Supercritical fluid extraction (SFE) as an effective tool in reducing auto-oxidation of dried pine sawdust for power generation†
Mehrdad
Arshadi
*a,
Andrew J.
Hunt
b and
James H.
Clark
b
aUnit of Biomass Technology and Chemistry, Swedish University of Agricultural Sciences, SE-90183 Umeå, Sweden. E-mail: mehrdad.arshadi@slu.se; Tel: +46 90 7868773
bDepartment of Chemistry, The University of York, Heslington, York, UK. E-mail: andrew.hunt@york.ac.uk; Fax: +44 (0)1904 432705; Tel: +44 (0)1904 322546
First published on 3rd January 2012
Abstract
Supercritical carbon dioxide was an ideal solvent for the extraction of fatty and resin acids from pine sawdust. This green extraction process significantly reduces the potential for uncontrolled auto-oxidation within this valuable fuel, thereby removing the risk of combustion during storage and processing. Experimental design was utilised to maximize the yield of the extractives. Within the experimental conditions investigated (74–250 bar and 40–60 °C), higher pressures and lower temperatures gave preferentially high extraction yields, with 97% of the fatty and resin acids in 2 h at 250 bar, 40 °C, 40 g min−1 and with 10% ethanol co-solvent. The calorific value of the pelletized sawdust was not significantly affected by the extraction process and the resulting extracts have demonstrated a significant potential for conversion to a biodiesel liquid fuel or higher value chemicals.
Introduction
The use of renewable resources to generate fuels and multiply high value chemical products through clean chemical technologies is an intrinsic part of an integrated bio-refinery.1–3 Soft wood such as pine or spruce are one such resource that are relatively inexpensive, available in large volumes, and does not compete with food applications. Pelletized pine or spruce is commonly used as fuel in homes and power stations throughout Sweden, where annual production of wood pellets is 1.7 million tons.4Significant problems are associated with the auto-oxidation of fatty and resin acids during storage and processing of the biomass.5–8 This can lead to uncontrolled heating and spontaneous combustion. Fatty acid oxidation can also form aldehydes that give rise to odour problems and a negative impact on the indoor environment.5–6 Feedstock properties can potentially be improved by removal of fatty and resin acids from fresh pine sawdust prior to pelletization. The utilisation of green technologies to extract fatty and resin acids, in addition to phenolics, sterols and terpenes can be utilised as part of an integrated bio-refinery.3 The extractives from softwood contain so called tall oil (up to 90% fatty and resin acids), a valuable product which can be used as raw material in biodiesel production.9–10
The extraction of plant lipids including fatty and resin acids has traditionally been conducted with volatile organic solvents such as hexane, acetone, petroleum ether, chloroform, dichloromethane and benzene.11Extractions with these solvents are typically unselective and concerns over their environmental and toxicological effects have prompted the use of greener solvent alternatives.12 One such alternative, supercritical carbon dioxide (scCO2) is non-flammable, relatively inexpensive, has low toxicity, a low critical point, readily available in high purity and large quantities as a by-product of industries such as fermentation, combustion or ammonia synthesis.13–14 Product isolation post extraction from scCO2, is achieved to total dryness simply by pressure release and evaporation. The solvent properties can be changed as a function of pressure and temperature.15 The addition of polar modifiers (such as ethanol) to scCO2 improves extraction yields.16 Supercritical extracts of both hard and softwood pulps contain fatty acids methyl esters and fatty acids, this is in stark contrast to Soxhlet extracts where only fatty acids were extracted.17 Due to the highly penetrating properties of supercritical fluids, they have been used commercially for the impregnation of wood.18
Herein, we demonstrate that scCO2 is an ideal solvent for the extraction of lipophilic compounds from softwood sawdust. This green extraction process reduces the potential for this valuable fuel to undergo uncontrolled auto-oxidation, which can result in spontaneous combustion during storage. Pine sawdust was utilised as a model substance to investigate the extraction from woody biomasses. Experimental designs have been used to maximize the yield of the extractives by optimization of different extractions parameters over the pressure of 74–250 bar and 40–60 °C.
Materials and methods
Fresh sawdust of pine (Pinus sylvestris L.) was obtained from Bioenergi i Luleå AB pellet mill in Luleå, Sweden and a local sawmill in the Umeå region of northeastern Sweden. The sawdust has been dried according to a standard method.19 The sawdust was milled by Brabender Wiley mill (Brabender GmbH & Co. KG, Duisburg, Germany) equipped with a 1 mm screen. The material was kept in sealed gastight plastic bags at +2 °C before the analyses.Soxhlet extractions
Fatty and resin acids in wood pellets were extracted in a Soxhlet apparatus (Universal Extraction System B-811 from Büchi Labortechnik AG, Flawil, Switzerland) with a mixture of petroleum ether (bp 40 to 60 °C) and acetone (90 to 10 v/v) as the solvent for 1 h.20Supercritical fluid extractions
scCO2 extraction was performed using a Thar SFE 500 extractor, with supercritical fluid grade carbon dioxide (99.99%). A typical extraction was conducted as follows:The 500 mL extraction vessel was charged with 50.0 g of sawdust. The extraction vessel was heated to the required temperature and allowed to equilibrate for 5 min. The automated back pressure regulator was set to the required pressure and CO2 was allowed to enter the extraction vessel. The required pressure was obtained through the use of an internal pump at the rate of 40 g min−1. Once the required pressure was reached, the CO2 passed into the collection vessel and the lipid isolated. On completion the system was depressurised over a period of 20 min.
Fatty and resin acids analyses
The extracts from Soxhlet and/or SFE method were weighed carefully and, after derivatization using bis(trimethylsilyl)trifluoroacetamide (Fluka, Sigma-Aldrich, Buchs SG, Schweiz) and trimethylchlorosilane (Fluka, Sigma-Aldrich, Buchs SG, Schweiz) at 70 °C, were analyzed by GC-MS according to a previously reported method.6,20Co-solvent application
Ethanol was utilized as co-solvent in the SFE process. Other parameters were kept constant at optimum levels.Experimental design and statistical analysis
The software used for the experimental design, and statistical calculations was MODDE 9.0.0.0 (Umetrics Inc, Kinnelon, NJ, USA). All significance tests were done at a 95% level.Results and discussion
Supercritical CO2 fluid extraction
Supercritical fluid extractions (SFE) of the samples were performed according to an experimental design shown in Table 1. The experiments for this study were designed by using response surface methodology (RSM). The most efficient extraction was the experiment number 1 (Table 1) and indicated that pressure had the greatest effect on extraction yield (Fig. 1). The maximum yield of extractives was obtained with 40 °C and 250 bar. Under these conditions it was possible to extract 77% of fatty and resin acids from the sawdust. Three extractions were conducted at the central conditions of the experimental design; this demonstrated good reproducibility with an average total lipid yield of 0.96% (±0.04). Compositions of extractions 1–5 were analysed and are presented the in the ESI (Table S1).†| Extraction | Temperature (°C) | Pressure (bar) | %Yield (total extracted from biomass) | %Fatty and resin acid extracted |
|---|---|---|---|---|
| a Conducted three times to demonstrate consistency of extraction. b Average of three repeats at these conditions. | ||||
| 1 | 40 | 250 | 1.556 | 77.1 |
| 2 | 60 | 74 | 0.029 | 16.2 |
| 3 | 60 | 250 | 0.846 | 46.3 |
| 4 | 40 | 74 | 0.099 | 15.5 |
| 5a | 50 | 162 | 0.96 (±0.04)b | 54.2 |
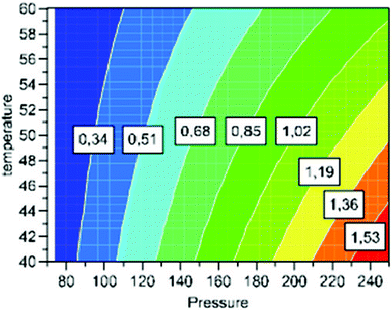 | ||
| Fig. 1 Contour diagram of optimal extraction conditions. | ||
The sawdust samples post extraction with scCO2 were analysed for remaining fatty and resin acids within the sawdust (Table 2). This was achieved by Soxhlet extraction of the sawdust residues. The fatty and resin acid analyses of the Soxhlet extracted residues indicated that the scCO2 extractions were incomplete and significant proportions of these molecules remained within the sawdust. Post extraction at 250 bar and 40 °C, a total of 1263 μg g−1 of fatty and resin acids remained within the sample. This is a considerable reduction compared to the untreated sawdust which contained a total of 5529 μg g−1 (as measured by Soxhlet extraction).
| Compound | μg g−1 remaining fatty and resin acids on sawdusta | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Raw material | |
| a μg g−1 remaining fatty and resin acids in sawdust post extraction was calculated by conducting a Soxhlet extraction on the residues with a mixture of petroleum ether (bp 40 °C to 60 °C) and acetone (90 to 10 v/v) as the solvent for 1 h. | ||||||
| Octanoic acid | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.6 |
| Nonanoic acid | 1.5 | 2.1 | 3.1 | 3.8 | 2.2 | 7.4 |
| Decanoic acid | 0.0 | 1.6 | 1.3 | 1.3 | 0.0 | 3.4 |
| Dodecanoic acid | 1.7 | 2.3 | 3.0 | 3.0 | 2.7 | 4.6 |
| Tridecanoic acid | 0.0 | 1.1 | 0.7 | 1.3 | 0.9 | 2.0 |
| 2-Undecenoic acid | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Tetradecanoic acid | 3.0 | 6.4 | 7.3 | 8.4 | 4.5 | 9.8 |
| Pentadecanoic acid | 4.1 | 8.0 | 7.4 | 9.7 | 5.1 | 11.5 |
| Pentadecanoic acid, anteiso | 2.6 | 4.1 | 4.3 | 4.6 | 2.6 | 5.2 |
| Hexadecanoic acid, anteiso | 3.5 | 8.6 | 7.5 | 9.4 | 5.4 | 11.2 |
| 9-Hexadecenoic acid | 0.0 | 7.1 | 7.8 | 8.7 | 4.1 | 9.0 |
| Hexadecanoic acid | 47.7 | 98.1 | 78.3 | 102.8 | 67.4 | 123.8 |
| Heptadecanoic acid, anteiso | 23.4 | 58.4 | 44.7 | 59.3 | 36.0 | 70.5 |
| 9-Octadecenoic acid | 10.8 | 0.0 | 0.0 | 26.6 | 12.7 | 32.9 |
| 9,12,15-Octadecatrienoic acid | 22.5 | 105.8 | 62.5 | 108.0 | 45.6 | 122.6 |
| 9,12-Octadecadienoic acid | 47.8 | 263.4 | 139.8 | 269.4 | 109.6 | 293.9 |
| 11-Octadecenoic acid | 235.8 | 648.0 | 462.3 | 656.9 | 408.2 | 733.5 |
| Octadecanoic acid | 13.9 | 44.4 | 16.4 | 46.7 | 21.1 | 0.0 |
| Pimaric acid | 61.9 | 399.2 | 193.4 | 400.4 | 145.9 | 534.4 |
| Pimaric acid, isomer | 9.0 | 72.2 | 35.5 | 72.5 | 25.2 | 86.0 |
| Isopimaric acid | 41.3 | 278.8 | 137.9 | 256.0 | 103.7 | 303.9 |
| Abietic acid | 0.0 | 291.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| Dehydroabietic acid | 466.1 | 1861.3 | 1289.3 | 1866.9 | 1093.3 | 2214.1 |
| Abietic acid, isomer | 64.4 | 0.0 | 88.8 | 299.0 | 93.8 | 351.4 |
| 7-Oxodehydroabietic acid | 197.7 | 428.9 | 347.1 | 412.2 | 311.3 | 531.5 |
| 11-Eicosenoic acid | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Docosanoic acid | 0.0 | 16.0 | 9.2 | 16.2 | 7.3 | 18.8 |
| Dehydroabietic acid, isomer | 4.2 | 21.9 | 17.4 | 23.7 | 16.1 | 14.1 |
| Arachidonic acid | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Undecanoic acid | 0.0 | 0.0 | 0.0 | 0.0 | 8.8 | 0.0 |
| Tricosanoic acid | 0.0 | 3.4 | 1.7 | 3.6 | 1.7 | 4.8 |
| 9-Octadecenoic acid | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 26.3 |
| Total (the sum) | 1263 | 4633 | 2967 | 4670 | 2535 | 5529 |
Experimental design was used to optimise temperature and pressure in order to obtain maximum extraction yield from the sawdust within the experimental parameters selected (74–250 bar and 40–60 °C). The results indicated that parameters such as time and %co-solvent also required investigation to extract all of the fatty and resin acids from the sawdust. Therefore, it was necessary to perform a second set of experiments, with additional extractions parameters.
Application of co-solvent
The second set of experiments investigated the effect of extraction duration and also the addition of co-solvent. For these experiments a new sample of pine was used in which the average total amount of fatty and resin acids in the wood was 7569 μg g−1 (as measured by Soxhlet extraction). After extraction over 2 h about 3799 μg g−1 of total fatty and resin acids are left and the yield is less than 50% (Fig. 2A). After 6 h of extraction the yield was 85.5% and after 8 h of extraction the yield is increased to 92.8%.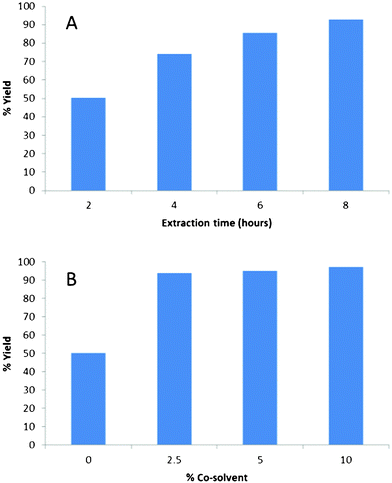 | ||
| Fig. 2 (A) Graph depicting %yield of fatty and resin acid extracted over 2, 4, 6 & 8 h. (B) Graph depicting %yield of fatty and resin acid extracted with 0, 2.5, 5 & 10% co-solvent (ethanol). | ||
By addition of a more polar solvent such as ethanol to scCO2, it is possible to more effectively extract the fatty and resin acids. The addition of 2.5% of ethanol increased the yield to 93.6% in just 2 h (Fig. 2). When the amount of ethanol was 10%, the yield of fatty and resin acids increased to 97% after 2 h of extraction. There are several advantages to the use scCO2 extraction compared to traditional Soxhlet extraction, including reduced toxicity and greater selectivity. Industrial scale use of scCO2 should be applicable within the biorefinery as it has already been employed commercially for hop extraction, decaffeination of coffee and dry cleaning.13 One drawback of scCO2 is the non-polar nature of this solvent; this can be resolved by the addition of a co-solvent such as ethanol, which can also be classed as a natural or bio-derived solvent. The calorific value of the pelletized sawdust was not significantly affected by the extraction process and the resulting extracts have demonstrated a significant potential for conversion to a biodiesel liquid fuel.9–10
It has been observed that wood pellets made from pine sawdust with reduced proportions of fatty and resin acids have improve durability and bulk density compared to pellets made from fresh pine sawdust.8 As such, extraction of these compounds viasupercritical fluid extraction demonstrates great potential as a method to improved properties of pelletized wood fuels.
Conclusions
To summarize, it is obvious that 2 h extractions using scCO2 with no co-solvent are not long enough to extract all fatty and resin acids in the material but, addition of 10% ethanol to the CO2 solvent improves the extraction efficiency to 97%. It is also possible to get a high yield (around 93%) by extracting with only CO2 over a longer time (8 h). Industrial scale supercritical extractions are a reality and as such could be applied to applications such as fatty and resin acid extraction from sawdust prior to pellet production. The extracted fatty and resin acids as a value added product can be used for biodiesel production. Sunpine Ltd in cooperation with Preem oil company already produces biodiesel from tall oil since May 2010.Acknowledgements
The authors would like to acknowledge the financial support of the Swedish Energy Agency, the Swedish Pellet Production Industry, the British Embassy in Sweden, the Swedish University of Agricultural Sciences. Thanks to Bioenergi i Luleå AB pellet mill in Luleå, Sweden for supply of the fresh pine sawdust.References
- M. Arshadi and A. Sellstedt, Production of Energy from Biomass, in Introduction to Chemicals from Biomass, ed. J. H. Clark and F. E. I. Deswarte, John Wiley & Sons, Ltd, 2008, pp. 143–178 Search PubMed.
- J. H. Clark, V. L. Budarin, F. E. I. Deswarte, J. J. E. Hardy, F. M. Kerton, A. J. Hunt, R. Luque, D. J. Macquarrie, K. Milkowski, A. Rodriguez, O. J. Samuel, S. J. Tavener, R. J. White and A. J. Wilson, Green Chem., 2006, 8, 853–860 RSC.
- V. L. Budarin, P. S. Shuttleworth, J. R. Dodson, A. J. Hunt, B. Lanigan, R. Marriott, K. J. Milkowski, A. J. Wilson, S. W. Breeden, J. Fan, E. H. K. Sin and J. H. Clark, Energy Environ. Sci., 2011, 4, 471–479 CAS.
- L. Antti, M. Finell, M. Arshadi and T. A. Lestander, Wood Material Science and Engineering, 2011, 6, 34–40 CrossRef CAS.
- M. Arshadi, P. Geladi, R. Gref and P. Fjällström, Ann. Occup. Hyg., 2009, 53, 797–805 CrossRef CAS.
- M. Arshadi and R. Gref, Forest Prod. J., 2005, 55, 132–135 CAS.
- M. Arshadi, D. Nilsson and P. Geladi, J. Near Infrared Spectrosc., 2007, 15, 379–386 CrossRef CAS.
- M. Arshadi, R. Gref, P. Geladi, S. A. Dahlqvist and T. Lestander, Fuel Process. Technol., 2008, 89, 1442–1447 CrossRef CAS.
- A. Keskin, M. Gürü and D. Altıparmak, Fuel, 2007, 86, 1139–1143 CrossRef CAS.
- D. Altıparmak, A. Keskin, A. Koca and M. Gürü, Bioresour. Technol., 2007, 98, 241–246 CrossRef.
- A. P. Tulloch, Chemistry of Waxes of Higher Plant, in Chemistry and Biochemistry of Natural Waxes, Elsevier, Amsterdam, 1976 Search PubMed.
- F. E. I. Deswarte, J. H. Clark, J. J. E. Hardy and P. M. Rose, Green Chem., 2006, 8, 39–42 RSC.
- A. J. Hunt, E. H. K. Sin, R. Marriott and J. H. Clark, ChemSusChem, 2010, 3, 306–322 CAS.
- R. S. Oakes, A. A. Clifford and C. M. Rayner, J. Chem. Soc., Perkin Trans. 1, 2001, 917–941 RSC.
- L. T. Taylor, Supercritical Fluid Extraction, John Wiley & Sons, New York, 1996 Search PubMed.
- L. Wang and C. L. Weller, Trends Food Sci. Technol., 2006, 17, 300–312 CrossRef CAS.
- L. H. McDaniel, M. Ashraf-Khorassani and L. T. Taylor, J. Supercrit. Fluids, 2001, 19, 275–286 CrossRef CAS.
- A. W. Kjellow and O. Henriksen, J. Supercrit. Fluids, 2009, 50, 297–304 CrossRef CAS.
- SIS-CEN/TS 14774-1:204 Solid biofuels-methods for determination of moisture content-oven dry method-part 1: total moisture-reference method.
- M. Finell, M. Arshadi, R. Gref, T. Scherzer, W. Knolle and T. Lestander, Radiat. Phys. Chem., 2009, 78, 281–287 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1ra00715g |
| This journal is © The Royal Society of Chemistry 2012 |
