Thiourea dioxide promoted cobalt-catalyzed hydrolysis of imines: dual activationvia organocatalysis and metal catalysis
Subodh
Kumar
,
Suman L.
Jain
* and
Bir
Sain
*
Chemical Sciences Division, Indian Institute of Petroleum (Council of Scientific and Industrial Research), Mohkampur, Dehradun, 248005, India. E-mail: suman@iip.res.in; birsain@iip.res.in; Fax: +91-135-2660202; Tel: +91-135-2525901
First published on 22nd November 2011
Abstract
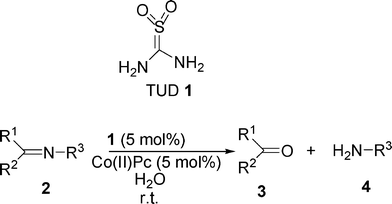
An unusual promoting effect of thiourea dioxide in the cobalt catalyzed hydrolysis of imines to the corresponding carbonyl compounds and amines under mild conditions is described. In the absence of thiourea dioxide the reaction does not proceed and unreacted imine is recovered at the end. This is the first report that describes the promoting effect of thiourea dioxide in metal catalyzed hydrolysis of imines without using acidic or basic conditions.
In recent years, the concept of combining transition metal catalysis and organocatalysis has attracted increasing attention in the synthetic community in order to develop increasingly efficient transformations.1 Although a variety of remarkably efficient catalytic systems have been developed so far by using the unique concept of dual catalysis, and their applications in various transformations have been reported,2 more of them undoubtedly remain to be explored. Metal catalyzed hydrolysis of imines to amines is an important synthetic transformation which is extensively used in the synthesis of bioactive complex molecules.3 This reaction mainly requires strong acidic or basic conditions, which are undesirable for valuable syntheses of several complex molecules. However, to the best of our knowledge, the catalytic hydrolysis of iminesvia the combined activation of a metal catalyst and an organocatalyst under neutral reaction conditions still remains unexplored. Thiourea dioxide (TUD) 1,4 easily prepared by the oxidation of thiourea with hydrogen peroxide is a well known reducing agent which has been extensively used for the reduction of various substrates such as organosulfur compounds,5nitro, azo, and azoxy compounds to amines,6quinones to hydroquinones7 and for the deoxygenation of α,β-epoxy ketones,8 and heteroatomic N-oxides9 under alkaline conditions. Thiourea dioxide, owing to its strong hydrogen bonding ability, has great potential to activate many functionalities through hydrogen bonding and can be established as a promising organocatalyst for organic reactions.10 During our ongoing research work, we aimed to use thiourea dioxide as a reducing agent for the conversion of imines to secondary amines in the presence of a base. However, the conversion of N-methyl benzylidene amine 2a into its corresponding amine failed and we could recover only the original substrates at the end. A recent report by Pogorelova et al.11 captured our interest and we focused our attention towards using the dual activation approach via using organocatalysis and metal catalysis to perform the reaction. Accordingly, we carried out the reaction using combined catalysis by using thiourea dioxide (5 mol%) and cobalt(II) phthalocyanine (5 mol%) as catalysts in aqueous medium at room temperature. Surprisingly, the reaction occurred faster and resulted in the almost quantitative conversion of imine 2a into benzaldehyde 3a and aniline 4a as the ultimate reaction products (Scheme 1).
 | ||
| Scheme 1 Combined metal and organocatalyst promoted hydrolysis of imines. | ||
After completion of the reaction, the reaction products were extracted from the aqueous layer using diethyl ether. The combined organic layers were dried and concentrated under reduced pressure to yield products. The remaining aqueous layer containing catalyst and thiourea dioxide was reused as such for the next reaction. The recycling of the developed catalytic system was checked for six runs, showing consistent activity and importantly no leaching was observed during this course. The developed method is advantageous in many ways, such as it is mild due to the absence of strong acidic or basic conditions, the catalyst is recyclable and water is used as the reaction medium. In order to confirm the role of TUD, we also performed the reaction without TUD under otherwise similar reaction conditions. The reaction did not take place and only the original substrate could be recovered even after prolonged reaction time (Table 1, entry 1).
| Entry | Substrate 2 | Product 3, 4 | Time/h | Conversionb/yieldc (%) | TOF/h−1 |
|---|---|---|---|---|---|
| a Reaction conditions: imine (1 mmol), TUD (0.1 mmol), CoPc (1 mol%), water (3 ml) at room temperature. b Determined by GC-MS. c Isolated yield. d Without TUD. e Without Co-catalyst. | |||||
| 1 |
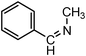
|
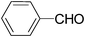
|
8.0d | — | — |
| 12.0e | — | — | |||
| 3.5 | 100/98 | 28.57 | |||
| 2 |
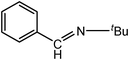
|
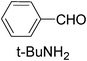
|
4.0 | 100/98 | 25.00 |
| 3 |
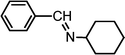
|
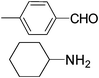
|
3.5 | 96/92 | 27.42 |
| 4 |
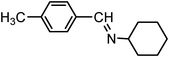
|
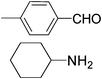
|
3.0 | 94/92 | 31.33 |
| 5 |
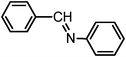
|
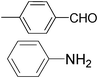
|
6.0 | 80/78 | 13.33 |
| 6 |
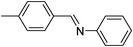
|
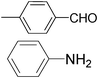
|
5.5 | 85/81 | 15.45 |
| 7 |
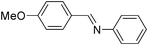
|
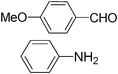
|
5.0 | 75/72 | 15.00 |
| 8 |
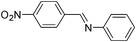
|
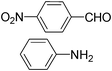
|
6.0 | 30 | 5.00 |
| 9 |
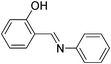
|
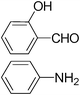
|
2.5 | 80/78 | 32.00 |
Furthermore to evaluate the effect of the CoPc catalyst, we studied the hydrolysis of N-methyl benzylidene amine by using TUD alone in the absence of catalyst under similar reaction conditions. The reaction did not take place and we could recover the original substrate at the end of the reaction (Table 1, entry 1). Similarly there was no reaction on using Co(II)Pc alone in the absence of TUD. These findings proved that the presence of the CoPc catalyst and thiourea dioxide are essential for this transformation. The conversion and selectivity of the reaction products were determined by GC-MS, however the identities of the products were established by comparing the spectral data with known compounds.
Furthermore, we extended the scope of the reaction by performing the hydrolysis of various imines under the described reaction conditions.12 Representative examples and their results are summarized in Table 1. The reaction occurred efficiently with the substrates having N-alkyl groups and provided almost quantitative yields of the corresponding carbonyl compounds and amines under similar reaction conditions. Imines having N-aromatic groups were found to be less reactive from the reaction-time point of view. Similarly, Schiff bases derived from salicylaldehyde and aromatic amines were efficiently converted to the corresponding hydrolyzed products using this protocol.
Although the exact mechanism of the reaction is not known at this stage, the probable mechanism may involve dual activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of imine and water molecules by the metal cation and thiourea dioxide through hydrogen bonding as shown in Scheme 2. This non-bonding association activates the water nucleophile towards attack on the imine bond, resulting in the cleavage of the imine to give the corresponding aldehyde and amine.
N bond of imine and water molecules by the metal cation and thiourea dioxide through hydrogen bonding as shown in Scheme 2. This non-bonding association activates the water nucleophile towards attack on the imine bond, resulting in the cleavage of the imine to give the corresponding aldehyde and amine.
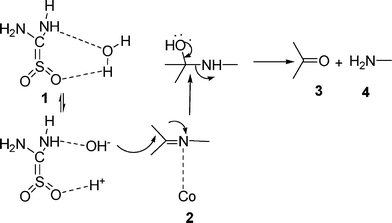 | ||
| Scheme 2 Probable mechanistic pathway. | ||
In summary we have described an efficient approach using a new concept of combined catalysis via transition metal catalysis and organocatalysis for the hydrolysis of imines using cobalt(II) phthalocyanine and thiourea dioxide as catalysts in aqueous medium. The developed protocol is advantageous in many respects, such as it does not need strong acidic or basic conditions, the catalyst is recyclable and it affords excellent product yields in short reaction times. To the best of our knowledge this is the first report on the combined activation of a metal catalyst and an organocatalyst for the hydrolysis of imines. The scope and limitations of the developed catalytic system for other transformations are currently under investigation.
Acknowledgements
We thank Director IIP for his kind permission to publish these results. SLJ kindly acknowledges DST, New Delhi for funding. SK acknowledges CSIR, New Delhi for his fellowships.References
- (a) T. Masahiro, Curr. Org. Chem., 2011, 15, 2227 Search PubMed; (b) Z. H. Shao and H. B. Zhang, Chem. Soc. Rev., 2009, 38, 2745 RSC and references cited therein.
- (a) J. Barluenga, N. Quinones, M. P. Cabal, F. Aznar and C. Valdes, Angew. Chem., Int. Ed., 2011, 50, 2350 CAS; (b) I. S. Santoro, F. Himo and A. Cordova, Adv. Synth. Catal., 2011, 353, 245 CrossRef CAS; (c) A. S. K. Hashmi and C. Hubbert, Angew. Chem., Int. Ed., 2010, 49, 1010 CrossRef CAS.
- (a) D. V. Prabhu, K. Lalitha and N. B. Laxmeshwar, J. Indian Chem. Soc., 2004, 81, 1103 Search PubMed; (b) P. Marcazzan, B. O. Patrick and B. R. James, Inorg. Chem., 2004, 43, 6838 CrossRef CAS; (c) K. C. Vichare, S. S. Shetye and V. D. Bhosale, Asian J. Chem., 2004, 16, 343 Search PubMed; (d) D. V. Prabhu and N. B. Laxmeshwar, Asian J. Chem., 1997, 9, 703 Search PubMed.
- O. Ohura, O. Fujimoto, U.S. Patent 4,233,238, 1980 Search PubMed.
- C. Daneault and C. Leduc, Cellul. Chem. Technol., 1994, 28, 205–217 Search PubMed.
- (a) S. Sambher, C. Basker, R. S. Dhillon Borgogno, G. Colonna, S. Fornasier and R. Renu, Synthesis, 1975, 529–530 Search PubMed; (b) J. Drabowicz and M. Mikolajczyk, Synthesis, 1978, 542 Search PubMed.
- R. B. dos Santos, T. J. Brocksom and U. Brocksom, Tetrahedron Lett., 1997, 38, 765–748 CrossRef CAS.
- R. Balicki and U. Chmielowiec, Monatsh. Chem., 2000, 131, 1105–1107 Search PubMed.
- P. H. Gore, Chem. Ind. (London, U. K.), 1954, 1355 Search PubMed.
- (a) S. Kumar, S. Verma, S. L. Jain and B. Sain, Tetrahedron Lett., 2011, 52, 3393 Search PubMed; (b) S. Verma, S. L. Jain and B. Sain, Tetrahedron Lett., 2010, 51, 6897 CrossRef CAS.
- A. S. Pogorelova, S. V. Makarov, E. S. Ageeva and R. Silagi Dumitresku., Russ. J. Phys. Chem. A, 2009, 83, 2050–2053 Search PubMed.
- General experimental procedure for the hydrolysis of imines: thiourea dioxide was prepared by following the literature procedure.4Co(II)phthalocyanine was synthesized according to the reported method with some modifications.13 Into a stirred mixture of imine (1 mmol), TUD (5 mol%, 0.05 mmol) in water (3 ml) was added Co(II)phthalocyanine (5 mol%, 0.05 mmol). The resulting mixture was stirred at room temperature for the time reported in Table 1. Completion of the reaction was analysed by TLC. After completion of the reaction, the products were separated by extraction with diethyl ether. The combined organic layers were washed with water and brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give corresponding products. The recovered aqueous layer containing Co(II) phthalocyanine and thiourea dioxide was reused for subsequent experiments. The conversion and selectivity were determined by GC-MS and the identity of the selected products was established by 1H NMR analysis.
- J. N. Weber and D. H. Busch, Inorg. Chem., 1965, 4, 469 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
