Spontaneous pattern formation on silica and titania dip-coating films prepared at extremely low substrate withdrawal speeds†
Hiroaki
Uchiyama
*,
Masaki
Hayashi
and
Hiromitsu
Kozuka
Faculty of Chemistry, Materials and Bioengineering, Kansai University, 3-3-35 Yamate-cho, Suita, 564-8680, Japan. E-mail: h_uchi@kansai-u.ac.jp; Fax: +81-6-6388-8797; Tel: +81-6-6368-1121 ex.5638
First published on 8th November 2011
Abstract
Linear patterns were spontaneously formed on the silica and titania dip-coating films prepared from tetramethyl orthosilicate (Si(OCH3)4) and titanium tetraisopropoxide (Ti(OC3H7i)4) solutions, respectively. In both films, the pattern formation occurred at extremely low substrate withdrawal speeds below 1.0 cm min−1, where the film thickness increased with decreasing substrate withdrawal speed for dip-coating. The linear patterns on micrometre scale were arranged perpendicular to the substrate withdrawal direction. The values of RZ (10 point height of irregularities) and S (mean spacing of local peaks) of the patterns increased with decreasing substrate withdrawal speed.
1. Introduction
Pattern formation through self-organization is a practically important and genuinely interesting phenomenon for many researchers in various technological and scientific fields. A spontaneous organization of molecules provides unique and sophisticated patterns on micrometre and nanometre scales. Many studies have focused on a self-organization as an alternative fabrication process of highly ordered patterns in thin films.1–16 Honeycomb patterns in polymer films were realized by casting polymer solutions under humid conditions.1–4 Periodic stripe patterns were achieved by controlling the wrinkling of the surface of the metal,5–6 organic7–8 and organic–inorganic hybrid films.9 Ordered web-like structures consisting of diblock copolymers were obtained through two consecutive self-assembly processes at different length scales.10 Such micron- and nano-patterned films prepared via self-organization have great potential for practical applications such as electronics,17–18 photonics,19–20 and bioengineering.21–24Sol–gel coating techniques are widely accepted as the methods for preparing metal oxide films. In the case of the dip-coating process, where the substrate is dipped into the coating solution and then vertically withdrawn at constant speeds, micrometre-sized patterns such as unevenness in thickness or ridges are formed on the surface of the films under certain conditions. Since the surface structure of films are responsible for scattering and reflecting of light, the mechanism of the formation of such patterns has been investigated by several approaches. We have reported that linear striations and cell-like patterns are formed parallel to the substrate withdrawal direction during dip-coating at high substrate withdrawal speeds of 70–140 cm min−1.25 The linear patterns thus obtained are caused by Bénard-Marangoni convection in the sol layer that occurs during solvent evaporation, and the size and shape of the patterns depends on the viscosity of the coating solutions and the substrate withdrawal speed. The spontaneous formation of such periodic patterns are regarded as a self-organization process, and the surface structure thus obtained hold great promise for the application to photonic devices such as diffraction gratings and microlens arrays.
Generally, the thickness of dip-coating films decreases with decreasing substrate withdrawal speed. On the other hand, Faustini et al. reported that the thickness of the sol–gel dip-coating films prepared at very low substrate withdrawal speeds below 0.01 mm s−1 (0.06 cm min−1) increases with decreasing substrate withdrawal speed, which is caused by what they call “capillary flow” of coating solutions induced by solvent evaporation at the edge of the meniscus.26 However, the precise mechanism of the capillary flow or the increase in thickness at such low substrate withdrawal speeds remains unclear, which allows us, on the other hand, to expect unique self-organization to take place under such extreme conditions. Recently, we have prepared sol–gel films at such low substrate withdrawal speeds, and found that linear patterns are formed on the surface of films, arranged perpendicular to the substrate withdrawal direction. In this work, we examined the pattern formation on alkoxide-derived dip-coating films prepared at very low substrate withdrawal speeds below 1.0 cm min−1. We prepared silica and titania films from Si(OCH3)4 and Ti(OC3H7i)4 solutions, respectively, and compared the size and shape of surface patterns between them. Moreover, an organic polymer, poly(vinylpyrrolidone) (PVP), was added into the coating solutions for the suppression of the cracking of films during the solvent evaporatrion.27 Here, we investigated the effect of the substrate withdrawal speed, the kind of metal oxides and the addition of PVP in the coating solutions on the size, shape and periodicity of the patterns, and discussed the mechanism of the pattern formation.
2. Experimental section
The starting materials were Ti(OC3H7i)4 (Wako Pure Chemical Industries, Osaka, Japan), Si(OCH3)4 (Shin-Etsu Silicones, Tokyo, Japan), acetylacetone (acac) (Wako Pure Chemical Industries), nitric acid (69 mass%, Wako Pure Chemical Industries), C2H5OH (Wako Pure Chemical Industries), poly(vinylpyrrolidone) (PVP) (K90, 6.3 × 105 in viscosity average molecular weight, Tokyo Kasei Kogyo Co., Tokyo, Japan), and ion-exchanged water. The compositions of the starting solutions are listed in Table 1. Starting solutions of molar compositions, Si(OCH3)4:H2O:HNO3:C2H5OH:PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01:20 : x (x = 0 and 0.5), and Ti(OC3H7i)4 : acac : H2O:HNO3:C2H5OH:PVP = 1
0.01:20 : x (x = 0 and 0.5), and Ti(OC3H7i)4 : acac : H2O:HNO3:C2H5OH:PVP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 : y (y = 0 and 0.5), were prepared by the following procedure, where the mole ratio for PVP was defined for the monomer (polymerizing unit). First, PVP was dissolved in a half of the prescribed amount of C2H5OH. For preparing titania films, acac was added in the PVP–C2H5OH solution in advance. And then, the alkoxide (Si(OCH3)4 or Ti(OC3H7i)4) was added to the solutions. The other half of C2H5OH was added to water, and then nitric acid was added. The H2O–HNO3–C2H5OH solution was added dropwise to the alkoxide solutions under stirring. The Si(OCH3)4 and Ti(OC3H7i)4 solutions thus prepared were kept standing at room temperature in a sealed glass container for 30 min and 3 h, respectively, and served as solutions for dip-coating. Hereafter, the Si(OCH3)4 solutions of x = 0 and 0.5, and the Ti(OC3H7i)4 solutions of y = 0 and 0.5 are denoted as Si0, Si0.5, Ti0 and Ti0.5, respectively. Gel films were deposited on Si(100) substrate (20 mm × 40 mm × 0.85 mm) using a dip-coater (MICRODIP MD-0408, SDI, Kyoto, Japan), where the substrates were withdrawn at 0.02–1.0 cm min−1 (Fig. 1a). After the deposition, the gel films were kept at room temperature for 30 min.
30 : y (y = 0 and 0.5), were prepared by the following procedure, where the mole ratio for PVP was defined for the monomer (polymerizing unit). First, PVP was dissolved in a half of the prescribed amount of C2H5OH. For preparing titania films, acac was added in the PVP–C2H5OH solution in advance. And then, the alkoxide (Si(OCH3)4 or Ti(OC3H7i)4) was added to the solutions. The other half of C2H5OH was added to water, and then nitric acid was added. The H2O–HNO3–C2H5OH solution was added dropwise to the alkoxide solutions under stirring. The Si(OCH3)4 and Ti(OC3H7i)4 solutions thus prepared were kept standing at room temperature in a sealed glass container for 30 min and 3 h, respectively, and served as solutions for dip-coating. Hereafter, the Si(OCH3)4 solutions of x = 0 and 0.5, and the Ti(OC3H7i)4 solutions of y = 0 and 0.5 are denoted as Si0, Si0.5, Ti0 and Ti0.5, respectively. Gel films were deposited on Si(100) substrate (20 mm × 40 mm × 0.85 mm) using a dip-coater (MICRODIP MD-0408, SDI, Kyoto, Japan), where the substrates were withdrawn at 0.02–1.0 cm min−1 (Fig. 1a). After the deposition, the gel films were kept at room temperature for 30 min.
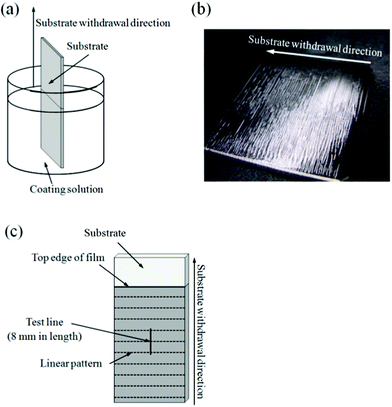 | ||
| Fig. 1 Schematic illustration of the dip-coating process (a), a typical dip-coating film prepared at extremely low substrate withdrawal speeds (b) and the test line employed in the surface roughness measurement (c). | ||
The viscosity of the sols was measured at 25 °C using an oscillating type viscometer (Model VM-1G, Yamaichi Electronics, Tokyo, Japan) at a frequency of 500 Hz. Microscopic observation of the surface of films was made using an optical microscope (KH-1300, HiROX, Tokyo, Japan). The process of pattern formation was observed in situ and stored as digital video images using the optical microscope, where 1.0 g of silicon powder (98 mass%, Wako Pure Chemical Industries) as the marker was added into 40 cm3 of the coating solutions in order to visualize the solution flow.
Surface roughness of the films was measured using a contact probe surface profilometer (SE-3400, Kosaka Laboratory, Tokyo, Japan). A typical dip-coating film prepared at extremely low substrate withdrawal speeds is shown in Fig. 1b, where linear patterns perpendicular to the substrate withdrawal direction are seen on the surface. The measurement was conducted on straight test line 8 mm in length and perpendicular to the substrate withdrawal direction at the center of the coating films as illustrated in Fig. 1c. Surface roughness parameters, RZ (ten point height of irregularities) and S (mean spacing of local peaks), the definitions of which are shown in Fig. 2, were calculated from the transverse profiles. RZ and S represent the amplitude and wavelength, respectively, of wavelike transverse profiles.
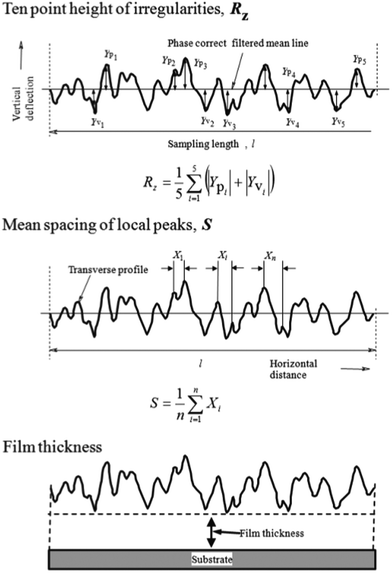 | ||
| Fig. 2 Surface roughness parameters, RZ (10 point height of irregularities) and S (mean spacing of local peaks of the profile), and the definition of film thickness. | ||
Film thickness was measured by the profilometer, where the thickness was defined as that at the valley part as shown in Fig. 2. A part of the gel film was scraped off with a surgical knife immediately after the gel film deposition, and the level difference between the coated part and the scraped part was measured after drying or heating.
3. Results
The Si(OCH3)4– and Ti(OC3H7i)4–derived coating solutions were colorless and yellow, respectively, and transparent. Table 2 shows the viscosity of the coating solutions, which increased with the addition of PVP.| Coating solutions | Viscosity/mPa s |
|---|---|
| Si0 | 1.6 |
| Si0.5 | 62.0 |
| Ti0 | 1.9 |
| Ti0.5 | 23.2 |
From the coating solutions without PVP (Si0 and Ti0), cracked gel films were obtained at substrate withdrawal speeds below 0.3 cm min−1. On the other hand, cracks were not observed for the gel films prepared from the solutions containing PVP (Si0.5 and Ti0.5).
Fig. 3 shows the dependence of the thickness on the substrate withdrawal speed for the crack-free silica and titania gel films prepared from the solutions of Si0.5 and Ti0.5, respectively. The film thickness increased with decreasing withdrawal speed. The silica films had larger thickness than the titania films.
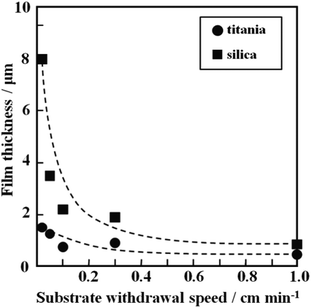 | ||
| Fig. 3 Dependence of thickness on substrate withdrawal speed for the silica and titania gel films prepared from Si0.5 and Ti0.5 solutions. | ||
The silica and titania gel films prepared from Si0.5 and Ti0.5 solutions, respectively, exhibited linear patterns perpendicular to the withdrawal direction when prepared at low withdrawal speeds of 0.02–0.3 cm min−1. Fig. 4 shows the optical micrographs of the titania gel films prepared from Ti0.5 solution at various withdrawal speeds. Smooth surface with inhomogeneity in interference color was found in the film prepared at 1 cm min−1 (Fig. 4a). On the other hand, linear patterns perpendicular to the withdrawal direction were formed on the surface of the films prepared at 0.02–0.3 cm min−1 (Fig. 4b–e). The spacing of the patterns on the titania films was 0.3–0.6 mm as seen in the figures. Fig. 5 shows the transverse profiles of the titania gel films prepared at various withdrawal speeds. Specific patterns were not observed at 1.0 cm min−1 (Fig. 5a). On the other hand, wavy patterns were formed at 0.02–0.3 cm min−1(Fig. 5b–e), which agreed with the optical microscopic observation shown in Fig. 4b–e. Fig. 6 and 7 show the optical micrographs and the transverse profiles of the silica gel films prepared from Si0.5 solution at various withdrawal speeds. The surface patterns observed in the optical micrographs and transverse profiles for the silica films were almost the same as those for the titania films. However, the silica films had larger spacing of the linear patterns than the titania films.
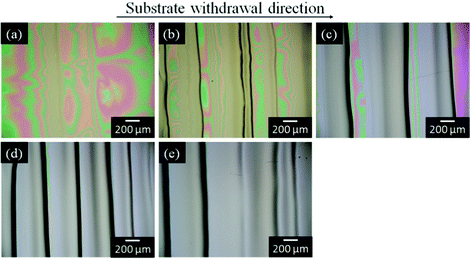 | ||
| Fig. 4 Optical micrographs of the titania gel films prepared from Ti0.5 solution at substrate withdrawal speeds of 1.0 (a), 0.3 (b), 0.1 (c), 0.05 (d) and 0.02 (e) cm min−1. | ||
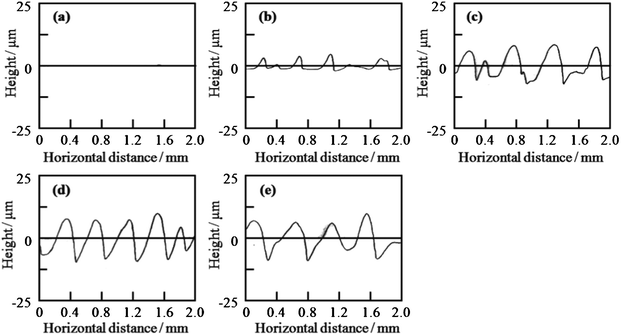 | ||
| Fig. 5 Transverse profiles of the titania gel films prepared from Ti0.5 solution at substrate withdrawal speeds of 1.0 (a), 0.3 (b), 0.1 (c), 0.05 (d) and 0.02 (e) cm min−1. | ||
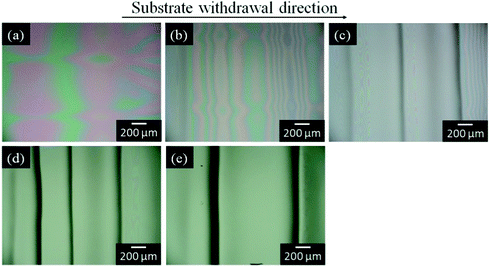 | ||
| Fig. 6 Optical micrographs of the silica gel films prepared from Si0.5 solution at substrate withdrawal speeds of 1.0 (a), 0.3 (b), 0.1 (c), 0.05 (d) and 0.02 (e) cm min−1. | ||
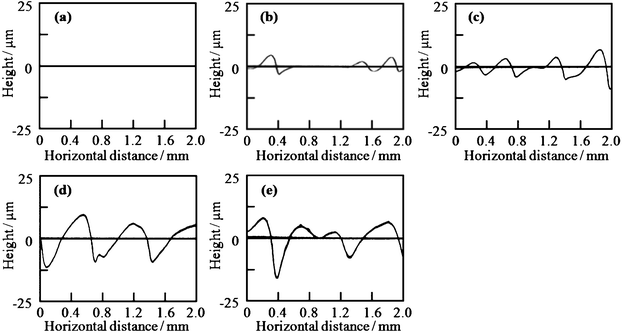 | ||
| Fig. 7 Transverse profiles of the silica gel films prepared from Si0.5 solution at substrate withdrawal speeds of 1.0 (a), 0.3 (b), 0.1 (c), 0.05 (d) and 0.02 (e) cm min−1. | ||
Fig. 8 shows the dependence of the roughness parameters, RZ and S, on the substrate withdrawal speed for the silica and titania gel films prepared from Si0.5 and Ti0.5 solutions, respectively. The decrease in the withdrawal speed led to an increase in RZ and S, i.e. in height and spacing of the linear patterns. The linear patterns were almost the same in height between the silica and titania films, while the spacing was larger for the silica films than for the titania films.
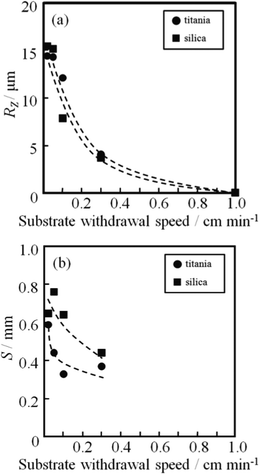 | ||
| Fig. 8 Dependence of film thickness (a), RZ (b) and S (c) on substrate withdrawal speed for the silica and titania gel films prepared from Si0.5 and Ti0.5 solutions. | ||
The formation of linear patterns was observed in situ, where the observation was made near the meniscus of the coating solution as shown Fig. 9a. The observation was performed on Si0.5 solution where silicon powders were added as the marker for visualizing the flow of solution and the substrate withdrawal speed was 0.05 cm min−1. As seen in the movie shown in the ESI† and as schematically drawn in Fig. 9, during the dip-coating process, silicon powders as the marker were locally gathering at the edge of the meniscus of the coating solution, and then linear pattern perpendicular to the substrate withdrawal direction was formed there, which were periodically repeated.
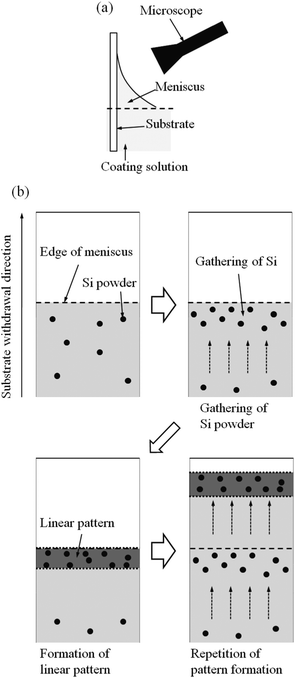 | ||
| Fig. 9 Experimental set-up for in situ observation (a) and the schematic illustration of the result of the observation (b) (silicon powders were used as the marker for visualizing the flow of solution). | ||
The patterned films prepared at 0.02–0.3 cm min−1 were cracked after being kept in the ambient atmosphere for several days. Moreover, the size of the patterns was responsive to humidity and temperature, while the effect of humidity and temperature should be investigated in the near future.
4. Discussion
4.1. Mechanism of the evolution of linear patterns at low substrate withdrawal speeds
At low substrate withdrawal speeds, the crack-free films were obtained from the solutions containing PVP (Si0.5 and Ti0.5). The addition of PVP suppresses the stress evolution attributed to the condensation reaction of metalloxane polymers in gel films, and consequently allows thick coatings to be formed without cracking.27The decrease in the substrate withdrawal speeds led to an increase in the film thickness in the present samples (Fig. 3). Faustini et al. have reported that the thickness of the dip-coating films prepared at very low substrate withdrawal speeds below 0.01 mm s−1 (0.06 cm min−1) increases with decreasing substrate withdrawal speed, which is caused by capillary flow of coating solutions.26 During the dip-coating of such low substrate withdrawal speeds, the solvents evaporate from the edge of the meniscus, and then the coating solutions is raised to the edge of the meniscus by capillary force. In our experiments, the capillary flow of the coating solution is suggested by the gathering of silicon powders at the edge of the meniscus (ESI†). As a result of the capillary flow, the edge of the coating solution is pinned to the substrate, and thus the downward flow of the solution by gravity is suppressed, leading in an increase in the film thickness. Such pinning phenomena of solutions due to the capillary flow are often observed at the edge of a droplet of colloidal suspensions where solvent evaporation occurs, which is commonly known as the coffee-ring effect.28–29
The decrease in the substrate withdrawal speed also led to the formation of linear patterns perpendicular to the withdrawal direction (Fig. 4–7). Spontaneous formation of patterns attributed to Bènard–Marangoni convections is known to occur in sol–gel-derived coating layer under certain conditions.25,30–32 We previously reported that the linear striations parallel to the substrate withdrawal direction are formed on dip-coating films prepared at high substrate withdrawal speeds of 70–140 cm min−1.25 The linear striations thus obtained were thought to be formed via the connection of cell-like Bénard–Marangoni convections and the down flow along the substrate withdrawal direction. On the other hand, the linear patterns obtained in the present work at low substrate withdrawal speeds of 0.02–0.3 cm min−1 were perpendicular to the substrate withdrawal direction. At such very low withdrawal speeds, the viscosity of the coating layer should be very high near the meniscus due to the solvent evaporation, which makes the convections hard to occur in the coating layer. Thus, the pattern formation at such low substrate withdrawal speeds is deduced not to be caused by the Bénard–Marangoni convections.
As shown in Fig. 9, which is the schematic drawings of what was observed in the ESI,† the linear patterns perpendicular to the substrate withdrawal direction were periodically formed at the edge of the meniscus of the coating solution. At the edge of the meniscus, the coating solution could be pinned to the substrate through the evaporation of the solvents and the capillary flow of the solutions. Such pinning phenomena often lead to the formation of periodic patterns through the repetition of the pinning of viscous solutions at their edge, which is called “stick-slip motion”.3,33–35
The formation of linear patterns at the low substrate withdrawn speeds observed in the present work could be caused by the “stick-slip motion” due to the capillary flow of the coating solutions. The schematic illustration of the pattern formation is shown in Fig. 10. Firstly, the gelation of the coating solution locally progresses at the edge of the meniscus due to the evaporation of the solvents. And then, the coating solution is continuously supplied to the edge of the meniscus by the capillary force during solvent evaporation. As a consequence, the thickness of the coating layer locally increases at the edge of the meniscus, leading to the formation of a convex gel part. The convex part is continuously raised with the substrate withdrawal, and progressively segregated from the meniscus. As a result, the edge of the coating solution moves downward like a receding tide until the next pinning occurs by the capillary flow. The linear patterns perpendicular to the withdrawal direction could thus be formed through the repetition of the pinning of the coating solutions at the edge of meniscus and the formation of convex parts due to the gelation of the solutions there.
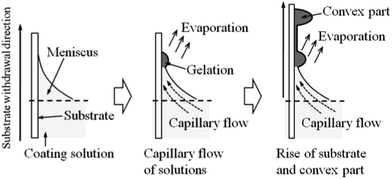 | ||
| Fig. 10 Schematic illustration of the pattern formation during the dip-coating of low substrate withdrawal speeds. | ||
The height and spacing of the linear patterns increased with decreasing withdrawal speed (Fig. 8). At low substrate withdrawal speeds, the gelled part at the edge of the meniscus is kept near the surface of the coating solutions for a long time, and as a result continuously receives the sufficient solution through the capillary flow. This is thought to result in the increase in the size of the patterns.
4.2. Comparison of linear patterns between silica and titania films
The silica gel films prepared from Si0.5 solution had a larger thickness than the titania gel films from the Ti0.5 solution. Si0.5 solution had higher viscosity due to the lower content of solvent (C2H5OH), which led to the formation of thicker gel films.The height of the patterns (Rz) was almost the same between the silica and titania films. The capillary flow to the edge of the meniscus and the gelation of the solution there are triggered by solvent evaporation. In both Si0.5 and Ti0.5 solutions, C2H5OH was used as the solvent, giving rise to identical solvent evaporation rates. This would lead to a similarity in the capillary flow behavior and the gelation of the solution between both coating solutions, which could provide the similar height of the patterns.
On the other hand, the spacing of the linear patterns formed on the silica gel films was larger than that on the titania gel films. After the complete gelation of the coating solution at the meniscus, the edge of the coating solution moves downward until the formation of the next gelled part. Since Si0.5 solution had higher viscosity than Ti0.5 solution, the downward flow would be slower for Si0.5 solution. This could make the cycle of the pattern formation longer in time scale for the silica gel films than for the titania gel films, resulting in the large spacing in the former.
5. Conclusion
Micron-scale linear patterns aligned perpendicular to the substrate withdrawal direction were formed at an extremely low withdrawal speed below 1 cm min−1 on the dip-coating films derived from Si(OCH3)4 and Ti(OC3H7i)4 solutions. The spontaneous pattern formation similarly occurred on both silica and titania films, and the height and spacing of the patterns increased with decreasing withdrawal speed. Such unique surface structures of dip-coating films were thought to form through the capillary rise of the coating solutions to the edge of the meniscus during solvent evaporation and the formation of convex parts due to the gelation there.Acknowledgements
This work has been funded by the High Technology Research Center of Kansai University.References
- M. H. Stenzel-Rosenbaum, T. P. Davis, A. G. Fane and V. Chen, Angew. Chem., Int. Ed., 2001, 40, 3428 CrossRef CAS.
- H. Yabu, R. Jia, Y. Matsuo, K. Ijiro, S. Yamamoto, F. Nishino, T. Takaki, M. Kuwahara and M. Shimomura, Adv. Mater., 2008, 20, 4200 CAS.
- H. Yabu and M. Shimomura, Adv. Funct. Mater., 2005, 15, 575 CrossRef CAS.
- H. Yabu and M. Shimomura, Langmuir, 2005, 21, 1709 CrossRef CAS.
- T. Ohzono and M. Shimomura, Langmuir, 2005, 21, 7230 CrossRef CAS.
- T. Ohzono and M. Shimomura, Phys. Rev., E, 2006, 73.
- E. P. Chan and A. J. Crosby, Adv. Mater., 2006, 18, 3238 CrossRef CAS.
- J. Y. Chung, A. J. Nolte and C. M. Stafford, Adv. Mater., 2009, 21, 1358 CrossRef CAS.
- M. Takahashi, T. Maeda, K. Uemura, J. X. Yao, Y. M. Tokuda, T. Yoko, H. Kaji, A. Marcelli and P. Innocenzi, Adv. Mater., 2007, 19, 4343 CrossRef CAS.
- S. W. Hong, J. Wang and Z. Q. Lin, Angew. Chem., Int. Ed., 2009, 48, 8356 CrossRef CAS.
- E. Ishow, R. Camacho-Aguilera, J. Guerin, A. Brosseau and K. Nakatani, Adv. Funct. Mater., 2009, 19, 796 CrossRef CAS.
- O. Karthaus, N. Maruyama, H. Yabu, T. Koito, K. Akagi and M. Shimomura, Macromol. Symp., 2000, 160, 137 CrossRef CAS.
- L. Zhao, M. D. Goodman, N. B. Bowden and Z. Lin, Soft Matter, 2009, 5, 4698 RSC.
- H. W. Hong, J. Xu, J. Xia, Z. Lin, F. Qiu and Y. Yang, Chem. Mater., 2005, 17, 6223 CrossRef CAS.
- J. Xu, J. Xia, S. W. Hong, Z. Lin, F. Qiu and Y. Yang, Phys. Rev. Lett., 2006, 96, 066104 CrossRef.
- L. Zhao, M. Byun, J. Rzayev and L. Zhiqun, Macromolecules, 2009, 42, 9027 CrossRef CAS.
- L. J. Guo, Adv. Mater., 2007, 19, 495 CrossRef CAS.
- W. Zhang and S. Y. Chou, Appl. Phys. Lett., 2003, 83, 1632 CrossRef CAS.
- M. S. Kim, J. S. Kim, J. C. Cho, M. Shtein, L. J. Guo and J. Kim, Appl. Phys. Lett., 2007, 90, 123113 CrossRef.
- D. Pisignano, L. Persano, M. F. Raganato, P. Visconti, R. Cingolani, G. Barbarella, L. Favaretto and G. Gigli, Adv. Mater., 2004, 16, 525 CrossRef CAS.
- H. Cao, Z. N. Yu, J. Wang, J. O. Tegenfeldt, R. H. Austin, E. Chen, W. Wu and S. Y. Chou, Appl. Phys. Lett., 2002, 81, 174 CrossRef CAS.
- D. Falconnet, D. Pasqui, S. Park, R. Eckert, H. Schift, J. Gobrecht, R. Barbucci and M. Textor, Nano Lett., 2004, 4, 1909 CrossRef CAS.
- L. J. Guo, X. Cheng and C. F. Chou, Nano Lett., 2004, 4, 69 CrossRef CAS.
- J. D. Hoff, L. J. Cheng, E. Meyhofer, L. J. Guo and A. J. Hunt, Nano Lett., 2004, 4, 853 CrossRef CAS.
- H. Uchiyama, W. Namba and H. Kozuka, Langmuir, 2010, 26, 11479 CrossRef CAS.
- M. Faustini, B. Louis, P. A. Albouy, M. Kuemmel and D. Grosso, J. Phys. Chem. C, 2010, 114, 7637 CrossRef CAS.
- H. Kozuka, J. Sol-Gel Sci. Technol., 2006, 40, 287 CrossRef CAS.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Nature, 1997, 389, 827 CrossRef CAS.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 756 CrossRef CAS.
- D. P. Birnie III, J. Mater. Res., 2001, 16, 1145 CrossRef CAS.
- H. Kozuka, S. Takenaka, S. Kimura, T. Haruki and Y. Ishikawa, Glass Technol., 2002, 43C, 265 Search PubMed.
- H. Kozuka, Y. Ishikawa and N. Ashibe, J. Sol-Gel Sci. Technol., 2004, 31, 245 CrossRef CAS.
- J. Xu, J. Xia and Z. Lin, Angew. Chem., Int. Ed., 2007, 46, 1860 CrossRef CAS.
- J. Huang, R. Fan, S. Connor and P. Yang, Angew. Chem., Int. Ed., 2007, 46, 2414 CrossRef CAS.
- H. S. Kim, C. H. Lee, P. K. Sudeep, T. Emrick and A. J. Crosby, Adv. Mater., 2010, 22, 4600 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Video image obtained in the in situ observation of the pattern formation during dip-coating. Si0.5 solution containing silicon powder as the marker was used, and the substrate withdrawal speed was 0.05 cm min−1. See DOI: 10.1039/c1ra00770j |
| This journal is © The Royal Society of Chemistry 2012 |
