The life and death of carbon nanotubes
Sebastian W.
Pattinson
a,
Kirsten
Prehn
b,
Ian A.
Kinloch
c,
Dominik
Eder
d,
Krzysztof K. K.
Koziol
a,
Karl
Schulte
b and
Alan H.
Windle
*a
aDepartment of Materials Science and Metallurgy, University of Cambridge, Pembroke Street, Cambridge, CB2 3QZ, UK. E-mail: ahw1@cam.ac.uk; Fax: +44 1223 335637; Tel: +44 (0)1223 334321
bInstitute of Polymer Composites, Technische Universität Hamburg-Harburg, Denickestrasse 15, 21073, Hamburg, Germany
cSchool of Materials, University of Manchester, Grosvenor Street, Manchester, M1 7HS, UK
dInstitut für Physikalische Chemie, Westfälische Wilhelms-Universität Münster, Corrensstrasse 28/30, 48149, Münster, Germany
First published on 13th February 2012
Abstract
We investigate the growth of aligned arrays of carbon nanotubes by the pyrolysis of ferrocene and toluene. These arrays are typically grown with ferrocene being introduced throughout the reaction. This continuous injection of a catalyst precursor is unusual when compared with other substrate bound carbon nanotube production routes. We have studied the activity and lifetime of the catalyst by switching from the ferrocene–toluene catalyst precursor solution to a pure toluene feedstock during growth which was monitored in situ using a laser micrometre. The catalysts' activity after the cessation of ferrocene injection was found to depend on both the concentration of the ferrocene in the initial feedstock and the time over which it was injected. The mode of growth of the array and the causes of carbon nanotube growth termination are elucidated from the data obtained.
Introduction
While the growth of nanotubes from planar substrates as aligned carpets has been reported for more than a decade, there are a number of issues which are still debated. Central amongst these are:(a) given that the nanotubes forming a carpet are seen to have a considerable range of diameters, then why do they all grow at the same rate?
(b) even though the catalyst precursor is continuously added at a constant rate, why does the carpet growth rate appear to vary systematically with time?
(c) what causes the growth to stop, limiting the nanotube length achievable?
This paper reports the results of experiments designed to address these fairly fundamental questions.
Carbon nanotubes have been synthesised using a wide variety of methods including electrical arc-discharge,1 laser vapourisation,2 catalytic chemical vapour deposition (CVD)3 and plasma-enhanced CVD.4 The most widely used route for industrial purposes is CVD on account of the comparatively low growth temperatures required and the high yield and purity of the material produced.5–9 Typically, the catalyst precursor is either deposited on a substrate prior to synthesis, or dissolved in a hydrocarbon feedstock and then injected directly into the reaction zone so that catalyst material is supplied continuously. In the latter case, the catalyst is formed in situ via the decomposition of a vapourised precursor such as a metallocene10 or an inorganic metal salt.11
The kinetics of individual carbon nanotube growth have been studied in situ by TEM.12 However, an advantage of CVD is that the nanotubes grow in a high number density, which forces them to align mutually and perpendicular to the substrate to form an array.13–20 The kinetics of such CNT growth have been measured in situ by laser diffractography,17 time resolved reflectivity,21,22in situ photography,23 optical interference24 and laser micrometry.25
The initiation of nanotube growth depends on the synthesis method used. In systems where catalyst is pre-deposited, the formation of the catalyst particles and growth of carbon nanotubes are two distinct steps.26 In the case of nanotubes grown with the catalyst being continually supplied from the vapour phase, this process is significantly more complex as catalyst particle formation, nanotube nucleation, and nanotube growth all occur simultaneously.27 This growth of arrays by a vapour phase catalyst has been studied ex situ by growing the arrays for differing amounts of time and then examining the samples produced by electron microscopy.27
Much of the work on CNT growth kinetics has been focused on discovering the reasons for CNT growth termination. Suggested causes include limitation of carbon diffusion through the CNT array to the catalyst;28 phase change in the catalyst particle;29 catalyst shrinkage due to Ostwald ripening and diffusion into the substrate;30 and poisoning by amorphous or otherwise uncatalysable carbon on the catalyst surface.31,32
In order to understand the initiation, growth, and eventual growth termination of the carbon nanotube array in our injected catalyst system, kinetic measurements of CNT array growth were taken by an in situ technique using a laser micrometre setup that was designed and built in-house and similar to that used by Yasuda et al.25 One crucial question is whether this continuous addition of catalyst is necessary for continuous growth as it has the disadvantage of increasing the metal impurities in the nanotubes produced and cost. To address this question, the growth rates were measured for a series of experiments in which catalyst and feedstock were intitially introduced and then after a given time the introduction of catalyst was stopped with only the feedstock introduction continuing. These measurements were then correlated with further examination of the nanotube arrays by scanning election microscopy (SEM), transmission electron microscopy (TEM) and energy dispersive X-ray (EDX) analysis.
Methods
The experimental setup was based upon that previously used by Singh et al.16 A solution of ferrocene catalyst precursor in toluene was injected into a pre-heated carrier gas stream at 180 °C. The gas stream then swept the evaporated feedstock into the reactor zone held at 760 °C where silica growth substrates were placed. To interrupt the ferrocene injection, a second syringe was used that contained pure toluene. The reaction started by injecting the ferrocene–toluene solution for a certain time, after which the solution flow was stopped and switched over to the pure toluene for the rest of the reaction. Typically both solutions were injected parallel for the duration of ferrocene and toluene injection to compensate for any dead-volume in the second syringe and ensure continuous injection of feedstock. For this work, ferrocene concentrations of 2 wt%, 5 wt% or 9.6 wt% in toluene were used. All of the feedstock was injected into a quartz tube furnace (diameter = 65 mm) at an overall injection rate of 5.6 ml hr−1, with Ar as the carrier gas flowing at 1.4 l min−1.A high-speed laser micrometre (MICRO-EPSILON optoCONTROL 2600) was used to measure the height of the nanotube arrays during growth. A significant issue with this approach was that the nanotubes grew on the reaction tubes' walls as well as the substrates, making the tubes opaque. This difficulty was overcome by using a reaction tube which had two side arms in line with the growth substrates. These side arms went through instrumentation access holes in the furnace elements and were terminated with optical windows. The laser micrometre could then be shone through these windows. The intrinsic precision of the method (∼ ± 1 μm) was in excess of the uniformity of the growth rates across the growing nanotube array. The greatest source of error was the possibility of the nanotubes at the front and back edges (w.r.t. the laser beam) of the array growing slightly more quickly and thus dominating the measurement. The effect was checked for by growing the nanotubes on the surface of a silica tube, some 3 cm in diameter, so that the measurements were tangential to the very gently curving array. The detector output was integrated over periods varying between 5 and 10 mins before data readout as shown by the time spacing of the data points in Fig. 1. Post-growth analysis of the nanotubes and their catalysts was conducted ex situ by field emission gun SEM (Hitachi S-5500, Jeol 6340) and TEM (FEI Tecnai F20, Jeol 200CX). Samples for analysing the catalysts on the silica were prepared by cutting the CNT array off the substrate using a razor blade and looking at the substrate in the SEM. The spatial distribution of the iron in the nanotubes was analysed in the SEM using EDX.
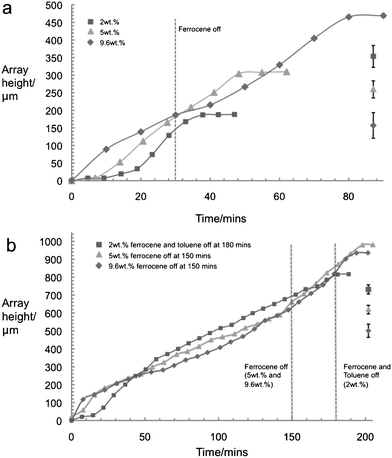 | ||
| Fig. 1 (a) Growth rates of nanotubes synthesized using ferrocene injected for 30 min at 2 wt.%, 5 wt.%, and 9.6 wt.%, followed by pure toluene injection till growth end. At 2 wt.% ferrocene, growth ceases very quickly after ferrocene injection is ended. At 5 wt.% and 9.6 wt.% growth is able to continue in the absence of ferrocene. Following injection at 9.6 wt.% ferrocene there is even a higher CNT array growth rate in pure toluene. (b) Growth rates of CNT arrays grown with ferrocene and toluene injection at 2 wt.% for 180 min, and at 5 wt.% and 9.6 wt.% for 150 min and then with pure toluene till growth end. The growth rate of the higher ferrocene concentration arrays begins to level off more quickly than it does at the lower concentration. Following this, there is a significant period of linear growth during which array growth rate is independent of ferrocene concentration. Once ferrocene and toluene injection ceases, growth ends quickly. The dotted line indicates the time at which injection was stopped. Error bars for the CNT array height are shown on each graph. | ||
Results and discussion
Fig. 1(a) shows the growth of the nanotubes with time for 3 different concentrations of ferrocene in toluene. For each experiment the feed of the ferrocene–toluene solution was switched to pure toluene after 30 min with the volumetric flow rate of feedstock being kept constant. In the case of the 2 wt.% ferrocene in toluene, there is an incubation period of 19 min. This is followed by steady growth until ferrocene injection is stopped. After 8 min of toluene only injection the growth rate decays to zero. For the case of 5 wt.% ferrocence in toluene, the incubation time is reduced to 7 min. During ferrocene and toluene injection the growth rate begins to decay. Once the injection has been switched to pure toluene (after 30 min, as in the 2 wt.% case), however, the growth rate initially decreases slightly, then recovers to almost its original value up until the point where growth ends, 20 min after the ferrocene was stopped. For the 9.6 wt% ferrocene solution the incubation time is too short to be measured accurately, but while the initial growth rate is not dissimilar from that at the two more dilute ferrocene solutions, it soon begins to decay even though ferrocene is still being added, reaching about half of its initial value at the point where the ferrocene is stopped. The growth rate then continues to decrease for a further two minutes, but, as with the 5% case, it then increases again reaching a value similar to the initial part of the curve after 60 min (30 min after ferrocene cessation). The final decay in rate (70 min) preceded cessation in growth 10 min later.Fig. 1(b) shows growth curves for longer injection times. The incubation period and initial decay in growth rate are identical to those in Fig. 1(a), as is to be expected. Following the decay in the initial growth rate, a constant linear growth rate of 3.8 ± 0.1 μm min−1 is established regardless of ferrocene concentration. The sample grown at 2 wt.% ferrocene in toluene for 180 min ceases growth almost immediately following the end of ferrocene and toluene injection. The sample grown at 5 wt.% ferrocene in toluene for 150 min is able to continue growth for 50 min after ferrocene injection is ended, significantly longer than the array grown with 5 wt.% ferrocene in toluene for only 30 min. The array grown at 9.6 wt.% ferrocene in toluene for 150 min is able to grow for 45 min during toluene only injection, which is slightly less than the duration of growth in toluene of the array grown with 9.6 wt.% ferrocene for 30 min.
The behaviour of these growth curves is sufficiently intriguing to warrant closer examination.
Incubation and establishment of entangled crust
The incubation stage is significant although not unexpected. It being the period in which the deposited iron has to establish nuclei of sufficient dimensions to enable tube growth itself to commence. The iron from ferrocene decomposition will arrive at random on the substrate as metal atoms, or possibly small particles resulting from gaseous collisions, and then migrate by surface diffusion to the nucleation points of the catalyst. These particles then grow to the size at which tube growth is established, the point being reached after different delay times for each particle. However, microscopic examination of the early stages of growth and that part of established nanotube carpets which arises from the early stages, i.e. the top of the carpet, show that the situation is more interesting. Fig. 2(a,b) show early stages of nanotube growth, where it is clear that the density of active nuclei is still low and that the packing fraction of the nanotubes is still too low to constrain them into alignment. The early nanotubes will thus entangle, which is shown clearly in Fig. 2(c), an SEM micrograph of the top of an established carpet. The entangled region, or crust, is clearly visible.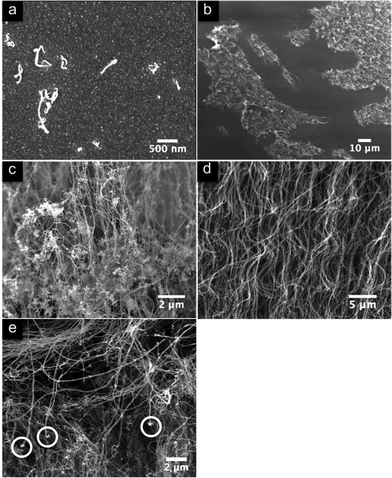 | ||
| Fig. 2 SEM images of the different stages of CNT growth. (a) After 2 min of 5 wt.% ferrocene in toluene injection catalyst particles have formed and some CNTs are growing. (b) After 7.5 min of this, islands of partially aligned CNTs have formed resulting from increasing packing density. (c) The top of a CNT array. Visible is the ‘crust’ of entangled CNTs formed during the early stages of growth. The crust also contains some single wall tubes, possibly nucleated from catalyst particles at the very early stages of growth. (d) A section of a carpet grown at 5 wt.% ferrocene in toluene. Visible are the slower growing nanotubes that grow straight, and the faster growing ones that are bent due to their attachment to the slower ones. (e) A part of a CNT array grown at 9.6 wt.% ferrocene in toluene for 30 mins and subsequently pure toluene for 60 min. Highlighted are ends of CNTs with catalyst particles attached that have been pulled off the substrate by their neighbours. The region examined is that close to the point of ferrocene cessation. | ||
The considerable significance of this entangled region is that it means that all the nanotubes will be constrained to grow at an average rate, so that some will be held back and others pulled. A further consequence is that the tubes which may naturally be growing faster will tend to buckle while those which are naturally slower growers will be stretched and appear particularly straight. This effect can be seen in Fig. 2(d). If the nanotube is stretched sufficiently it will be lifted off the substrate as in Fig. 2(e). The ends of the catalyst particles in Fig. 2(e) most likely did not grow by tip growth, since under this model the nanotube would be growing in the direction opposite to that of the array surrounding it.
Furthermore, CNTs that buckle are unlikely to exert significant force on the array, whilst CNTs growing more slowly than the array have a limited ability to straighten and, like a taut string, will exert a stronger force on the array. The growth rate of the array as a whole will therefore be dominated by the slowest growing CNTs within it.
Linear growth
A significant aberration is that the rate of growth decreases from its initial value with time, even though the ferrocene injection is still continuing. This decrease is more rapid at higher ferrocene concentrations. As we shall see later the growth rate accelerates again after the ferrocene injection ceases, so it cannot be an issue of diffusion of the reactants through the carpet of increasing thickness as previously indicated.10 Instead, we suggest that the ever increasing size of the iron catalyst particle means that the dissolving carbon cannot diffuse to the growing ends of the multiwall nanotube sufficiently rapidly.After this initial decay, the arrays reach a linear growth rate that appears independent of the rate at which the catalyst is delivered. This is particularly visible in Fig. 1(b), which shows plots of array growth for up to 205 min. The injection at 2 wt.%. ferrocene in toluene was continued for 180 min followed by cessation of both ferrocene and toluene injection. Mixtures of 5 wt.% and 9.6 wt.% ferrocene in toluene were injected for 150 min followed by pure toluene till growth end. The identical growth rate suggests that the slowest growing CNTs, which dominate array growth rate, grow at similar rates regardless of ferrocene concentration. That this growth rate reaches a steady level suggests that the distance for carbon to diffuse from catalyst surface to the growing CNT edge equilibrates in these CNTs.
Comparing Fig. 1(a) and (b) it is also notable that additional ferrocene injection at 9.6 wt.% is not able to increase the time the array is able to grow in pure toluene. In combination with the more rapid decay in initial CNT growth rate seen at higher ferrocene concentrations, this suggests that there is excess iron delivered to the system at these concentrations. The next question concerns what happens to this iron.
Excess iron
There are three possibilities. Firstly, the iron may be diffusing into the silica substrate. We looked for evidence of this by stripping off an array from the substrate with a razor blade and then carrying out an EDX scan. This showed only trace amounts of iron combined with the silica surface layers. There is, however, very clear evidence of iron contained within the nanotubes, and also smaller catalyst particles deposited on their outsides. Fig. 3(a) shows an iron particle contained within the tube, implying that there is a continuous flow of iron, in excess to that required to maintain the catalyst particle, inwards from the periphery of this particle towards its core where components are detached by surface tension forces into the core of the tube and move upwards as nanotube growth continues. Fig. 3(b) shows a small catalyst particle on the outside of a CNT. We suggest that the excess external iron has been deposited from the vapour phase. The third possibility is that, as a result of the continually arriving iron, the catalyst particles simply get bigger. Fig. 4(a–c) show that the size of the catalyst particles, from which the nanotubes have been mechanically removed, increases with the series 2 wt.%, 5 wt.% and 9.6 wt% ferrocene, as one would expect given that CNT diameters increase as well.16 Whilst a significant proportion of the excess iron arriving at the substrate is removed by becoming incorporated in the tube cores, even this is not enough to keep pace with the rate of arrival of new iron, and this additional metal simply adds to the size of the particles and the diameter of the CNTs that grow from them. The implications of Fig. 4(d) will be discussed below. | ||
| Fig. 3 (a) An iron catalyst particle that has been pulled into a CNT during growth. (b) An image of catalyst on the outer wall of a CNT. | ||
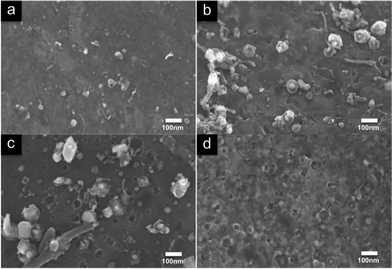 | ||
| Fig. 4 Secondary electron images of catalyst produced by (a) 2 wt.% (b) 5 wt.% and (c) 9.6 wt.% ferrocene injection for 1 h. Catalyst size increases with ferrocene concentration, the active particles are most likely the larger ones which often feature a dark ring where the nanotube was and a bright spot in the centre. (d) The substrate after 1 h of injection at 5 wt.% ferrocene followed by toluene injection till growth end. The catalyst appears covered with carbon. | ||
Ferrocene termination
That for the 5 wt.% and 9.6 wt.% ferrocene in toluene samples, tube growth continued for significant periods after ferrocene was terminated is not surprising, and additional support for the conclusion that the initial slowing of growth rate is the result of excessive iron build up on the catalyst particle. Even after ferrocene injection has ceased, iron is still being taken up as discrete particles into the core of the nanotubes. While these can be detected by TEM, it is also shown by the line of EDX sampling points (Fig. 5) across both the ferrocene and non-ferrocene sectors of a 9.6 wt.% ferrocene sample. Although the iron is reduced within the nanotubes once the ferrocene is stopped, it does not reduce to zero. The iron remaining in the tubes can only have come from the terminal particle which must therefore be shrinking. Thus the terminal particle will reduce to the point where carbon diffusion is no longer rate controlling, and the growth curves steepen to regain their original slope. | ||
| Fig. 5 EDX line scan for iron taken along the length of an array grown at 9.6 wt.% ferrocene in toluene for 30 min, with toluene injection till growth ends. The iron readings are shown at the position on the array where they were taken. The bright band in the secondary electron image is the result of CNT ends protruding from the array and charging in the electron beam. | ||
Another aspect is that close examination of the region close to the point at which the ferrocene was terminated in the 9.6 wt.% sample, shows a number of nanotubes which have obviously been pulled away from the substrate complete with their catalyst particle. A few such terminations are highlighted in Fig. 2(e), noting in passing that when the nanotubes are pulled off at room temperature, as opposed to the synthesis temperature, the iron is left behind on the substrate (Fig. 4). The highlighted band in the region of ferrocene termination in Fig. 5 is thought to arise from charging of these tube ends. The cessation of ferrocene injection appears to slow the growth rate for some nanotubes to a point where they are pulled from the substrate by their less tardy neighbours, the stress being transferred via the entangled crust (Fig. 2(c)). The reasons for this termination of nanotube growth are discussed next.
Final termination of growth
Once the second phase linearity in array growth is established, there comes a final point when the growth stops relatively rapidly even though hydrocarbon is still being provided (Fig. 1). One possibility is that all the catalyst iron has found its way into the tube cores, and thus the process has run out of terminal catalyst from which the tubes grow. However, such a process would require that the surface tension forces drawing the iron up the nanotube are sufficient to draw the iron away from the edges of the graphite cylinders at which growth is continuing. In any case, the high EDX iron reading at the base of the array still indicates the presence of basal catalyst particles. A second possibility is that the catalyst is poisoned in some way, and a clue is found in Fig. 4(d). Here the nanotubes have been intentionally stripped from the substrate of a 5 wt.% sample after cessation of growth some 35 min after the ferrocene injection was terminated. The catalyst particles are still visible, however, they appear to be buried under a carbon film, identified by an EDX scan. Indeed it appears that throughout the process carbon, presumably amorphous and likely to be compounded with hydrogen, has deposited on the substrate between the catalyst particles. It is likely that this takes place during both the catalyst injection phase, as well as during pure toluene injection. A check on this possibility was made by injecting toluene without any iron at all. Of course there were no nanotubes, but a steady build up of carbon on the substrate resulting from the pyrolysis of the toluene. Once the iron is no longer arriving to build up the catalyst particles, the gradually rising ‘tide’ of amorphous carbon eventually drowns the iron particles, denying access of active carbon species capable of increasing the activity of carbon in the iron beyond that which would form an equilibrium with the growing nanotubes. This view is supported by the fact that growth termination occurs relatively quickly after the period of toluene only injection, in fact as quickly as when both toluene and ferrocene are switched off together (Fig. 1(b)).Conclusion
A model is presented which is based on the origin and role of an entangled crust on the surface. It explains why all the nanotubes of a carpet, at first sight, appear to grow at the same rate. A combination of characterization techniques has demonstrated that the growth rate of carbon nanotubes in an array varies considerably, but is dependent on the size of the iron particle at their base. We have also elucidated the precise form in which this can be affected: the rate of iron addition, the loss of the catalyst into the nanotube, and drowning of the catalyst by amorphous carbon formed as a by product of pyrolysis of the carbon source. Such indications help to focus future strategies for more precise control of nanotube growth.Acknowledgements
SWP would like to thank the EPSRC for funding. IAK woud like to thank the RAE. KK would like to thank the Royal Society.References
- E. G. Gamaly and T.W. Ebbesen, Phys. Rev. B: Condens. Matter, 1995, 52, 2083 CrossRef CAS.
- A. Thess, R. Lee, P. Nikolaev, H. Dai, P. Petit, J. Robert, C. Xu, Y. H. Lee, S. G. Kim, A. G. Rinzler, D. T. Colbert, G. E. Scuseria, D. Tomanek, J. E. Fischer and R. E. Smalley, Science, 1996, 273, 483 CAS.
- M. José-Yacamán, M. Miki-Yoshida, L. Rendon and J. G. Santiesteban, Appl. Phys. Lett., 1993, 62, 657 CrossRef.
- Z. F. Ren, Z. P. Huang, J. W. Xu, J. H. Wang, P. Bush, M. P. Siegal and P. N. Provencio, Science, 1998, 282, 1105 CrossRef CAS.
- D.-H. Kim, H.-S. Jang, C.-D. Kim, D.-S. Cho, H.-S. Yang, H.-D. Kang, B.-K. Min and H.-R. Lee, Nano Lett., 2003, 3, 863 CrossRef CAS.
- H. Cui, G. Eres, J. Y. Howe, A. Puretkzy, M. Varela, D. B. Geohegan and D. H. Lowndes, Chem. Phys. Lett., 2003, 374, 222 CrossRef CAS.
- I. Kinloch, M. S. P. Shaffer, Y. M. Lam and A. H. Windle, Carbon, 2004, 42, 101 CrossRef CAS.
- C. Gommes, S. Blacher, C. Bossuot, P. Marchot, J. B. Nagy and J.-P. Pirard, Carbon, 2004, 42, 1473 CrossRef CAS.
- M. Bronikowski, Carbon, 2006, 44, 2822 CrossRef CAS.
- C. Singh, M. S. P. Shaffer, K. K. K. Koziol, I. A. Kinloch and A. H. Windle, Chem. Phys. Lett., 2003, 372, 860 CrossRef CAS.
- H. Hou, A. K. Schaper, Z. Jun, F. Weller and A. Greiner, Chem. Mater., 2003, 15, 580 CrossRef CAS.
- R. Sharma, P. Rez, M. M. J. Treacy and S. J. Stuart, J. Electron Microsc. Tech., 2005, 54, 231 CAS.
- R. Andrews, D. Jacques, A. M. Rao, F. Derbyshire, D. Qian, X. Fan, E. C. Dickey and J. Chen, Chem. Phys. Lett., 1999, 303, 467 CrossRef CAS.
- C. Singh, M. Shaffer, I. Kinloch and A. Windle, Phys. B, 2002, 323, 339 CrossRef CAS.
- S. B. Sinnott, R. Andrews, D. Qian, A. M. Rao, Z. Mao, E. C. Dickey and F. Derbyshire, Chem. Phys. Lett., 1999, 315, 25 CrossRef CAS.
- C. Singh, M. S. P. Shaffer and A. H. Windle, Carbon, 2003, 41, 359 CrossRef CAS.
- L. M. Dell'Acqua-Bellavitis, J. D. Ballard, P. M. Ajayan and R. W. Siegel, Nano Lett., 2004, 4, 1613 CrossRef CAS.
- C. P. Deck and K. Vecchio, Carbon, 2005, 43, 2608 CrossRef CAS.
- M. Pinault, V. Pichot, H. Khodja, P. Launois, C. Reynaud and M. Mayne-L'Hermite, Nano Lett., 2005, 5, 2394 CrossRef CAS.
- Y. J. Jung, B. Wei, R. Vajtai and P. M. Ajayan, Nano Lett., 2003, 3, 561 CrossRef CAS.
- A. A. Puretzky, D. B. Geohegan, S. Jesse, I. N. Ivanov and G. Eres, Appl. Phys. A: Mater. Sci. Process., 2005, 81, 223 CrossRef CAS.
- D. B. Geohegan, A. A. Puretzky, I. N. Ivanov, S. Jesse, G. Eres and J. Y. Howe, Appl. Phys. Lett., 2003, 83, 1851 CrossRef CAS.
- A. A. Puretzky, G. Eres, C. M. Rouleau, I. N. Ivanov and D. B. Geohegan, Nanotechnology, 2008, 19, 5560 CrossRef.
- D.-H. Kim, H.-S. Jang, C.-D. Kim, D.-S. Cho, J.-G. Jee and H.-R. Lee, Nanotechnology, 2003, 14, 46 CrossRef CAS.
- S. Yasuda, D. N. Futaba, M. Yumura, S. Ijima and K. Hata, Appl. Phys. Lett., 2008, 93, 143115 CrossRef.
- W. Z. Li, S. S. Xie, L. X. Qian, B. H. Chang, B. S. Zou, W. Y. Zhou, R. A. Zhao and G. Wang, Science, 1996, 274, 1701 CrossRef CAS.
- I. Kunadian, R. Andrews, D. Qian and M. P. Mengüç, Carbon, 2009, 47, 384 CrossRef CAS.
- G. Zhong, T. Iwasaki, J. Robertson and H. Kawarada, J. Phys. Chem. B, 2007, 111, 1907 CrossRef CAS.
- X. Wang, Y. Feng, H. E. Unalan, G. Zhong, P. Li, H. Yu, A. I. Akinwande and W. I. Milne, Carbon, 2011, 49, 214 CrossRef CAS.
- S. M. Kim, C. L. Pint, P. B. Amama, D. N. Zakharov, R. H. Hauge, B. Maruyama and E. A. Stach, J. Phys. Chem. Lett., 2010, 1, 918 CrossRef CAS.
- M. Stadermann, S. P. Sherlock, J.-B. In, F. Fornasiero, H. G. Park, A. B. Artyukhin, Y. Wang, J. J. De Yoreo, C. P. Grigoropoulos, O. Bakajin, A. A. Chernov and A. Noy, Nano Lett., 2009, 9, 738 CrossRef CAS.
- L. Ni, K. Kuroda, L.-P. Zhou, T. Kizuka, K. Ohta, K. Matsuishi and J. Nakamura, Carbon, 2006, 44, 2265 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
