The crystal structure of a biogenic aragonite from the nacre of an ammonite shell
S. M.
Antao
*
Department of Geoscience, University of Calgary, Calgary, Alberta, Canada T2N 1N4. E-mail: antao@ucalgary.ca
First published on 10th November 2011
Abstract
The crystal structure of a biogenic aragonite from the nacre of an ammonite shell was obtained using synchrotron high-resolution powder X-ray diffraction (HRPXRD) data and Rietveld structure refinement. The well-preserved ammonite sample is from Alberta, Canada, and is from the Cretaceous period. The aragonite structure was refined in space groupPmcn, Z = 4, and the cell parameters obtained are a = 4.96265(2), b = 7.97016(4), c = 5.74474(3) Å, and V = 227.222(2) Å3. The chemical analyses indicate a formula of [Ca0.995Sr0.004Ba0.001]∑=1.0(CO3). The average <Ca–O> and <C–O> distances are 2.5281(3) and 1.2871(6) Å, respectively, and the average <O–C–O> angle is 119.94(8)°. The CO3 groups are non-planar. Based on crystal-structure data for biogenic and non-biogenic aragonite samples, aragonite from ammonite nacre has minimal structural distortions and is very similar to non-biogenic aragonite, in particular, a sample from Spain.
1. Introduction
Calcium carbonate, CaCO3, in the form of calcite, aragonite, and vaterite is abundant in the Earth and has implications for carbon cycling. Aragonite (metastable under ambient conditions) is the stable phase at high pressure and at low temperature. Several marine organisms (e.g., molluscs, bivalves, corals, etc.) secrete biogenic aragonite (or calcite) in their strong shells, which can withstand harsh environments and provide protection from their predators. The high mechanical performances (e.g., enhanced fracture resistance) of biogenic aragonite, compared to non-biogenic aragonite, make it intriguing in nanotechnology, industrial, biomedical, and domestic applications.1–4 Bio-organisms can control crystal morphology and polymorphism on a nano-meter scale and are attracting the attention of materials scientists.The shells of marine organisms have nacre linings that are composed of polygonal aragonite tablets that are cemented by a thin organic matrix interface with nano-scale aragonite crystals, often referred to as a “brick–bridge–mortar” arrangement.5,6 The aragonite structure consists of layers of nine-coordinated Ca2+ cations that are parallel to (001), and occur approximately in the positions of hexagonal close-packing (Fig. 1). This is in contrast with the deformed cubic close-packed arrangement of Ca2+ cations in calcite, and gives rise to the pseudo-hexagonal symmetry. Successive CO32−groups along c point alternately to the +b and −b directions. Although the Ca2+ cations are nearly in a hexagonal array, the arrangement of the CO32−groups lowers the symmetry to orthorhombic, and the CO32−groups are slightly aplanar. The nacre lamellae have a preferred crystallographic orientation perpendicular to the c axis.7
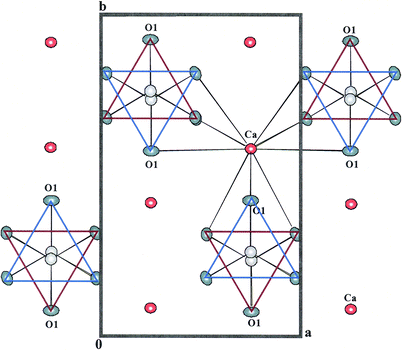 | ||
| Fig. 1 Projection of the aragonite structure showing the approximate hexagonal close-packing of the O atoms and the coordination of a Ca atom to nine O atoms of six different CO3 groups. The O2 atoms are unlabelled. | ||
Researchers have investigated the crystal structure of biogenic aragonite.8–11 Different shell layers in the same bio-composite organism have different textural and structural parameters.8–10 All textured layers show unit-cell anisotropy, and the most deformed cell parameters depend on the layer type.9 The relative intensities of the minor peaks of the carbon XANES spectra are different for biogenic and non-biogenic aragonite.12 Biogenic and non-biogenic aragonite have different bond distances, atom configurations, and especially aplanarity resulting from the interaction between organic macromolecules and the growing crystallites during biomineralization.11 Bio-composites are mechanically stressed/strained and mild annealing leads to pronounced structural relaxation.13–15 The anisotropic unit-cell distortions in biogenic aragonite are largest along the c axis.11,13,16 However, these distortions in some non-powderized layers of biogenic aragonite from bivalves and gastropods are larger along the other unit-cell directions.9
The calcite–aragonite phase equilibrium is often used as a geobarometer and geothermometer.17–19 Based on structural differences, biogenic aragonite transforms to calcite at lower temperatures than non-biogenic aragonite.5,15,20–26 The aragonite–calcite transition in snail shells is mainly governed by time and trace elements, Mg atoms in particular inhibit this transition.27
Shell mineralogy plays a primary role by placing limits on possible chemical compositions.28 Trace-element content in skeletal parts of bio-organisms varies systematically with the environment in which the organism grows.29 Variations in the physical and chemical characteristics of aragonite and calcite in fossils serve as a useful tool to reconstruct paleo-environment, paleo-climate, and paleo-ecology.30–31
The differences between trace element contents of fossil carbonate shells and their recent counterparts have been attributed to diagenetic alteration, difference in chemistry of the paleoenvironment, and biochemical evolution of the organism over geological time. However, the best preserved fossils generally seem to approach the composition of their recent counterparts.32 For example, upper Cretaceous ammonites have a shell composition similar to modern mollusks,33 whereas diagenetically modified Triassic sponge spicules have higher amounts of Fe, Mg, and Mn atoms compared to recent spicules.34 Well preserved ammonites have a shell composition of pure aragonite.35 However, pure aragonite in fossil shells is often rare in older geological deposits because of diagenetic modification.33
This study investigates the crystal structures of a biogenic aragonite from the nacre of a well-preserved ammonite shell. The aim is to compare the crystal structure of biogenic and non-biogenic aragonite samples from various sources.
2. Experimental
2.1 Sample description
The well-preserved Cretaceous ammonite sample is from Alberta, Canada. The fossil is over 65 million years old and showed no detectable traces of secondary mineral growth. The shell has a pearly luster that is indicative of good preservation. The iridescent, red-orange, plate like crystals of aragonite from the well-developed nacre layer were scraped with a knife for the experiments.2.2 Electron microprobe analyses
The aragonite sample from the ammonite nacre was analyzed using a JEOL JXA–8200 electron microprobe and the JEOL operating program on a Solaris platform. The operating conditions were: a 15 kV accelerating voltage, a 10 nA beam current, a beam diameter of 5 μm, and various standards [e.g., fluorapatite (CaKα, PKα, FKα), barite (BaKα, SKα), hornblende (FeKα, Mg-Kα), scapolite (ClKα), rhodonite (MnKα), strontianite (SrKα)]. The chemical analysis is given in Table 1, and it corresponds to nearly pure CaCO3 with small amounts of Sr and Ba replacing Ca atoms, as indicated by the formula, [Ca0.995Sr0.004Ba0.001]∑=1.0(CO3). Aragonite often incorporates Sr2+, instead of Mg2+ cations, into its structure.| Compounds of ammonite nacre | wt. % | Element | apfua |
|---|---|---|---|
| a Atoms per formula unit. b CO2 is calculated by difference. Based on ∑M = 1, the number for the CO3 group is also 1. The formula is [Ca0.995Sr0.004Ba0.001]∑=1.0(CO3). | |||
| CaO | 54.27 | Ca | 0.995 |
| MgO | 0.01 | Mg | 0.000 |
| SrO | 0.42 | Sr | 0.004 |
| BaO | 0.10 | Ba | 0.001 |
| P2O5 | 0.03 | P | 0.000 |
| F | 0.01 | F | 0.000 |
| b CO2 | 45.17 | ||
| Total | 100.00 | ∑M | 1.000 |
2.3 Synchrotron high-resolution powder X-ray diffraction (HRPXRD)
Aragonite from a small portion of a nacre layer was finely crushed in an agate mortar and pestle for synchrotron high-resolution powder X-ray diffraction (HRPXRD) experiment that was performed at beamline 11-BM, Advanced Photon Source, Argonne National Laboratory. The sample was loaded into a Kapton capillary and rotated during the experiment at a rate of 90 rotations per second. The data were collected to a maximum 2θ of about 40° [(sinθ)/λ = 0.8261 Å−1] with a step size of 0.001° and a step time of about 0.1 s step−1. Additional details of the experimental set-up are given elsewhere.36–382.4 Rietveld structure refinement
The crystal structure of aragonite was modeled using the Rietveld method39 that is incorporated in the GSAS program,40 the EXPGUI interface,41 and initial structural parameters.42 Scattering factors for neutral atoms were used. The structure refinement was carried out by varying parameters in the following sequence: scale factor, background, cell, zero shift, profile (type 3 in the GSAS program with Lorentzian size and strain broadening terms), atom positions, and isotropic displacement parameters. Finally, all variables were refined simultaneously until convergence was achieved. The HRPXRD trace indicates that the sample is 99.996(1)% aragonite and one peak (very low intensity and broad) of calcite was visible (Fig. 2). Table 2 contains the Rietveld refinement statistics and unit-cell parameters. Table 3 contains atom positions and isotropic displacement parameters, and Table 4 contains bond distances.![The HRPXRD trace for aragonite, CaCO3, from ammonite nacre, together with the calculated (continuous line) and observed (crosses) profiles. The difference curve (Iobs − Icalc) is shown at the bottom at the same scale as intensity. The short vertical lines indicate allowed reflection positions. The intensities for the trace and difference curve beyond 20° 2θ are multiplied by a factor of 10. The strongest (111) peak at 6.99° 2θ has a FWHM of 0.016° and an intensity of about 14000 counts. Only one small broad peak for calcite is observed [peak (104) at 7.82° 2θ has a FWHM of 0.107° and an intensity of about 250 counts].](/image/article/2012/RA/c1ra00568e/c1ra00568e-f2.gif) | ||
| Fig. 2 The HRPXRD trace for aragonite, CaCO3, from ammonite nacre, together with the calculated (continuous line) and observed (crosses) profiles. The difference curve (Iobs − Icalc) is shown at the bottom at the same scale as intensity. The short vertical lines indicate allowed reflection positions. The intensities for the trace and difference curve beyond 20° 2θ are multiplied by a factor of 10. The strongest (111) peak at 6.99° 2θ has a FWHM of 0.016° and an intensity of about 14000 counts. Only one small broad peak for calcite is observed [peak (104) at 7.82° 2θ has a FWHM of 0.107° and an intensity of about 250 counts]. | ||
| Biogenic | Non-biogenic | |
|---|---|---|
| Ammonite Nacre | aCuenca, Spain42 | |
| a For comparison, non-biogenic aragonite from Cuenca, Spain42 is displayed in Tables 2–4. b R (F 2)= R-structure factor based on observed and calculated structure amplitudes = [∑(F2o − F2c)/∑(F2o)]1/2. Nobs = number of observed reflections; and the number of data points is 36499. Space group is Pmcn and the number of formula units per unit cell, Z = 4. | ||
| a/Å | 4.96265(2) | 4.96174(2) |
| b/Å | 7.97016(4) | 7.96918(3) |
| c/Å | 5.74474(3) | 5.74265(2) |
| V/Å3 | 227.222(2) | 227.070(1) |
| λ/Å | 0.41399(2) | 0.40245(2) |
| b R (F2) | 0.0361 | 0.0369 |
| R wp | 0.0901 | 0.0888 |
| χ 2 | 1.967 | 3.329 |
| N obs | 641 | 699 |
| 2θ range | 3.5–40 | 3.5–40 |
| Biogenic | Non-biogenic | ||
|---|---|---|---|
| Atom | Ammonite Nacre | Cuenca, Spain42 | |
| a Atom positions: Ca, C, and O1 at 4c (¼, y, z), and O2 at 8d (x, y, z). b Calculated using the formula, ΔZC–O1 (Å) = |zC – zO1|c.11 | |||
| Ca | y | 0.41509(4) | 0.41508(3) |
| z | 0.75935(7) | 0.75953(5) | |
| U | 0.854(9) | 0.735(6) | |
| C | y | 0.7611(2) | 0.7612(1) |
| z | −0.0838(3) | −0.0842(2) | |
| U | 1.29(4) | 1.07(1) | |
| O1 | y | 0.9223(2) | 0.9228(1) |
| z | −0.0947(2) | −0.0947(1) | |
| U | 1.21(3) | 1.07(1) | |
| O2 | x | 0.4748(1) | 0.4743(1) |
| y | 0.68050(9) | 0.68059(6) | |
| z | −0.0870(1) | −0.0871(1) | |
| U | 1.10(2) | 1.07(1) | |
| bAplanarity (Å) | 0.0626 | 0.0603 |
| Biogenic | Non-biogenic | ||
|---|---|---|---|
| Ammonite Nacre | Cuenca, Spain42 | ||
| a Number of bond lengths/angles used to compute the average. | |||
| Ca–O1 | ×1 | 2.4117(11) | 2.4097(7) |
| Ca–O1 | ×2 | 2.6562(4) | 2.6560(3) |
| Ca–O2 | ×2 | 2.5494(8) | 2.5478(6) |
| Ca–O2 | ×2 | 2.4469(8) | 2.4478(6) |
| Ca–O2 | ×2 | 2.5182(8) | 2.5186(6) |
| <Ca–O> [9]a | 2.5281(3) | 2.5278(2) | |
| C–O1 | ×1 | 1.2857(18) | 1.289(1) |
| C–O2 | ×2 | 1.2878(9) | 1.2851(6) |
| <C–O> [3] | 1.2871(6) | 1.2864(4) | |
| O1–C–O2 | ×2 | 119.86(7) | 119.92(5) |
| O2–C–O2 | ×1 | 120.1(2) | 120.0(1) |
| <O–C–O> [3] | 119.94(8) | 119.95(4) |
3. Results and discussion
Aragonite, which is metastable under ambient conditions, is well preserved in this ammonite fossil shell from Alberta. Based on the ammonite shell appearance, the small amount of calcite [0.004(1)%], and the low Mg/Ca ratio (Table 1), the ammonite sample was not diagenetically modified and can be classified as a well-preserved fossil.33The unit-cell parameters for aragonite from the ammonite nacre are a = 4.96265(2), b = 7.97016(4), c = 5.74474(3) Å, and V = 227.222(2) Å3 (Table 2). These parameters are similar to those of non-biogenic aragonite samples from Cuenca, Spain,42 and Tuscany, Italy,42 and also to samples from Sefrou, Morocco.11,43 Very small positive distortions are observed between biogenic (ammonite nacre) and non-biogenic (Spain) aragonite samples (Δa/a = 0.0002, Δb/b = 0.0001, Δc/c = 0.0004, and ΔV/V = 0.0007). Positive distortions along the a and c axes, and a negative distortion along the b axis was obtained for mollusk samples.11,13,16 In order to diminish interactions between the organic and mineral phase, the mollusk samples were bleached.11,13 However, the unit-cell parameters are practically insensitive to the bleach treatment and structural distortions originate primarily within the crystallites.11,13 Based on these results, the ammonite sample used in this study was unbleached. It should be noted that distortions arising from textural effects are removed in powderized samples.8–10 Therefore, results from this study can be compared to other powder diffraction studies.
The chemical formula for the ammonite nacre is [Ca0.995Sr0.004Ba0.001]∑=1(CO3) and that for the aragonite sample from Spain is [Ca0.985Sr0.014]∑=1(CO3).42 The Ca2+ is substituted mainly by a larger Sr2+ cation (radius of Ca2+ = 1.0 and Sr2+ = 1.18 Å44), which causes minor differences in the cell parameters for the two aragonite samples. The non-biogenic sample from Spain has a larger combined cation radius, but has slightly smaller cell parameters than the biogenic aragonite nacre, which is unusual. The larger cell parameters for ammonite nacre are probably the result of the stress/strain in the biogenic aragonite. Data for aragonite samples from various studies are displayed in Fig. 3. Except for a fresh water snail,25 a gastropod,8–10 and a bivalve,9 most biogenic aragonite samples have larger cell parameters compared to non-biogenic samples (Fig. 3).
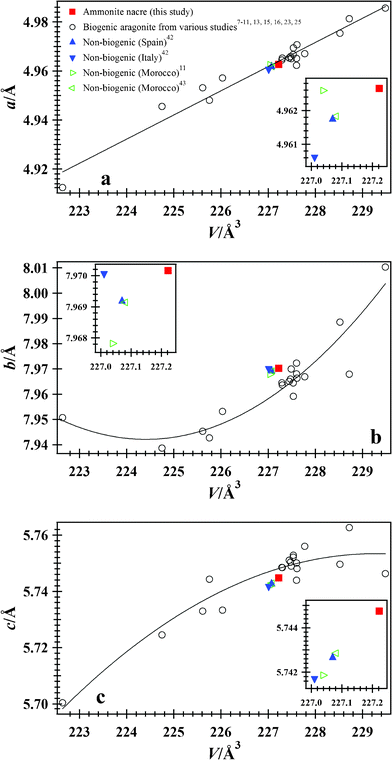 | ||
| Fig. 3 Cell parameters vs. V: (a) a parameter, (b) b parameter, and (c) c parameter for biogenic aragonite from ammonite nacre, and including data from various studies;7–11,13,15,16,23,25 solid line is a least-squares fit to these biogenic aragonite data. Data for non-biogenic aragonite samples from Cuenca, Spain,42 Tuscany, Italy,42 and Sefrou, Morocco11,43 are also included for comparison. Error bars from this study are smaller than the symbols. Cell parameters for ammonite nacre are similar to other non-biogenic aragonite samples (see inserts). For the biogenic aragonite samples, the acell parameter varies linearly with volume, whereas the b and ccell parameters show non-linear behavior with some scatter. | ||
The aplanarity of the CO3 group is the distance of the C atom from the plane formed by the three O atoms. In calcite, the CO3 groups are planar, but in aragonite they are non-planar. For isostructural aragonite-type carbonates, as the substituting cation size increases, the CO3 group becomes more symmetrical and less aplanar from CaCO3 to SrCO3 to BaCO3.42 Biogenic aragonite samples are closer to planarity than non-biogenic samples.11 Using a formula11 for aplanarity, [ΔZC–O1 (Å) = |zC − zO1|c], ammonite nacre has a value of 0.0626 Å, which is a little higher than 0.0603 Å for the non-biogenic aragonite from Spain (Table 3). A value of 0.056 Å was obtained for a non-biogenic aragonite from Serfou, Morocco,11 and biogenic aragonite samples had values as low as 0.031 Å.11 Using the above formula for aplanarity, values ranging from 0.00029 to 0.111 Å were obtained for gastropod and bivalve shells.8–10 Shell layers within the same gastropod had different aplanarity values (0.00029 Å for the outer comarginal, 0.04335 Å for the radial, and 0.1066 Å for the comarginal layer from top to bottom).8–10 It seems that the geometry of the CO3 group for the various biogenic aragonite samples is different and is probably a result of the different amounts of stress/strain in the un-annealed samples used in the various studies [shells from gastropod, bivalve, cephalopod – coleoidea, and cephalopod – ammonoidea (this study)].
The average <Ca–O> and <C–O> distances, and the average <O–C–O> angle for ammonite nacre [2.5281(3) and 1.2871(6) Å, and 119.94(8)°] and the non-biogenic aragonite sample from Spain [2.5278(2) and 1.2864(4) Å, and 119.95(4)°] are very similar (Table 4, Fig. 4). Very small positive distortions for the average bond distances are observed between biogenic (ammonite nacre) and non-biogenic (Spain) aragonite (Δ<Ca–O>/<Ca–O> = 0.0001 and Δ<C–O>/<C–O> = 0.0005). Aragonite samples from bivalve and gastropod shells have average <Ca–O> distances varying from 2.5278(3) to 2.529(1) Å and average <C–O> distances varying from 1.282(1) to 1.2871(3) Å.11 Other biogenic aragonite samples have a larger variation for these average distances.8–10 Bivalve shells have a small average <C–O> distance = 1.242 Å, and gastropod shells have average <C–O> distances varying between 1.260 to 1.288 Å, even for different layers within the same gastropod.8–10 Average <Ca–O> distances vary between 2.524 to 2.543 Å.8–10 Therefore, the <C–O> and <Ca–O> distances probably reflect differences in stress/strain relations in biogenic aragonite from various organisms.
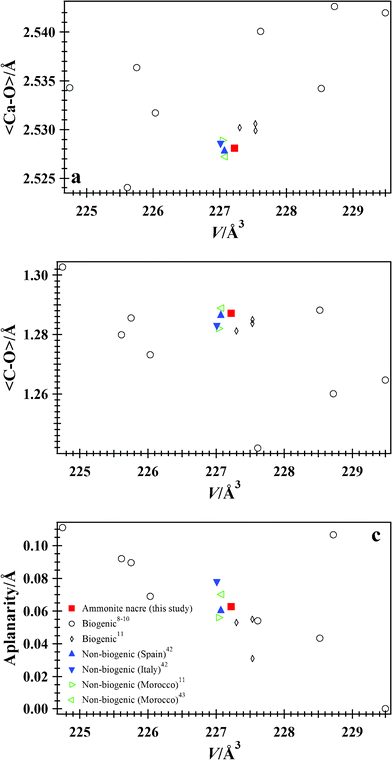 | ||
| Fig. 4 (a) <Ca–O>, (b) <C–O>, and (c) aplanarity vs. V for biogenic aragonite from ammonite nacre, and including data from various studies.8–11 Data for non-biogenic aragonite samples from Cuenca, Spain,42 Tuscany, Italy,42 and Sefrou, Morocco,11,43 are also included for comparison. Error bars from this study are smaller than the symbols. Bond distances and aplanarity for ammonite nacre are similar to a non-biogenic aragonite from Spain. | ||
The C–O1 and Ca–O2 distances showed substantial variations for biogenic samples.11 These distances between biogenic (ammonite nacre) and non-biogenic (Spain) aragonite (ΔC–O1/C–O1 = −0.3% and ΔCa–O2/Ca–O2 = 0 to 0.1%) are nearly unchanged, indicating that the two aragonite structural parameters are similar.
In conclusion, this study presents an accurate structure of aragonite from an ammonite nacre that has minimal unit-cell distortions and bond-distance variations, and is very similar to a non-biogenic aragonite from Spain. Does this imply that the age of biogenic samples has a role to play in structural distortions? Ammonites are excellent index fossils. The sample used in this study is from the Cretaceous period and is over 65 million years old. Another factor to consider is the effect of diagenesis on the structural distortions of bio-composites over geological time. The well-preserved ammonite sample used in this study is diagenetically unmodified based on the absence of secondary mineralization, the small amount of calcite, and the low Mg/Ca ratio. Nature-inspired biominerals continue to marvel researchers and further investigation on a variety of biogenic samples is needed to understand the structural distortions in aragonite.
Acknowledgements
The two reviewers and the editor T.N. Guru Row are thanked for their comments that helped improve this manuscript. R. Marr is thanked for his help with the microprobe analyses. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. This work was supported by a Discovery grant from the National Science and Engineering Research Council of Canada and an Alberta Ingenuity New Faculty Award.References
- L. Addadi and S. Weiner, Nature, 1997, 389, 912 CrossRef CAS.
- R. T. Chiroff, R. A. White, E. W. White, J. N. Weber and D. Roy, J. Biomed. Mater. Res., 1977, 11, 165 CrossRef CAS.
- S. Kamat, X. Su, R. Ballarini and A. H. Heuer, Nature, 2000, 405, 1036 CrossRef CAS.
- S. I. Stupp and P. V. Braun, Science, 1997, 277, 1242 CrossRef CAS.
- F Ren, X Wan, Z Ma and J Su, Mater. Chem. Phys., 2009, 114, 367 CrossRef CAS.
- F. Song, A. K. Soh and Y. L. Bai, Biomaterials, 2003, 24, 3623 CrossRef CAS.
- E. Zolotoyabko and J. P. Quintana, J. Appl. Crystallogr., 2002, 35, 594 CrossRef CAS.
- D. Chateigner, S. Ouhenia, C. Krauss, M. Belkhir and M. Morales, Nucl. Instrum. Methods Phys. Res., Sect. B, 2010, B268, 341 CrossRef.
- D. Chateigner, S. Ouhenia, C. Krauss, C. Hedegaard, O. Gil, M. Morales, L. Lutterotti, M. Rousseau and E. Lopez, Mater. Sci. Eng., A, 2010, A528, 37–51 CrossRef CAS.
- S. Ouhenia, D. Chateigner, M. A. Belkhir and E. Guilmeau, J. Struct. Biol., 2008, 163, 175–184 CrossRef CAS.
- B. Pokroy, J. S. Fieramosca, R. B. Von Dreele, A. N. Fitch, E. N. Caspi and E. Zolotoyabko, Chem. Mater., 2007, 19, 3244 CrossRef CAS.
- D. Zhou, R. A. Metzler, T. Tyliszczak, J. H. Guo, M. Abrecht, S. N. Coppersmith and P. U. P. A. Gilbert, J. Physical Chem., 2008, B112, 13128 Search PubMed.
- B. Pokroy, A. N. Fitch, P. L. Lee, J. P. Quintana, E. N. Caspi and E. Zolotoyabko, J. Struct. Biol., 2006, 153, 145 CrossRef CAS.
- B. Pokroy, A. N. Fitch and E. Zolotoyabko, Cryst. Growth Des., 2007, 7, 1580 CAS.
- J. Stolarski, R. Przenioslo, M. Mazur and M. Brunelli, J. Appl. Crystallogr., 2007, 40, 2 CrossRef CAS.
- B. Pokroy, J. P. Quintana, E. N. Caspi, A. Berner and E. Zolotoyabko, Nat. Mater., 2004, 3, 900 CrossRef CAS.
- W.D. Carlson, Mineral. Soc. Am. Rev. Mineral., 1983, 11, 191 CAS.
- S. A. T. Redfern, E. Salje and A. Navrotsky, Contrib. Mineral. Petrol., 1989, 101, 479 CrossRef CAS.
- E. Salje and K. Viswanathan, Contrib. Mineral. Petrol., 1976, 55, 55 CrossRef CAS.
- S. M. Antao and I. Hassan, Can. Mineral., 2010, 48, 1225 CrossRef CAS.
- J. L. Irigaray, H. Oudadesse, H. El Fadl, T. Sauvage, G. Thomas and A. M. Vernay, J. Therm. Anal., 1993, 39, 3 CrossRef CAS.
- A. Lucas, M. Mouallem-Bahout, C. Carel, J. Gaudé and M. Matecki, J. Solid State Chem., 1999, 146, 73 CrossRef CAS.
- J. E. Parker, S. P. Thompson, A. R. Lennie, J. Potter and C. C. Tang, CrystEngComm, 2010, 12, 1590 RSC.
- N. Passe-Coutrin, Ph. N'Guyen, R. Pelmard, A. Ouensanga and C. Bouchon, Thermochim. Acta, 1995, 265, 135 CrossRef CAS.
- N. Udomkan, P. Limsuwan and Y. Chaimanee, Int. J. Mod. Phys. B, 2006, B20, 1097 CrossRef.
- D. Wardecki, R. Przenioslo and M. Brunelli, CrystEngComm, 2008, 10, 1450 RSC.
- X. Sheng, J. Chen, Y. Cai, Y. Chen and J. Ji, Chin. Sci. Bull., 2005, 50, 891 CrossRef CAS.
- F. Leutwein and R. Waskowial, N. Jahrb. Mineral., 1962, Abh. 99, 45 Search PubMed.
- J. N. Weber, Geochim. Cosmochim. Acta, 1973, 37, 2173 CrossRef CAS.
- G. Leone, F. Bonadonna and G. Zanchetta, Palaeogeogr., Palaeoclimatol., Palaeoecol,, 2000, 163, 115 CrossRef.
- X. Sheng, J. Chen, J. Ji and Y. Sui, Geochem. J., 2005, 39, 61 CrossRef CAS.
- J.A. Speer, Mineral. Soc. Am. Rev. Mineral., 1983, 11, 145 Search PubMed.
- B. Burchardt and S. Weiner, Sedimentology, 1981, 28, 423 CrossRef.
- J. Veizer and J. Wendt, N. Jahrb. Geol. Paleont. Monat., 1976, 1976, 558 Search PubMed.
- A. Hallam and N. B. Price, Nature, 1966, 212, 25 CrossRef CAS.
- S.M. Antao, I. Hassan, J. Wang, P.L. Lee and B.H. Toby, Can. Mineral., 2008, 46, 1501 CrossRef CAS.
- P.L. Lee, D. Shu, M. Ramanathan, C. Preissner, J. Wang, M.A. Beno, R.B. Von Dreele, L. Ribaud, C. Kurtz, S.M. Antao, X. Jiao and B.H. Toby, J. Synchrotron Radiat., 2008, 15, 427 CAS.
- J. Wang, B.H. Toby, P.L. Lee, L. Ribaud, S.M. Antao, C. Kurtz, M. Ramanathan, R.B. Von Dreele and M.A. Beno, Rev. Sci. Instrum., 2008, 79, 085105 CrossRef.
- H.M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65 CrossRef CAS.
- A.C. Larson and R.B. Von Dreele, Los Alamos National Laboratory Report, 2000, LAUR 86–748 Search PubMed.
- B.H. Toby, J. Appl. Crystallogr., 2001, 34, 210 CrossRef CAS.
- S.M. Antao and I. Hassan, Can. Mineral., 2009, 47, 1245 CrossRef CAS.
- E. N. Caspi, B. Pokroy, P. L. Lee, J. P. Quintana and E. Zolotoyabko, Acta Crystallogr., Sect. B: Struct. Sci., 2005, B61, 129 CAS.
- R.D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, A32, 751 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
