DOI:
10.1039/C2RA00643J
(Paper)
RSC Adv., 2012,
2, 2914-2922
Theoretical investigation of hydrogen adsorption in all-metal aromatic clusters†
Received
29th August 2011
, Accepted 11th December 2011
First published on 13th February 2012
Abstract
Molecular hydrogen adsorption in binary all-metal aromatic systems has been explored using ab initio quantum chemical calculations. For this study, we have considered different classes of bimetallic clusters, viz. Be3M2, Mg3M2 and Al4M2 (M = Li, Na and K). In all these bimetallic clusters, the interaction energies of the alkali metal ion with the base metal surface are quite high and the alkali metal sites are found to carry partial positive charges which enhance the adsorption enthalpies of molecular hydrogen on them. Among the three classes considered here, Mg3M2 are found to have poor hydrogen adsorption enthalpies as compared to the other two classes due to less charge on the alkali metals. Although the charge developed on K is more than that developed on Li and Na, the hydrogen adsorption in Be3K2 and Al4K2 is found to be weak in comparison to their Li and Na doped counterparts. In the case of Be3Li2 and Be3Na2, the hydrogen adsorption energies are found to be quite comparable to the optimum adsorption energy proposed for ambient temperature hydrogen storage and the gravimetric density of hydrogen is found to be 22.64 and 14.12 wt% respectively, with three H2 molecules adsorbed per alkali metal atom. In the case of Al4M2, the positive charges on the alkali metal atoms as well as the hydrogen adsorption energies are found to be higher as compared to those in Be3M2 clusters. The gravimetric densities of hydrogen in hydrogenated Al4Li2 and Al4Na2 are found to be respectively 11.59 and 9.4 wt% with four H2 molecules adsorbed per alkali metal atom.
1. Introduction
Hydrogen is considered to be an ideal alternative energy carrier for the presently existing energy systems because of its nonpolluting nature and the possibility of an abundant source from water. However, finding an efficient hydrogen storage system continues to be a bottleneck for the commercialization of hydrogen energy. An ideal hydrogen storage material should satisfy certain conditions, viz. high volumetric and gravimetric densities, fast kinetics for adsorption as well as desorption of molecular hydrogen at ambient conditions, recyclability and cost effectiveness of the material. Physical hydrogen storage as a gas or liquid is technically challenging and requires high pressures and low temperatures, respectively in order to have a good energy density. To achieve the reversible hydrogen uptake and release at near ambient conditions, the hydrogen binding energy should be somewhat intermediate between that of physisorption and chemisorption, with the hydrogen being stored mainly in molecular form. Bhatia et al.1 proposed that the adsorption enthalpy for ambient temperature storage of hydrogen and delivery between 30 bar and 1.5 bar pressure should be −3.6 kcal mol−1. Although a large number of materials have been tested for hydrogen storage, none of the presently existing materials meet all the requirements to achieve the target of the Department of Energy (DOE).2 Therefore, the exploration of new material for hydrogen storage has been an important and active area of research.
In recent years, hydrogen adsorption has been tested3–10 in a large number of materials such as carbon nanomaterials, alanates, borates, zeolites, metal hydrides, metal–organic frameworks, covalent organic frameworks etc. It has been shown that merely van der Waals surfaces are not good enough for efficient hydrogen adsorption and the presence of a charged site can significantly improve the adsorption energy. One of the scenarios used to incorporate ionic sites is metal doping. Zhao et al.11 and Yildirim et al.12 studied hydrogen storage in transition metal doped fullerenes and single-walled carbon nanotubes respectively and found that there is a significant improvement in the hydrogen interaction energy. Though there are a number of studies13–15 on transition metal doped molecules proposed for hydrogen storage, the problem with transition metals is that they tend to aggregate and form clusters at high temperatures. However, recently it was shown11,16 that the clustering can be prevented by doping with boron which acts as a glue. Later, it was shown17,18 that the alkali metal cations can also interact with molecular hydrogen which has been further explored in carbon-based materials, particularly the alkali metal-doped fullerenes. In recent times, hydrogen adsorption in high surface area micro-porous materials like metal–organic frameworks (MOFs), porous carbon materials, zeolitic imidazolate frameworks (ZIFs), covalent organic frameworks (COFs), porous silica etc. is found to be a good approach towards the hydrogen storage problem.19–22 Though MOFs and COFs have huge surface areas, the hydrogen binding energies in these systems are very poor. To improve this hydrogen binding energy, the organic linkers are decorated with metal atoms like lithium where the metal atom acquires charge and can bind molecular hydrogen more strongly.23
In the present study, we propose hydrogen adsorption in all-metal aromatic complexes. It has also been shown24 that systems which are electron deficient for becoming aromatic can be doped with electropositive metals like alkali metal atom and can be used for hydrogen adsorption. In 2001, Li et al.25 reported both experimental and theoretical evidence of aromaticity in all-metal molecules MAl4− (M = Li, Na and Cu). Since then, aromaticity and anti-aromaticity in a large number of all-metal systems have been studied26–33. It has been shown34 that all-metal aromatic systems tend to be more electron deficient as compared to the corresponding aromatic hydrocarbons. Recently, the aromaticity in simple molecular (metal clusters) systems35 like Be32−, Mg32− and their bimetallic species as well as their multi-decker sandwich complexes36 have been investigated. Based on these studies, one can expect that the bimetallic complexes like Al4M2, Be3M2, Mg3M2etc. will have a positive charge on the metallic site, which can be used as a hydrogen adsorption site. Here, we explore the molecular hydrogen adsorption in all-metal aromatic systems Al4M2, Be3M2 and Mg3M2 (M = Li, Na and K). We have calculated the interaction energy of alkali metal ions with the di-anionic metal surface (Be32−, Mg32− and Al42−) and found it to be very high. We have also carried out a systematic study on the interaction of molecular hydrogen with these bimetallic aromatic clusters, by considering different number of hydrogen molecules, that lead to the complexes Be3M2(H2)2n and Al4M2(H2)2n (M = Li and Na).
2. Computational details
Electronic structure theory based GAMESS37 software has been used for all the energy calculations and geometry optimization of the molecular systems. We have employed the density functional theory (DFT) with pure Hartree–Fock exchange and Lee–Yang–Parr (HLYP)38 correlation energy functionals. We have also employed the second order Møller–Plesset (MP2) perturbation method for all the systems to include the proper electron correlation effects as the molecular hydrogen interaction is weak in nature. We have used Grimme's38b DFT-D3 method for obtaining the dispersion correction for calculation all hydrogen interaction energies. We have used the extensive split-valence basis sets with diffuse and polarization functions, 6-31++G(2d,2p) for optimization as well as the Hessian calculation, and the single point energy of the optimized structure was calculated by the Dunning type correlation-consistent basis set, aug-cc-pVTZ. All the NICS calculations are carried out using the Gaussian03 package39 at MP2/6-31++G(2d,2p) level of theory. The initial geometries and all the reported structures have been obtained using the graphical software GABEDIT40 and MOLDEN.41 The highest occupied molecular orbital (HOMO)-lowest unoccupied molecular orbital (LUMO) gaps are calculated as the energy difference between the corresponding orbitals. The interaction energy (IE) between a metal cation and Xm2− (X = Be, Mg and Al and m = 3 or 4) is calculated using eqn (1) and the hydrogen adsorption energy is calculated using eqn (2), as given below.| | | IE(Xm—M2) = ½[E(XmM2) − {E(Xm2−) + 2 × E(M+)}] | (1) |
| | | IE(XmM2—H2) = 1/n[E(XmM2(H2)n − {E(XmM2) + n × E(H2)}] | (2) |
We have further assessed the thermodynamic stability of the systems through the calculated values of their chemical hardness (η) and electrophilicity (ω) values using molecular electronic structure principles like the principles of maximum hardness (PMH)42,43 and minimum electrophilicity (MEP).44 For a system of N electrons, the electronegativity45,46 (χ) and hardness47,48 (η) are defined as follows:
| | 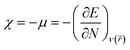 | (3) |
and
| | 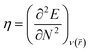 | (4) |
where,
E is the total energy of the system,
μ is the chemical potential and
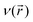
is the external potential. Electrophilicity can be calculated as
49| | 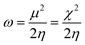 | (5) |
A finite difference approximation to eqn (3) and (4) can be expressed as46,47
and
where
I and
A represent the ionization potential and electron affinity of the system, respectively.
Ionization potentials (I) are obtained as the difference between the positive ion energy and the neutral system energy at the neutral geometry and electron affinities (A) are calculated as the difference between the negative ion energy and the neutral system energy at the neutral geometry, viz.
.
3. Results and discussion
3.1 All-metal aromatic clusters
All the metal aromatic systems considered in the present study are Be3M2, Mg3M2 and Al4M2 where M = Li, Na and K. The geometries of these systems were optimized using both DFT and MP2 methods and the MP2/6-31++G(2d,2p) optimized structures are shown in Fig. 1. In all the cases of these bimetallic clusters, one of the alkali metal atoms is placed above and another below the planar triangular (in Be and Mg cases) or square (in Al case) surface of the base metal system (Be3 or Mg3 or Al4). In the case of Be3Li2, the interaction energy between Be32− and Li+ is found to be −205.5 kcal/mole whereas in the case of Na and K doped systems, this interaction energy is found to be −185.5 and −155.2 kcal mol−1 respectively. These large interaction energies indicate the stability of the complexes towards dissociation which is required for a recyclable hydrogen storage material. The optimized geometries of all these Be3M2 clusters are found to have the symmetry D3d. In all the alkali metal doped beryllium clusters, the alkali metal sites are found to carry partial positive charges and the calculated charges on Li, Na and K are 0.56, 0.61 and 0.64 a.u. respectively. The gradual increase in charge on the metal from Li to K can be expected due to an increase in electropositivity from Li to K.
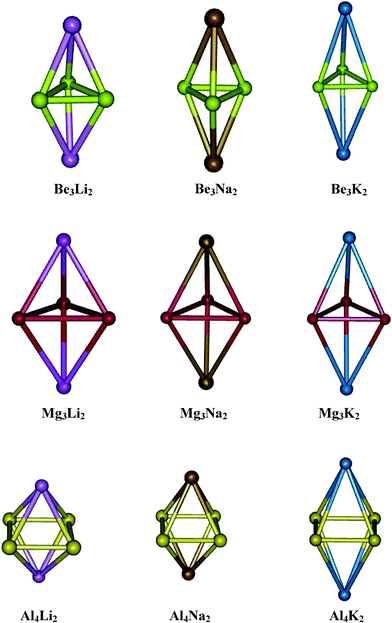 |
| | Fig. 1 Optimized geometries of Be3M2, Mg3M2 and Al4M2 (M = Li, Na and K) | |
The next family of clusters considered is Mg3M2 (M = Li, Na and K) and the corresponding optimized geometries at MP2/6-31++G(2d,2p) level of theory are again shown in Fig. 1. All these Mg3M2 geometries are also found to have D3d symmetry similar to the earlier case. The interaction energy of Li+ with Mg32−, calculated at MP2/aug-cc-pVTZ, is found to be −177.7 kcal mol−1 per lithium ion and that corresponding to Na+ is calculated to be −163.5 kcal mol−1. If we observe the charge on alkali atoms in these clusters, each Li, Na and K are found to carry positive charges of 0.14, 0.31 and 0.41 a.u. respectively. The positive charges developed on the alkali metal in these clusters are very less when it is compared with the same in the case of Be3M2 clusters. As the difference in electronegativity between Be and M is high as compared to that between Mg and M, the electron transfer from the alkali metal (M) to Be is more as compared to that for M to Mg and therefore, higher positive charge on the alkali metal in Be3M2 is expected in comparison to Mg3M2. The other family of clusters considered here, viz. Al4M2 (M = Li, Na and K), has been extensively studied as all-metal aromatic systems.25,33b All MP2/6-31++G(2d,2p) optimized geometries are shown in Fig. 1 and the corresponding results are given in Table 1. The Li+ interaction energy with Al42− calculated using MP2/aug-cc-pVTZ method is found to be −165.8 kcal mol−1 per Li ion and that corresponding to Na+ and K+ in the cases of Al4Na2 and Al4K2 are found to be −153.5 and −117.7 kcal mol−1 per alkali metal ion respectively. In the case of Al4Li2, the charge on each Li is found to be +0.69 a.u. and the corresponding charges on Na and K in Al4Na2 and Al4K2 are found to be 0.76 and 0.85 a.u. respectively. As the interaction energies based on anions/cations may be grossly distorted Coulombically, neutral fragments are also considered and the interaction energy of the alkali metal atoms with neutral Be3 and Al4 in Be3M2 and Al4M2 have been calculated and reported in Table 1. The calculated interaction energies of Li, Na, and K with Be3 are found to be −57.8, −46.4, and −41.5 kcal mol−1 per metal atom respectively. In the case of Al4M2, the interaction energies of Li, Na, and K are found to be −55.5, −51.3, and −33.0 kcal mol−1 respectively. In the cases of Be3M2 and Al4M2, the alkali metal interaction energies are well above the cohesive energies of the respective metals.
Table 1 Calculated parameters for interaction of alkali metal ions (Li, Na and K) with Be32−, Mg32− and Al42−
| System |
Interaction energy (kcal mol−1) |
Charge on metal (a.u.) |
Min. vib. freq. (cm−1) |
HOMO–LUMO gap (eV) |
NICS (ppm) |
| 0.0 |
0.5 |
1.0 |
| |
MP2 |
MP2b |
HLYP |
MP2 |
HLYP |
|
Not considered in the present study. Please see the text for details.
The interaction energy per metal is calculated as IE(Xm—M2) = ½[E(XmM2) − {E(Xm) + 2 × E(M)}].
|
| Be3Li2 |
−204.5 |
−57.8 |
−209.1 |
0.565 |
0.557 |
182.1 |
5.97 |
−44.16 |
−39.92 |
−26.44 |
| Be3Na2 |
−185.1 |
−46.4 |
−189.1 |
0.608 |
0.593 |
96.0 |
5.30 |
−45.86 |
−41.18 |
−28.73 |
| Be3K2 |
−155.2 |
−41.5 |
a
|
0.635 |
0.649 |
74.4 |
4.32 |
a
|
a
|
a
|
| Mg3Li2 |
−177.7 |
a
|
−182.8 |
0.136 |
0.269 |
144.1 |
5.14 |
a
|
a
|
a
|
| Mg3Na2 |
−163.5 |
a
|
−169.2 |
0.312 |
0.368 |
72.9 |
4.69 |
a
|
a
|
a
|
| Mg3K2 |
a
|
a
|
a
|
0.410 |
0.431 |
52.2 |
3.73 |
a
|
a
|
a
|
| Al4Li2 |
−165.8 |
−55.5 |
−168.6 |
0.692 |
0.693 |
113.3 |
5.27 |
26.73 |
24.83 |
22.30 |
| Al4Na2 |
−153.5 |
−51.3 |
−155.1 |
0.760 |
0.774 |
80.3 |
5.00 |
3.82 |
1.20 |
−3.56 |
| Al4K2 |
−117.7 |
−33.0 |
−133.0 |
0.848 |
0.848 |
47.2 |
4.55 |
a
|
a
|
a
|
All the bimetallic clusters considered here, viz. Be3M2, Mg3M2 and Al4M2 are found to have large HOMO–LUMO gaps which indicate the stability of these clusters through the principle of maximum hardness (PMH). In order to verify whether these clusters are stable or not, we have also calculated the Hessian for all these complexes and found that none of them is having imaginary frequency which indicates that all these structures belong to one of the local minima on the respective potential energy surfaces. According to Hoffmann et al.,50 to assign a complex as a stable one, the complex should not only have no imaginary frequencies, but the smallest vibrational frequency calculated should also be reasonably large. From our results reported in Table 1, one can observe that the smallest vibrational frequencies in the complexes, Be3Li2, Be3Na2 and Be3K2 are 182.05, 95.98 and 74.4 cm−1 respectively and the corresponding frequencies in Al4Li2, Al4Na2 and Al4K2 are found to be 113.25, 80.28 and 47.2 respectively indicating the stability of these complexes. We have also calculated the nucleus-independent chemical shift (NICS) values for all the clusters at the centre as well as at 0.5 Å and 1.0 Å away from the centre, as reported in Table 1. From the reported results, it can be seen that all the NICS values of Be3Li2 and Be3Na2 are found to be highly negative, which confirms the aromatic nature of these bimetallic clusters. For Al4Li2, the NICS(0) value is found to be positive but the NICS(0.5) and NICS(1.0) are negative at the B3LYP level. However, all such NICS values are positive at the MP2 level. In the case of Al4Na2, both NICS(0) and NICS(0.5) are positive and only NICS(1.0) is negative. There have been a large number of studies on the aromaticity/antiaromaticity of four membered aluminum and its lithium complexes which have shown25–27 Al4Li− and Al4Li2 as aromatic. Li et al.25 studied the aromaticity in Al4Li−, Al4Li2, Al4Li3− and Al4Li4 using CCSD/TZP level of theory and reported that Al4Li− and Al4Li2 have diatropic ring currents whereas Al4Li3− and Al4Li4 have paratropic ring currents suggesting Al42− and its Li complex to be aromatic and Al44− and its lithium complex as antiaromatic in nature. Based on canonical molecular orbital (CMO)-NICS analysis, Chen et al.51 showed that the π antiaromaticity in several Al44− based clusters is overtaken by their σ aromaticity and found the Al44− clusters to be net aromatic rather than antiaromatic. From these earlier studies33b based on high level theory, we can expect the Al4M2 bimetallic clusters to be aromatic even though the calculated NICS values are slightly positive as predicted in our calculations. It may be noted that certain systems show different aromatic behavior in different spin states.52 Hence, we have also calculated the NICS (0) and NICS (1.0) values for Al4Li2 at a triplet state and found the values to be 35.9 and 47.1 respectively.
3.2 Hydrogen adsorption in all-metal aromatic systems
Although molecular hydrogen does not have a dipole moment, it has a strong quadrupole moment and polarizability. Hence, despite the fact that the molecular hydrogen adsorption on a simple van der Waals surface is poor, presence of a positive charge can improve the adsorption enthalpies significantly as the charged site can bind with the H2 molecule through ion-quadrupole and ion-induced dipole interactions. Since all these bimetallic clusters considered here have cationic alkali metal sites, we have explored their hydrogen adsorption properties. In the case of Mg3M2, the positive charge on the alkali metals is found to be much less which leads to a very weak interaction with the molecular hydrogen and the interaction energies are found to be around −0.5 kcal mol−1 per hydrogen and hence we have not studied the hydrogen adsorption extensively in the case of Mg3M2. We have studied the hydrogen adsorption mainly in Be3M2 and Al4M2 and the results are discussed below.
In the families of Be3M2 and Al4M2 clusters, the potassium doped ones are found to interact with molecular hydrogen very weakly and the interaction energies are found to be around −0.6 kcal mol−1 per H2 molecule and hence we omitted the systematic hydrogen adsorption study in the cases of both Be3K2 and Al4K2 clusters. In the Be3M2 family, the two clusters studied for hydrogen adsorption here are Be3Li2 and Be3Na2. In both the cases, we have allowed different numbers of hydrogen molecules per metal site, by increasing it systematically as Be3M2(H2)2n (n = 1–4). The optimized structures of Be3Li2(H2)2n for n = 1–4 are shown in Fig. 2 and the corresponding results are given in Table 2. In the case of Be3Li2, the first H2 per metal site is found to get adsorbed with an interaction energy of −3.1 kcal mol−1 per H2 molecule. The second and third molecules are found to interact with interaction energies of −2.5 and −2.3 kcal mol−1 per H2 molecule respectively. On introducing the fourth H2 molecule, it is found that it moves away from the metal site instead of being bound to Li sites which shows that each Li can adsorb a maximum of three H2 molecules resulting into the species Be3Li2(H2)6 which corresponds to 22.64 wt% of hydrogen adsorption. The optimized geometries of Be3Na2(H2)2n using MP2/6-31++G(2d,2p) for n = 1–4 are shown in Fig. 3. The hydrogen adsorption energies in Be3Na2(H2)2n for n = 1–3 are found to be −2.0, −1.7 and −1.8 kcal mol−1 per molecular hydrogen respectively. The fourth H2 molecule placed near each Na site is found to be moving away from it as shown in Fig. 3 which shows that in this case also the maximum number of H2 that can be adsorbed per Na is only three which corresponds to 14.12 wt% of hydrogen adsorption. Both ΔH (calculated at 298.15 K) and Δω values are negative confirming the feasibility of the process. However, ΔG (calculated at 298.15 K) is found to be positive due to loss of entropy as is the case with the majority of the hydrogen storage materials, where H2 is adsorbed in its molecular form. Accordingly low temperature (adsorption in the gaseous form) or high pressure (adsorption in the liquid form) are often recommended. It may also be noted that although ΔG0 values are positive, it is possible to have a negative ΔG by adjusting the concentration, which in this case amounts to higher pressure of hydrogen. Very few systems like for glass,53 where H2 is adsorbed in the atomic form, however, there is a marginal increase in entropy, although this process faces practical problems. The majority of the present day research on this subject rather deals with the reaction enthalpy.
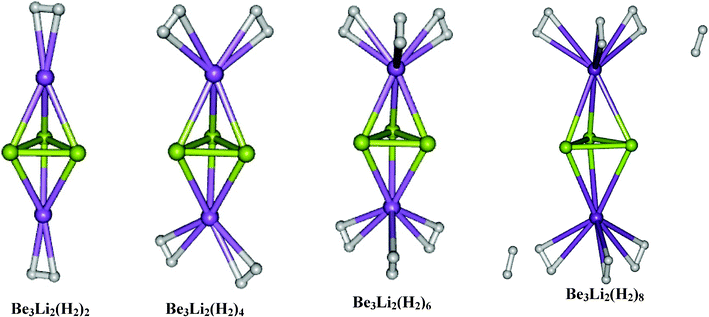 |
| | Fig. 2 Equilibrium geometries of Be3Li2(H2)2n (n = 1–4). | |
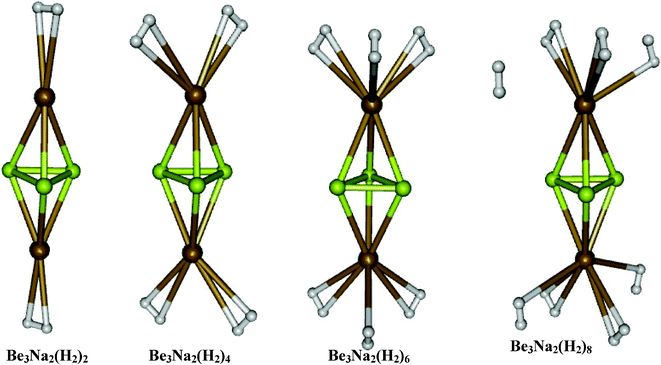 |
| | Fig. 3 Equilibrium geometries of Be3Na2(H2)2n (n = 1–4). | |
Table 2 Molecular hydrogen adsorption energies, charge on alkali metal, HOMO–LUMO gaps, reaction electrophilicity, reaction enthalpy, reaction free energy and NICS parameters for Be3M2(H2)2n (M = Li, Na and n = 1–3)
| System |
Interaction energy (kcal mol−1) |
Charge on metal (a.u.) |
HOMO–LUMO gap (eV) |
Δω (eV) |
ΔHa (kcal mol−1) |
ΔGa (kcal mol−1) |
NICS (ppm) |
| 0.0 |
0.5 |
1.0 |
| |
MP2 |
HLYP |
HLYP-Db |
MP2 |
HLYP |
MP2 |
MP2 |
MP2 |
|
The reaction enthalpy (ΔH) and reaction free energy (ΔG) are calculated at 298.15 K.
HLYP interaction energies after incorporating the dispersion correction and the interaction energy per H2 is calculated as IE(XmM2—H2) = 1/n[E(XmM2(H2)n − {E(XmM2) + E(nH2)}].
|
| Be3Li2 + 2H2 = Be3Li2–(H2)2 |
−3.1 |
−2.7 |
−3.0 |
0.36 |
0.355 |
6.39 |
−0.42 |
−1.4 |
+ 2.8 |
−42.09 |
−38.41 |
−25.70 |
| Be3Li2 + 4H2 = Be3Li2–(H2)4 |
−2.5 |
−2.2 |
−2.7 |
0.28 |
0.294 |
6.40 |
−0.59 |
−0.7 |
+ 4.9 |
−41.73 |
−38.20 |
−25.75 |
| Be3Li2 + 6H2 = Be3Li2–(H2)6 |
−2.3 |
−2.0 |
−2.6 |
0.44 |
0.383 |
6.34 |
−0.67 |
−0.6 |
+ 5.0 |
−42.30 |
−38.65 |
−26.08 |
| Be3Na2 + 2H2 = Be3Na2–(H2)2 |
−2.0 |
−1.1 |
−1.3 |
0.52 |
0.515 |
5.69 |
−0.44 |
−0.1 |
+ 3.5 |
−45.60 |
−40.98 |
−28.56 |
| Be3Na2 + 4H2 = Be3Na2–(H2)4 |
−1.7 |
−1.0 |
−1.2 |
0.48 |
0.452 |
5.83 |
−0.59 |
−0.1 |
+ 3.8 |
−45.26 |
−40.73 |
−28.45 |
| Be3Na2 + 6H2 = Be3Na2–(H2)6 |
−1.8 |
−1.1 |
−1.2 |
0.52 |
0.467 |
5.74 |
−0.70 |
−0.1 |
+ 4.1 |
−45.11 |
−40.61 |
−28.24 |
Next, we discuss the hydrogen adsorption in Al4Li2 and Al4Na2 clusters. The optimized structures of Al4Li2(H2)2n for n = 1–5 are shown in Fig. 4 and the corresponding results are reported in Table 3. The first molecular hydrogen on each Li site is found to be adsorbed with an interaction energy of −3.2 kcal mol−1 per H2 molecule which is higher than that observed in the case of Be3Li2 case and can be ascribed to the high positive charge on Li in Al4Li2 as compared to that in Be3Li2. The second, third and fourth molecular hydrogens are adsorbed with interaction energies of −3.2, −3.3 and −3.0 kcal mol−1 respectively and the fifth H2 is found to be moving away from the Li site as shown in Fig. 4. This result shows that each Li can bind four H2 molecules as in Al4Li2(H2)8 which corresponds to a gravimetric density of 11.59 wt% hydrogen. Interestingly, the maximum number of hydrogen molecules that can be adsorbed per metallic site is also higher in Al4Li2 as compared to that in Be3Li2 cluster. All the optimized structures of Al4Na2(H2)2n for n = 1–5 are shown in Fig. 5 and the corresponding results are reported in Table 3. In the case of Al4Na2 also, we found that each Na can adsorb a maximum of four H2 molecules which corresponds to a gravimetric density of 9.4 wt% hydrogen, whereas the fifth H2 molecule if attempted to be adsorbed moves away far from the sodium site. The interaction energies of molecular hydrogen with Al4Na2 forming Al4Na2(H2)2n for n = 1–4 are, −2.5, −2.3, −2.6 and −2.7 kcal mol−1, respectively. Negative values of ΔH and Δω confirm the feasibility of this process. From all the reported hydrogen adsorption enthalpies in Tables 2 and 3, it can be seen that the HLYP values without dispersion correction are less than the MP2 values and after incorporating the dispersion correction using DFT-D3 approach, the values are closer to the MP2 interaction energies which show the importance of dispersion correction in the case of weakly interacting situations.
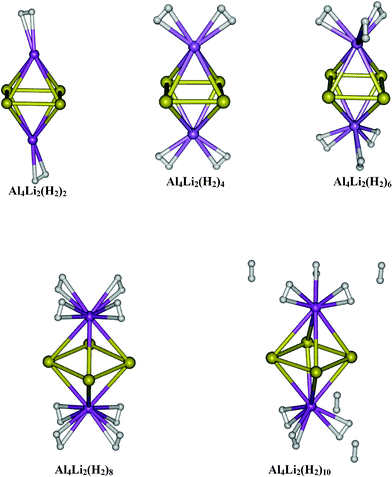 |
| | Fig. 4 Equilibrium geometries of Al4Li2(H2)2n (n = 1–5). | |
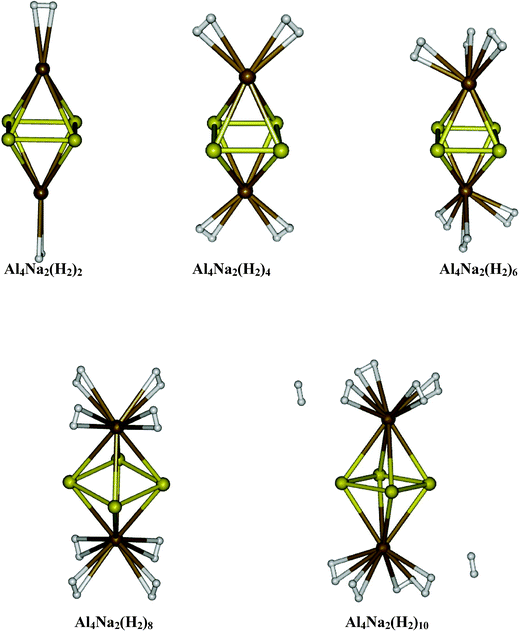 |
| | Fig. 5 Equilibrium geometries of Al4Na2(H2)2n (n = 1–5). | |
Table 3 Molecular hydrogen adsorption energies, charge on alkali metal, HOMO–LUMO gaps, reaction electrophilicity, reaction enthalpy, reaction free energy and NICS parameters of Al4M2(H2)2n (M = Li, Na and n = 1–4)
| System |
Interaction energy (kcal mol−1) |
Charge on metal (a.u.) |
HOMO–LUMO gap (eV) |
Δω (eV) |
ΔHa (kcal mol−1) |
ΔGa (kcal mol−1) |
NICS (ppm) |
| 0.0 |
0.5 |
1.0 |
| |
MP2 |
HLYP |
HLYP-Db |
MP2 |
HLYP |
MP2 |
MP2 |
MP2 |
|
The reaction enthalpy (ΔH) and reaction free energy (ΔG) are calculated at 298.15 K.
HLYP interaction energies after incorporating the dispersion correction and the interaction energy per H2 is calculated as IE(XmM2—H2) = 1/n[E(XmM2(H2)n − {E(XmM2) + E(nH2)}].
|
| Al4Li2 + 2H2 = Al4Li2–(H2)2 |
−3.2 |
−3.0 |
−3.4 |
0.577 |
0.666 |
5.44 |
−0.70 |
−1.6 |
3.8 |
43.13 |
39.08 |
32.84 |
| Al4Li2 + 4H2 = Al4Li2–(H2)4 |
−3.2 |
−2.8 |
−3.2 |
0.567 |
0.524 |
5.17 |
−0.14 |
−1.3 |
5.0 |
36.17 |
29.83 |
19.65 |
| Al4Li2 + 6H2 = Al4Li2–(H2)6 |
−3.3 |
−2.8 |
−3.6 |
0.529 |
0.525 |
5.52 |
−0.39 |
−1.2 |
5.2 |
16.35 |
10.28 |
0.79 |
| Al4Li2 + 8H2 = Al4Li2–(H2)8 |
−3.0 |
−2.4 |
−3.4 |
0.657 |
0.593 |
5.47 |
−0.44 |
−0.9 |
5.8 |
3.37 |
−1.76 |
−9.42 |
| Al4Na2 + 2H2 = Al4Na2–(H2)2 |
−2.5 |
−1.5 |
−1.8 |
0.713 |
0.754 |
5.24 |
−0.30 |
−0.5 |
4.5 |
7.48 |
4.42 |
−1.18 |
| Al4Na2 + 4H2 = Al4Na2–(H2)4 |
−2.3 |
−1.5 |
−1.8 |
0.711 |
0.699 |
5.22 |
−0.34 |
−0.5 |
4.0 |
9.75 |
6.18 |
−0.33 |
| Al4Na2 + 6H2 = Al4Na2–(H2)6 |
−2.6 |
−1.6 |
−1.9 |
0.722 |
0.717 |
5.29 |
−0.47 |
−0.5 |
4.9 |
10.05 |
6.07 |
−1.00 |
| Al4Na2 + 8H2 = Al4Na2–(H2)8 |
−2.7 |
−1.5 |
−2.0 |
0.837 |
0.896 |
5.24 |
−0.50 |
−0.5 |
4.9 |
9.25 |
5.02 |
−2.58 |
For all the hydrogenated complexes of Be3M2 and Al4M2 (M = Li and Na), we have calculated the HOMO–LUMO gaps and reported the results in Tables 2 and 3 respectively. These calculated HOMO–LUMO gaps are found to be reasonably large to confirm that these complexes are the stable ones. We have also calculated the Hessian for all the hydrogenated complexes and found that all systems with bound hydrogen are associated with real frequencies, which clearly show that these are stable structures belonging to one of the local minima on the PES. For testing the aromatic nature of these hydrogenated complexes, we have calculated the NICS parameters at three different points as calculated for metal clusters and discussed earlier and the corresponding results are tabulated in Tables 2 and 3. If we observe the variation in NICS values with increase in the number of molecular hydrogens adsorbed as shown in Fig. 6, this variation is much less in the case of Be3M2(H2)2n whereas it is considerable in the case of Al4M2(H2)2n.
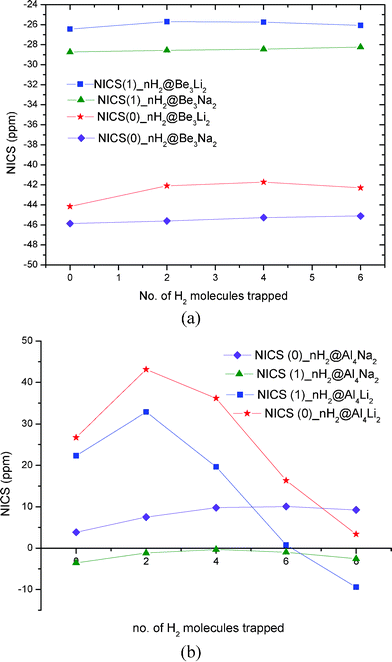 |
| | Fig. 6 Variation of NICS (0) and NICS (1) for (a) H2 trapped Be3M2 (M = Li, Na) (b) H2 trapped Al4M2 (M = Li, Na) systems with increase in the number of adsorbed molecular hydrogen. | |
The total energy and important global reactivity descriptors such as electronegativity (χ), hardness (η) and electrophilicity (ω) for all the Be3M2 and Al4M2 metal clusters as well as their hydrogenated complexes are calculated and reported in Table 4. From the total energy values reported in Table 4, it can be seen that there is a gradual decrease in the total energy after hydrogenation which shows the bonded nature of molecular hydrogen on these metal clusters. If we observe the variation of electrophilicity (ω) with increase in the number of hydrogen molecules adsorbed per metal site, there is a gradual decrease in the values. The hardness (η) value also increases in most cases, simultaneously confirming the validity of the principles of maximum hardness (PMH) and minimum electrophilicity (MEP). This trend demonstrates the stabilization of the metal clusters after hydrogenation, thereby providing some theoretical justification for the use of such all-metal aromatic systems as hydrogen storage materials. The important frontier molecular orbitals (FMOs) of the metal clusters, Be3M2 and Al4M2 and their molecular hydrogen adsorbed counterparts are shown in Fig. S2–S5 (ESI†). From these molecular orbital pictures, it is evident that there is a distinct electron delocalization throughout the Be32− and Al42− rings within the complexes. All the structures have both sigma and pi-character in bonding. It is also transparent from these pictures that the qualitative features of the frontier molecular orbitals do not change much on molecular hydrogen loading for all the complexes.
Table 4 Total energy (E), electronegativity (χ), hardness (η) and electrophilicity (ω) of the studied bimetallic clusters and their hydrogenated complexes calculated using MP2/6-31++(2d,2p) level of theory.
| System |
E (a.u.) |
χ (eV) |
η (eV) |
ω (eV) |
| Be3Li2 |
−58.85974 |
3.35 |
6.31 |
0.89 |
| Be3Li2–(H2)2 |
−61.18778 |
3.24 |
6.60 |
0.80 |
| Be3Li2–(H2)4 |
−63.51336 |
3.08 |
6.68 |
0.71 |
| Be3Li2–(H2)6 |
−65.83923 |
2.98 |
6.70 |
0.66 |
| Be3Na2 |
−367.64722 |
2.96 |
6.11 |
0.72 |
| Be3Na2–(H2)2 |
−369.97182 |
2.99 |
6.39 |
0.70 |
| Be3Na2–(H2)4 |
−372.29622 |
2.90 |
6.44 |
0.65 |
| Be3Na2–(H2)6 |
−374.62088 |
2.70 |
6.21 |
0.59 |
| Al4Li2 |
−982.78928 |
3.68 |
4.95 |
1.37 |
| Al4Li2–(H2)2 |
−985.11814 |
3.58 |
4.86 |
1.32 |
| Al4Li2–(H2)4 |
−987.44683 |
3.50 |
4.80 |
1.28 |
| Al4Li2–(H2)6 |
−989.77480 |
3.26 |
5.16 |
1.03 |
| Al4Li2–(H2)8 |
−992.09907 |
3.18 |
5.16 |
0.98 |
| Al4Na2 |
−1291.59293 |
3.18 |
5.08 |
0.99 |
| Al4Na2–(H2)2 |
−1293.91909 |
3.12 |
5.06 |
0.96 |
| Al4Na2–(H2)4 |
−1296.24506 |
3.12 |
4.96 |
0.98 |
| Al4Na2–(H2)6 |
−1298.57088 |
3.00 |
5.12 |
0.88 |
| Al4Na2–(H2)8 |
−1300.89651 |
2.98 |
5.10 |
0.87 |
From the results discussed above, one can understand that even though both lithium and sodium doped all-metal aromatic complexes are found to adsorb same number of H2 molecules, the hydrogen adsorption energies are higher in the case of Li decorated ones. In addition to the adsorption energy, the wt% is also more in Li doped clusters due to light weight of Li as compared to Na. Between Be3Li2 and Al4Li2, in terms of wt%, Be3Li2 is preferable; however, if we check the adsorption energies, Al4Li2 seems to be a more preferable hydrogen storage material. It might be further possible to construct multidecker sandwich type of materials using these Al4M2 as building blocks.
4. Conclusions
Using the ab initio electronic structure based theoretical calculations, we have explored the hydrogen adsorption properties of different all-metal aromatic systems viz. Be3M2, Mg3M2 and Al4M2 (M = Li, Na and K). In all these bimetallic clusters, the alkali metal sites are found to carry partial positive charges and the order of the charge is in accordance with the electropositivity of the alkali metal as well as the electronegetivity difference between the alkali metal and the other metal species. The potassium containing clusters are found to have very poor hydrogen adsorption capacity as compared with lithium and sodium containing clusters due to its large ionic radius and less charge density. All the Mg3M2 clusters are also found to have poor adsorption properties as a result of less charge transfer from alkali metal atom to the Mg3 ring which can be attributed to the less electronegativity difference between alkali metal and Mg as compared to alkali metal and Be or Al. Be3Li2 and Be3Na2 are found to trap a total of six hydrogen molecules each whereas in the case of Al4Li2 and Al4Na2 the number of H2 molecules adsorbed is eight. Although in terms of gravimetric density, Be3Li2 is advantageous over Al4Li2, in terms of binding energy Al4Li2 is preferable for better hydrogen storage. Reaction enthalpy and reaction electrophilicity lend additional support.
Acknowledgements
We thank the BARC computer center for providing the high performance parallel computing facility (Ameya and Ajeya Systems). This work has been supported by the INDO-EU project HYPOMAP, in the area of Computational Materials Science. SKG and PKC thank DST, New Delhi for Sir J. C. Bose Fellowships.
References
- S. K. Bhatia and A. L. Myers, Langmuir, 2006, 22, 1688 CrossRef CAS.
-
http://www1.eere.energy.gov/hydrogenandfuelcells/.
.
-
(a) A. C. Dillon, K. M. Jones, T. A. Bekkedahl, C. H. Kiang, D. S. Bethune and M. J. Heben, Nature, 1997, 386, 377 CrossRef CAS;
(b) G. E. Froudakis, J. Phys.: Condens. Matter, 2002, 14, R453 CrossRef CAS;
(c) S. Patchkovskii, J. S. Tse, S. N. Yurchenko, L. Zhechkov, T. Heine and G. Seifert, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10439 CrossRef CAS.
-
(a) A. Züttel, P. Sudan, Ph. Mauron, T. Kiyobayashi, Ch. Emmenegger and L. Schlapbach, Int. J. Hydrogen Energy, 2002, 27, 203 CrossRef;
(b) J. B. Martin, I. A. Kinloch and R. A. W. Dryfe, J. Phys. Chem. C, 2010, 114, 4693 CrossRef CAS;
(c) A. Du, Z. Zhu and S. C. Smith, J. Am. Chem. Soc., 2010, 132, 2876 CrossRef CAS.
-
(a) C. P. Baldé, B. P. C. Hereijgers, J. H. Bitter and K. P. de Jong, Angew. Chem., Int. Ed., 2006, 45, 3501 CrossRef;
(b) W. Grochala and P. P. Edwards, Chem. Rev., 2004, 104, 1283 CrossRef CAS;
(c) S. Chaudhuri, J. Graetz, A. Ignatov, J. J. Reilly and J. T. Muckerman, J. Am. Chem. Soc., 2006, 128, 11404 CrossRef CAS;
(d) I. Ljubić and D. C. Clary, Phys. Chem. Chem. Phys., 2010, 12, 4012 RSC.
-
(a) G. E. Froudakis, Nano Lett., 2001, 1, 531 CrossRef CAS;
(b) K. R. S. Chandrakumar, K. Srinivasu and S. K. Ghosh, J. Phys. Chem. C, 2008, 112, 15670 CrossRef CAS;
(c) T. A. Maark and S. Pal, Int. J. Hydrogen Energy, 2010, 35, 12846 CrossRef CAS.
-
(a) K. Srinivasu and S. K. Ghosh, J. Phys. Chem. C, 2011, 115, 1450 CrossRef CAS;
(b) M. Prakash, M. Elango and V. Subramanian, Int. J. Hydrogen Energy, 2011, 36, 3922 CrossRef CAS;
(c) Q. Sun, Q. Wang and P. Jena, Appl. Phys. Lett., 2009, 94, 013111 CrossRef;
(d) A. Datta and S. K. Pati, J. Phys. Chem. C, 2007, 111, 4487 CrossRef CAS.
-
(a)
J. J. Reilly, Metal Hydrides as Hydrogen Storage and theirApplications; CRC Press: Cleveland, OH, 1977; 2, p 13 Search PubMed;
(b) F. Schúth, Nature, 2005, 434, 712 CrossRef;
(c) S.-I. Orimo, Y. Nakamori, J. R. Eliseo, A. Züttel and C. M. Jensen, Chem. Rev., 2007, 107, 4111 CrossRef CAS.
-
(a) N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'keeffe and O. M. Yaghi, Science, 2003, 300, 1127 CrossRef CAS;
(b) J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670 CrossRef CAS;
(c) J. L. C. Rowsell and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 1304 CrossRef CAS;
(d) S. S. Han, J. L. Mendoza-Cortés and W. A. Gaddard, Chem. Soc. Rev., 2009, 38, 1460 RSC.
-
(a) K. Srinivasu, K. R. S. Chandrakumar and S. K. Ghosh, Phys. Chem. Chem. Phys., 2008, 10, 5832 RSC;
(b) A. Du. Z. Zhu and S. C. Smith, J. Am. Chem. Soc., 2010, 132, 2876 CrossRef;
(c) K. S. Subrahmanyam, P. Kumar, U. Maitra, A. Govindaraj, K. P. S. S. Hembram, U. V. Waghmare and C. N. R. Rao, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2674 CrossRef CAS.
- Y. Zhao, Y.-H. Kim, A. C. Dillon, J. M. Heben and S. B. Zhang, Phys. Rev. Lett., 2005, 94, 155504 CrossRef.
- T. Yildirim and S. Ciraci, Phys. Rev. Lett., 2005, 94, 175501 CrossRef CAS.
- A. B. Phillips and B. S. Shivaram, Phys. Rev. Lett., 2008, 100, 105505 CrossRef CAS.
-
(a) Q. Sun, Q. Wang, P. Jena and Y. Kawazoe, J. Am. Chem. Soc., 2005, 127, 14582 CrossRef CAS;
(b) B. Kiran, A. K. Kandalam and P. Jena, J. Chem. Phys., 2006, 124, 224703 CrossRef.
-
(a) A. K. Kandalam, B. Kiran and P. Jena, J. Phys. Chem. C, 2008, 112, 6181 CrossRef CAS;
(b) S. Bhattacharya, C. Majumder and G. P. Das, J. Phys. Chem. C, 2008, 112, 17487 CrossRef CAS.
- A. K. Singh, A. Sadrzadeh and B. I. Yakobson, J. Am. Chem. Soc., 2010, 132, 14126 CrossRef CAS.
- K. R. S. Chandrakumar and S. K. Ghosh, Chem. Phys. Lett., 2007, 447, 208 CrossRef CAS.
-
(a) Q. Sun, P. Jena, Q. Wang and M. Marquez, J. Am. Chem. Soc., 2006, 128, 9741 CrossRef CAS;
(b) K. R. S. Chandrakumar and S. K. Ghosh, Nano Lett., 2008, 8, 13 CrossRef CAS.
-
(a) S. S. Han, W.-Q. Deng and W. A. Goddard, Angew. Chem., Int. Ed., 2007, 46, 6289 CrossRef CAS;
(b) J. L. C. Rowsell, A. R. Millward, K. S. Park and O. M. Yaghi, J. Am. Chem. Soc., 2004, 126, 5666 CrossRef CAS;
(c) A. Kuc, T. Heine, G. Seifert and H. A. Duarte, Chem.–Eur. J., 2008, 14, 6597 CrossRef CAS.
-
(a) I. Cabria, M. J. López and J. A. Alonso, Phys. Rev. B, 2008, 78, 075415 CrossRef;
(b) A. Kuc, L. Zhechkov, S. Patchkovskii, G. Seifert and T. Heine, Nano Lett., 2007, 7, 1 CrossRef CAS.
-
(a) H. Wu, W. Zhou and T. Yildirim, J. Am. Chem. Soc., 2007, 129, 5314 CrossRef CAS;
(b) R. B. Rankin, J. Liu, A. D. Kulkarni and J. K. Johnson, J. Phys. Chem. C, 2009, 113, 16906 CrossRef CAS;
(c) M. Zhou, Q. Wang, L. Zhang, Y.-C. Liu and Y. Kang, J. Phys. Chem. B, 2009, 113, 11049 CrossRef CAS.
-
(a) S. S. Han, H. Furukawa, O. M. Yaghi and W. A. Goddard, J. Am. Chem. Soc., 2008, 130, 11580 CrossRef CAS;
(b) E. Klontzas, E. Tylianakis and G. E. Froudakis, Nano Lett., 2010, 10, 452 CrossRef CAS;
(c) E. Klontzas, E. Tylianakis and G. E. Froudakis, J. Phys. Chem. C, 2009, 113, 21253 CrossRef CAS.
-
(a) A. Blomqvist, C. M. Araújo, P. Srepusharawoot and R. Ahuja, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20173 CrossRef CAS;
(b) S. Giri, S. Bandaru, A. Chakraborty and P. K. Chattaraj, Invited Article: Special Issue of PCCP on Aromaticity, Electron Delocalization and Related Molecular Properties, Phys. Chem. Chem. Phys., 2011 10.1039/C1CP21752F.
- K. Srinivasu, K. R. S. Chandrakumar and S. K. Ghosh, ChemPhysChem, 2009, 10, 427 CrossRef CAS.
- X. Li, A. E. Kuznetsov, H.-F. Zhang, A. I. Boldyrev and L.-S. Wang, Science, 2001, 291, 859 CrossRef CAS.
- A. E. Kuznetsov, K. Birch, A. I. Boldyrev, X. Li, H. Zhai and L.-S. Wang, Science, 2003, 300, 622 CrossRef CAS.
-
(a) A. E. Kuznetsov, J. D. Corbet, L.-S. Wang and A. I. Boldyrev, Angew. Chem., Int. Ed., 2001, 40, 3369 CrossRef CAS;
(b) A. E. Kuznetsov, A. I. Boldyrev, X. Li and L.-S. Wang, J. Am. Chem. Soc., 2001, 123, 8825 CrossRef CAS.
-
(a) B. Schiemenz and G. Huttner, Angew. Chem., Int. Ed. Engl., 1993, 32, 297 CrossRef;
(b) R. B. King, T. Heine, C. Corminboeuf and P. v. R. Schleyer, J. Am. Chem. Soc., 2004, 126, 430 CrossRef CAS.
-
(a) H. J. Zhai, A. E. Kuznetsov, A. I. Boldyrev and L. S. Wang, ChemPhysChem, 2004, 5, 1885 CrossRef CAS;
(b) J. M. Mercero and J. M. Ugalde, J. Am. Chem. Soc., 2004, 126, 3380 CrossRef CAS.
-
(a) S. Shetty, D. G. Kanhere and S. Pal, J. Phys. Chem. A, 2004, 108, 628 CrossRef CAS;
(b) R. W. A. Havenith, P. W. Fowler, E. Steiner, S. Shetty, D. G. Kanhere and S. Pal, Phys. Chem. Chem. Phys., 2004, 6, 285 RSC;
(c) A. Saieswari, U. D. Priyakumar and G. N. Sastry, THEOCHEM, 2003, 663, 145 CrossRef CAS.
-
(a) A. Datta and S. K. Pati, J. Am. Chem. Soc., 2005, 127, 3496 CrossRef CAS;
(b) A. Datta, S. S. Mallajosyulu and S. K. Pati, Acc. Chem. Res., 2007, 40, 213 CrossRef CAS;
(c) S. S. Mallajosyulu, A. Datta and S. K. Pati, J. Phys. Chem. B, 2006, 110, 20098 CrossRef.
-
(a) P. K. Chattaraj and S. Giri, THEOCHEM, 2008, 865, 53 CrossRef CAS;
(b) A. Chakraborty, S. Giri and P. K. Chattaraj, THEOCHEM, 2009, 913, 70 CrossRef CAS;
(c)
S. Giri, R. P. S. AbhijithKumar, A. Chakraborty, D. R. Roy, S. Duley, R. Parthasarathi, M. Elango, R. Vijayraj, V. Subramanian, G. Merino and P. K. Chattaraj, Bonding, Aromaticity and Possible Bond-Stretch Isomerism in an “All-Metal” cluster–[Be6Zn2]2− in “Aromaticity and Metal Clusters” ed. P. K. Chattaraj, Taylor & Francis: Florida, 2010.
-
(a) D. R. Roy, S. Duley and P. K. Chattaraj, Proc. Ind. Natl. Sci. Acad. Part-A., 2008, 74, 11 CAS;
(b) P. K. Chattaraj, D. R. Roy, M. Elango and V. Subramanian, J. Phys. Chem. A, 2005, 109, 9590 CrossRef CAS.
- A. I. Boldyrev and L.-S. Wang, Chem. Rev., 2005, 105, 3716 CrossRef CAS.
-
(a) A. E. Kuznetsov and A. I. Boldyrev, Chem. Phys. Lett., 2004, 388, 452 CrossRef CAS;
(b) D. R. Roy and P. K. Chattaraj, J. Phys. Chem. A, 2008, 112, 1612 CrossRef CAS;
(c) S. Giri, A. Chakraborty, S. Duley, G. Merino, V. Subramanian and P. K. Chattaraj, ICCMSE, AIP Issue, 2009 Search PubMed (In Press);
(d) S. Giri, D. R. Roy, S. Duley, A. Chakraborty, R. Parthasarathi, M. Elango, R. Vijayraj, V. Subramanian, R. Islas, G. Merino and P. K. Chattaraj, J. Comp. Chem., 2010, 31, 1815 CAS.
- P. K. Chattaraj and S. Giri, Int. J. Quantum Chem., 2009, 109, 2373 CrossRef CAS.
- M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunga, K. A. Nguyen, S. J. Su, M. Dupuis and J. A. Montgomery, J. Comput. Chem., 1993, 14, 1347 CrossRef CAS.
-
(a) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785 CrossRef CAS;
(b) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- Gaussian 03W, revision B.03; Gaussian, Inc.: Pittsburgh, PA, 2003.
- A. R. Allouche, Gabedit—A graphical user interface for computational chemistry softwares, J Comput. Chem., 2011, 32, 174 CrossRef CAS , http://gabedit.sourceforge.net/..
- G. Schaftenaar and J. H. Noordik, Molden: a pre- and postprocessing program for molecular and electronic structures. J. Comput.-Aided Mol. Des., 2000, 14, 123 CAS.
- R. G. Parr and P. K. Chattaraj, J. Am. Chem. Soc., 1991, 113, 1854 CrossRef CAS.
- P. W. Ayers and R. G. Parr, J. Am. Chem. Soc., 2000, 122, 2010 CrossRef CAS.
- E. Chamorro, P. K. Chattaraj and P. Fuentealba, J. Phys. Chem. A, 2003, 107, 7068 CrossRef CAS.
- P. K. Chattaraj, J Indian Chem Soc, 1992, 69, 173 CAS.
- R. G. Parr, R. A. Donnelly, M. Levy and W. E. Palke, J. Chem. Phys., 1978, 68, 3801 CrossRef CAS.
- R. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 1983, 105, 7512 CrossRef CAS.
-
R. G. Pearson, Chemical hardness: Applications from molecules to solids; Wiley- VCH: Weinheim, 1997 Search PubMed.
- R. G. Parr, L. v. Szentpály and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922 CrossRef CAS.
- R. Hoffmann, P.v.R. Schleyer and H. F. Schaefer III, Angew. Chem., Int. Ed., 2008, 47, 7164 CrossRef.
- Z. Chen, C. Corminboeuf, T. Heine, J. Bohmann and P.v. R. Schleyer, J. Am. Chem. Soc., 2003, 125, 13930 CrossRef CAS.
- S. Paul and A. Misra, Inorg. Chem., 2011, 50, 3234 CrossRef CAS.
- J. H. de Boer, Adv. Catal., 1957, 9, 472 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra00643j/ |
| ‡ Current address: CIMAT, Universidad de Chile and QTC, Pontificia Universidad Católica de Chile, Avenida Vicuña Mackenna 4860, Santiago, Chile. |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 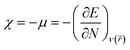
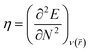
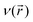 is the external potential. Electrophilicity can be calculated as49
is the external potential. Electrophilicity can be calculated as49