.6 | 77.2 | 75.0 | |
| Chitosan-g-PAM-g-PDMC | Measured | 8.5 ± 1.5 | 82.9 ±2.0 | 93.5 ± 1.8 | 81.3 ± 1.5 |
| Calculated | 7.1 | 84.3 | 93.9 | 80.1 | |
The optimal conditions for chitosan-g-PAM-g-PDMC as flocculants obtained from the Eqn (4) were coagulant dosage of 1109 mg L−1, flocculant dosage of 11.3 mg L−1 and pH 6.5. Under the optimal conditions, the treatment efficiency, i.e., the turbidity after being treated, the efficiency of lignin removal, water recovery and the efficiency of COD removal were estimated to be 7.1 NTU, 84.3%, 93.9% and 80.1% according to their individual regression equation respectively (Table 3). Under the optimal treatment conditions, although the flocculant dosage was less when chitosan-g-PAM-g-PDMC used as flocculant compared with PAM, the treatment efficiency was higher. The grafting of the two monomers onto chitosan backbone was in favor of the improvement of bridging ability, charge neutralization and sweep-floc ability. Therefore, the flocculation ability of the graft copolymer was better than that of PAM, especially presented in the higher removal efficiency even at lower flocculant dosage. The results indicated that the flocculant prepared in the experiment was superior to PAM from the application point of view.
To confirm the validity of the statistical experimental strategies, two runs of additional confirmation experiments were conducted under each of the operation conditions. The chosen conditions for coagulant dosage, flocculant dosage and pH are the optimal conditions according to their respectively desirability function regression equations. The measured turbidity, removal efficiency of lignin, water recovery and removal efficiency of COD were close to those estimated using RSM (Table 3). This also testifies that the RSM approach was appropriate for optimizing the operational conditions of the coagulation-flocculation process.
Conclusions
A new efficient flocculant was synthesized by grafting two monomer, AM and DMC onto chitosan, the based polymer, using gamma radiation method under acid-water conditions. The cationic degree of the copolymer was determined as 1.02 × 10−3 meqv g−1 using the colloid titration method. Results of the flocculation property evaluated using 0.25 wt% kaolin suspensions demonstrated that the grafted copolymer, chitosan-g-PAM-g-PDMC, was superior to PAM and chitosan from application point of view. The results of zeta potential demonstrated that the flocculation mechanism of the copolymer was distinct when it was used as flocculant under different conditions. Under acidic and neutral conditions, charge neutralization was the main effect for the flocculation. While under alkaline conditions, the flocculation was the synergetic effect of charge neutralization and bridging mechanism. The floccules from kaolin suspensions treated by the graft copolymer were denser, larger and settled more quickly than that treated by chitosan because of the increase of the bridging ability and the compression of the double electrical layer. Jar tests of the treatment of the papermaking wastewater demonstrated that the graft copolymer was superior to chitosan, because it achieved a higher flocculation efficiency at a lower flocculant dosage.Acknowledgements
We wish to thank the Key Special Program on the S&T for the Pollution Control (2008ZX07103-001 and 2008ZX07010-003) for the partial support of this study.References
- G. V. Franks, J. Colloid Interface Sci., 2005, 292, 598 CrossRef CAS.
- A. Metes, D. Kovacevic, D. Vujevic and S. Papic, Water Res., 2004, 38, 3373 CrossRef CAS.
- H. K. Shon, S. Vigneswaran, H. H. Ngo and R. B. Aim, Water Res., 2005, 39, 147 CrossRef CAS.
- H. N. Xiao, R. Pelton and A. Hamielec, J. Colloid Interface Sci., 1995, 175, 166 CrossRef CAS.
- W. Y. Yang, J. W. Qian and Z. Q. Shen, J. Colloid Interface Sci., 2004, 273, 400 CrossRef CAS.
- S. Bratskaya, S. Schwarz and D. Chervonetsky, Water Res., 2004, 38, 2955 CrossRef CAS.
- R. Divakaran and V. N. S. Pillai, Water Res., 2001, 35, 3904 CrossRef CAS.
- S. B. Seo, T. Kajiuchi, D. I. Kim, S. H. Lee and H. K. Kim, Macromol. Res., 2002, 10, 103 CrossRef CAS.
- N. K. Lazaridis, G. Z. Kyzas, A. A. Vassiliou and D. N. Bikiaris, Langmuir, 2007, 23, 7634 CrossRef CAS.
- T. Vincent and E. Guibal, Environ. Sci. Technol., 2004, 38, 4233 CrossRef CAS.
- D. O. Krentz, C. Lohmann, S. Schwarz, S. Bratskaya, T. Liebert, J. Laube, T. Heinze and W. M. Kulicke, Starch-Starke, 2006, 58, 161 CrossRef CAS.
- J. P. Wang, Y. Z. Chen, X. W. Ge and H. Q. Yu, Chemosphere, 2007, 66, 1752 CrossRef CAS.
- J. P. Wang, Y. Z. Chen, S. J. Yuan, G. P. Sheng and H. Q. Yu, Water Res., 2009, 43, 5267 CrossRef CAS.
- E. Loubaki, M. Ourevitch and S. Sicsic, Eur. Polym. J., 1991, 27, 311 CrossRef CAS.
- C. L. de Vasconcelos, P. M. Bezerril, T. N. C. Dantas, M. R. Pereira and J. L. C. Fonseca, Langmuir, 2007, 23, 7687 CrossRef CAS.
- Operational control of coagulation and filtration processes, ed. J. Ritter, Colorado: American Water Works Association, Denver, 2010.
- C. L. Schauer, M. S. Chen, R. R. Price, P. E. Schoen and F. S. Ligler, Environ. Sci. Technol., 2004, 38, 4409 CrossRef CAS.
- S. Bratskaya, S. Schwarz, J. Laube, T. Liebert, T. Heinze, O. Krentz, C. Lohmann and W. M. Kulicke, Macromol. Mater. Eng., 2005, 290, 778 CrossRef CAS.
- J. P. Wang, Y. Z. Chen, X. W. Ge and H. Q. Yu, Colloids Surf., A, 2007, 302, 204 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Table for levels of the variable tested in the 23 central composite designs, XRD patterns of chitosan and chitosan-g-PAM-g-PDMC, comparison of settling rate with different flocculants and images of floccules from treated kaolin suspension. |
| This journal is © The Royal Society of Chemistry 2012 |