Silver oxide particles/silver nanoparticles interconversion: susceptibility of forward/backward reactions to the chemical environment at room temperature†
Oscar A. Douglas
Gallardo
,
Raquel
Moiraghi
,
Micaela A.
Macchione
,
Jorge A.
Godoy
,
Manuel A.
Pérez
*,
Eduardo A.
Coronado
and
Vicente A.
Macagno
INFIQC—Departamento de Fisicoquímica, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Ciudad Universitaria, 5000, Córdoba, Argentina. E-mail: mperez@fcq.unc.edu.ar; Fax: +54 351 4334188; Tel: +54 351 4334180
First published on 13th February 2012
Abstract
The thermal stability of the silver oxide particles (Ag2O)/metallic silver nanoparticles (AgNPs) system in aqueous and gaseous environments is investigated with UV-Visible spectroscopy, TEM, SEM and DLS as characterisation techniques, and with calculations using electromagnetic theory. Thermal decomposition of aqueous Ag2O colloids to produce AgNPs is conclusively demonstrated and used as a base reaction to produce clean AgNPs without any external reducing agent. Such a spontaneous character of Ag2O decomposition in alkaline aqueous/water-enriched environments at room temperature makes the formation of silver oxide films on silver nanoparticles/nanostructures unlikely, keeping the silver surface oxide-free, a crucial feature in determining the silver catalytic and Raman enhancing properties. The synthetic suitability of this reaction to develop new routes to produce AgNPs is explored by analyzing the effect of temperature, complexing agents, and environment polarity on the AgNPs size/shape control. Thermal decomposition of Ag2O colloids in aqueous/water-enriched environments offers the possibility to produce AgNPs at low cost, with easy, clean, safe and green chemistry procedures.
1 Introduction
With applications in oxidation catalysis,1 sensors,2 fuel cells,3 photovoltaic cells,4 all-optical switching devices and optical data storage systems,5 silver oxide (Ag2O) is a versatile material. In gaseous environments, it is well known that Ag2O is stable below 170 °C,6a the temperature at which its thermal decomposition takes place to produce O2 and metallic silver. In contrast with the numerous studies about Ag2O thermal decomposition in gaseous environments,6 there is no record about the stability of Ag2O in aqueous conditions, to the best of our knowledge. The information available suggests that thermal Ag2O stability up to 170 °C for gaseous environments might have led to the idea of similar behaviour in aqueous environments. Despite the speculative character of such a statement, there are clues supporting it. a) Precipitation starting from aqueous silver salt solutions and strong alkali is a procedure commonly used to produce silver oxide.6a,6b,7 b) Concerning metallic silver substrates, an important effort is devoted to studying the surface-enhanced Raman spectroscopy (SERS) activity, mostly considering the effect of either the Ag2O generated by silver oxidation with O2 dissolved in aqueous environment8a or protective layers that can prevent that oxidation.8b The preservation of the surface properties of AgNPs is also an important aspect considered for their use in catalysis.9In such a context we have come across a simple fact that seems to have gone unnoticed for a very long time: aqueous colloids of Ag2O undergo decomposition to give AgNPs at room temperature. On the one hand, the relevance of such a finding lies on the importance of silver oxide as a material itself, but also on the synthetic use of this reaction for AgNPs production. On the other hand, due to the role AgNPs play in many areas such as catalysis, optolectronics, plasmonics, etc.,10 it is nowadays of topmost importance to have clean methods to produce them. Better synthesis routes involving low cost, easy, clean, safe and green chemistry procedure is a topic under constant development.11
In the present work, we demonstrate for the first time that aqueous Ag2O colloids undergo thermal decomposition at room temperature to give AgNPs. This new evidence is discussed in formulating a more general reaction scheme, which considers silver oxidation and Ag2O thermal decomposition from a unified viewpoint. In light of this scheme, the stability of silver oxide-free surface is analysed, given the key role in catalysis and enhanced Raman spectroscopy that having a clean silver nanoparticle/nanostructure surface plays.8,9 Finally, the potential application of the Ag2O thermal decomposition as base reaction to develop new synthetic routes to produce AgNPs is analysed.
2 Experimental and theoretical simulations
2.1 Experimental
All stock solutions were prepared from AR chemicals and purified water (Milli Ro-Milli Q system). Soluble silver salts solutions (AgNO3, AgClO4) were kept in darkness to prevent any photochemical reaction involving the silver ion (Ag+).| 2Ag+(aq) + 2OH−(aq) → Ag2O(s) + H2O(l) | (1) |
Two cases of interest were investigated, namely: either the ions concentration product is slightly greater than Ksp ([Ag+] × [OH−] > Ksp) or it largely exceeds Ksp ([Ag+] × [OH−] ≫…> Ksp). In accordance with the concept of chemical equilibrium, the amount of Ag2O colloid is directly related to the difference between the species initial concentration values (i.e. [Ag+] × [OH−]) and those equilibrium concentration values, let us say [Ag+]e and [OH−]e, that satisfy Ksp = [Ag+]e × [OH−]e. Thus, in the last case, the formation of the colloid in the silver (I)/alkali solution is evident since silver oxide microparticles (Ag2O–MPs) exhibit a characteristic brown color. In the first case, the amount of Ag2O–MPs formed is very low and no remarkable extinction is observed in the visible range. As it will be shown later the decomposition takes place almost irrespective of the amount of Ag2O–MPs and, consequently, all Ag+/OH− solutions with [Ag+] × [OH−] over Ksp will be referred to as silver (I)/alkali solutions. Therefore, to study the aqueous Ag2O thermal stability, silver (I)/alkali solutions containing different amounts of Ag2O–MPs were aged in darkness at 2 °C and at 25 °C.
2.2 Characterisation
UV-Visible spectra were recorded with a Shimadzu UV-1200 spectrometer either by using a 1 cm quartz cell at room temperature to periodically analyze the silver (I)/alkali solution optical behaviour along the reaction time or by directly measuring optical properties of deposits on glass substrates. The time scale of the thermal decomposition of the Ag2O largely exceeds the time elapsed during the spectra acquisition; therefore silver (I)/alkali solutions incubated at 2 °C were warmed up to room-temperature before recording the spectra.Selected experimental samples were characterized with Transmission Electron Microscopy (TEM, JEM-JEOL 1120 microscope), preparing samples without any purification treatment by seeding many drops of the colloidal solutions onto a Formvar-covered cooper grid and evaporating it in air at room temperature. Samples for characterization with Scanning Electron Microscopy (SEM, Carl Zeiss-Σigma, Field Emission) were prepared with the already described deposition procedure by driving the complete dry-up of silver (I)/alkali solutions.
An additional source of information on size distribution of Ag2O–MPs and AgNPs was obtained from Dynamic Light Scattering (DLS) by using a Beckman Coulter-Delsa Nano C to perform measurements with a laser light source at 658 nm and a detection angle of 165°. A digital correlator was used to develop an autocorrelation function, analysed with the method of cumulants resulting in a z-averaged diameter, dz (intensity-weighted mean hydrodynamic diameter). Results are referred to in the discussion but included only in the Electronic Supplementary Information material.†
2.3 Theoretical modelling
The extinction spectra of silver spheres were calculated using the exact electrodynamic solution as given by Mie theory13 using the dielectric constant of silver tabulated by Palik.14a The effect of electron confinement (surface scattering) on the dielectric constant was not considered, given that this effect is negligible for the 30–60 nm diameter range.15 All the spectra were scaled to match the intensity value of the experimental data as the purpose of this calculation is to show mainly the shape and position of the plasmon peak of silver nanoparticles with sizes representative of those determined by TEM. For the particular case of a Ag2O sphere, the dielectric constant values reported by different literature sources14b–d were used as input parameter for Mie theory calculations. In order to interpret the optical behavior of Ag2O deposits attached to glass substrates by assuming a thin slab, the use of effective medium theory was necessary, because real samples exhibit a non homogeneous distribution of material (ESI†).3 Results and discussion
3.1 Ag2O aqueous colloids
Ag2O precipitation driven with Ag+ and a strong alkali (eqn (1)) is a very well known reaction that has been used for a long time. In many works that study Ag2O stability in gaseous environments,6 the starting material is frequently obtained in a three-step preparation protocol: by driving Ag2O colloid precipitation, performing decantation and drying. The consideration of silver oxide colloids only as a raw material might lead researchers to overlook its thermo-lability that remained unnoticed for a long time. Here we will provide evidence proving the existence of a thermally activated decomposition of aqueous Ag2O colloids at room temperature. Freshly prepared silver (I)/alkali solutions with [Ag+] × [OH−] slightly over Ksp, where only very small Ag2O particles (sizes in the sub-micrometre range) are expected to be present, exhibit a profile with small extinction values and no remarkable spectral features in the visible range. Low extinction values in the 400–1100 nm range indicate that precipitation does not produce significant amounts of Ag2O–MPs. The spectral characteristics remain unaffected when this silver(I)/alkali solution is aged over a month at 2 °C (Fig. 1a, black dashed line), a fact indicating that Ag2O colloid is stable at this temperature. The aging at 25 °C of silver (I)/alkali solutions with similar composition during the same period leads to a drastically different spectral evolution (Fig. 1a, color line series). A broad maximum at ca. 413 nm can be noticed already after one day of reaction (Fig. 1a, green line). As time elapses, the peak extinction gradually increases, whereas its wavelength value remains almost constant (Fig. 1a, evolution indicated by the arrow). The interpretation of this optical feature can be made by using very well known and basic chemistry to perform tests on samples of the silver (I)/alkali solution at the stage of 30 days of aging at room temperature (Fig. 1a and b, black line). The addition of concentrated NH3 does not produce any changes in the spectroscopic profile (Fig. 1b, red line). This result clearly indicates that the extinction peak at 413 nm is not related to Ag2O particles; otherwise the peak should vanish, since silver oxide is selectively dissolved by ammonia according to:| Ag2O(s) + 4NH3(aq) + H2O(l) →2[Ag(NH3)2]+(aq) + 2OH−(aq) | (2) |
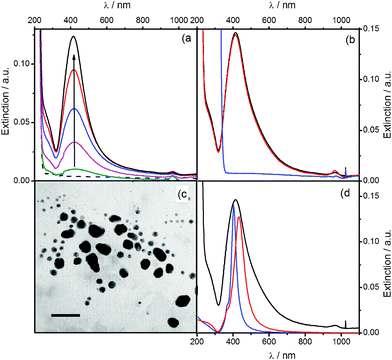 | ||
| Fig. 1 (a) UV-Vis extinction spectra for 0.1 mM AgNO3, 1 mM NaOH solutions: (---) after 30 days of aging at 2 °C, and during 30 days of aging at 25 °C: (green) 1, (pink) 6, (blue) 9, (red) 15, and (black) 30 days. (b) UV-Vis extinction spectra of (black) 30 days-aged 0.1 mM AgNO3, 1 mM NaOH solution after its reaction with: (red) NH3 and (blue) HNO3. (c) TEM image of AgNPs produced in a 0.1 mM AgNO3, 1 mM NaOH solution aged 30 days at 25 °C (scale bar: 50 nm). (d) Extinction profiles obtained with Mie theory for silver spheres with diameter of: (blue) 30 and (red) 60 nm. (black) UV-Vis spectrum of a 30 days-aged 0.1 mM AgNO3, 1 mM NaOH solution. | ||
The peak at 413 nm must be, instead, associated with the surface plasmon resonance (SPR) of silver metallic nanoparticles generated by the decomposition of the aqueous Ag2O colloids. This interpretation can be easily corroborated with the addition of concentrated HNO3, which drives the oxidation of AgNPs, according to
| 3Ag(s) + 4HNO3(aq) →3Ag+(aq) + 3NO−3(aq) + NO(g) + 2H2O(l) | (3) |
Of course, the oxidation of the AgNPs causes the complete vanishing of the peak at 413 nm (Fig. 1b, blue line). This evidence and its interpretation are consistent with TEM photographs obtained from a sample of the silver (I)/alkali solution aged 30 days at room temperature, where metallic AgNPs can be observed (Fig. 1c). Sizes representative of those in the TEM image were used to perform calculations with Mie theory for silver spheres. The simulations are in qualitative agreement with the peak observed experimentally at ca. 413 nm (Fig. 1d, black and red lines), providing additional support to the interpretation that associates this optical feature with the SPR of metallic AgNPs.13,15 With the presence of metallic AgNPs unambiguously proven only after an aging period at 25 °C of silver (I)/alkali solutions, which do not contain any reducing agent, the only consistent explanation is to acknowledge the existence of a thermal decomposition of Ag2O colloids.
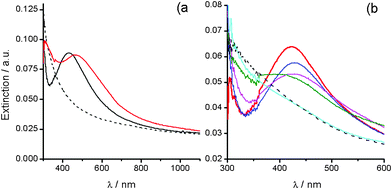
The decomposition process of colloidal Ag2O at 25 °C is also observed for silver (I)/alkali solutions containing microparticles (Ag2O–MPs). Setting [Ag+] × [OH−] to largely exceed Ksp leads to silver (I)/alkali solutions containing Ag2O–MPs, which appear as brown suspensions. These silver (I)/alkali solutions exhibit an extinction spectrum with a broad maximum at 650 nm (Fig. 2a, black line), associated with Ag2O–MPs. This interpretation is easily corroborated with the addition of concentrated NH3 that leads to the complete vanishing of this broad band, according to eqn (2). Sizes of Ag2O–MPs were estimated to be around 200 nm using DLS (ESI-Fig. 1†), however, a detailed size characterization is a task that involves methodological limitations (ESI-B†).
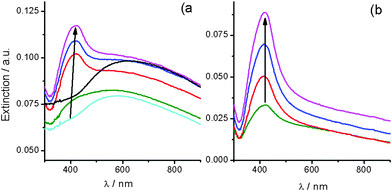 | ||
| Fig. 2 (a) UV-Vis extinction spectra evolution of a silver (I)/alkali solution containing Ag2O–MPs during its aging at 25 °C, for: (black) 0 (freshly prepared), (cyan) 3, (green) 10, (red) 24, (blue) 33, and (pink) 38 days. (b) Extinction spectra of metallic AgNPs produced by the decomposition at 25 °C of a Ag2O–MPs containing silver (I)/alkali solution, revealed with concentrated ammonia addition for increasing reaction time: (green) 17, (red) 24, (blue) 38, and (pink) 50 days. | ||
As the aging time at 25 °C elapses, an extinction peak at ca. 420 nm is gradually resolved, indicating the formation of metallic AgNPs (Fig. 2a, arrow in the color line series). Since the extinction band of Ag2O–MPs (ca. 650 nm) remains during the aging, spectra correspond to the contribution of both, Ag2O–MPs and metallic AgNPs. The addition of concentrated NH3 into aliquots extracted for increasing reaction times allows us to selectively dissolve the remaining Ag2O–MPs and reveal the contribution to spectra coming only from AgNPs. Spectra obtained following this procedure, quite impressively, provide a clear picture of how the formation and growth of metallic AgNPs evolves with time (Fig. 2b). The increase of the SPR peak at ca. 420 nm (Fig. 2b, pointed by the arrow) indicates a growing number of AgNPs with sizes below 50 nm. The extinction tail that follows the peak for longer wavelength values could be associated with particles larger than 50 nm and/or with non spherical shapes.16 This evidence is consistent with a reaction scheme involving continuous nucleation and growth processes taking place simultaneously.16
3.2 Ag2O deposits
Deposits prepared from silver (I)/alkali solution dry-up help us to obtain additional evidence on the thermal decomposition of Ag2O colloids. Regarding the conclusions of the previous section, the only process expected during the drying of aqueous silver (I)/alkali solutions at 2 °C should be the Ag2O deposition (Fig. 3, black solid line). Ag2O deposits on glass exhibit a profile of decreasing extinction as the wavelength increases, interpretation easily corroborated by performing simple chemical tests. The immersion of deposits in concentrated ammonia leads to the Ag2O dissolution, according to eqn (2), and a flat extinction profile corresponding to the glass substrate is obtained (Fig. 3a, red line).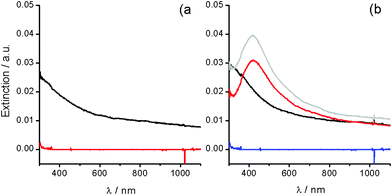 | ||
| Fig. 3 UV-Vis extinction profile for (black) deposits on glass obtained from a silver (I)/alkali solution drying at 2 °C. (a) (red) after two minutes immersion in concentrated NH3 solution. (b) (grey) after annealing for 2 min. at ca. 200 °C, and after the subsequent immersion in: (red) concentrated NH3 solution, and (blue) concentrated HNO3 solution. | ||
Ag2O deposits undergo thermal decomposition by annealing and the characteristic AgNPs' SPR profile is obtained (Fig. 3b, grey line). The treatment with ammonia of these annealed deposits leads to a small decrease of the extinction (Fig. 3b, red line), that might be associated with the dissolution of soluble electrolyte species co-deposited (NaNO3, NaOH). The profile shape remains unaffected, however, in consistency with the specificity of NH3 in dissolving Ag2O only. AgNPs are only dissolved by treatment with solutions containing oxidizing agents like HNO3 (Fig. 3b, blue line). SEM characterization helps us to corroborate the conclusions derived from spectral analysis (Fig. 4). Despite the variety of topological features observed at low magnification, the detection of electrons backscattered (Z-contrast) allows us to identify those regions in the sample that are enriched with elements heavier than the aluminum of the substrate (Fig. 4a and 4c). Thus, Ag2O particles can be chemically recognized (Fig. 4a, white structures) all over the substrate surface (dark grey background). Without covering the whole substrate surface, Ag2O deposition generates cluster structures with almost regular distribution. At higher magnification, the topography of these cluster structures are revealed to be formed by flake-like Ag2O particles arranged in a sort of micrometre flower-like shape (Fig. 4b). The thermal annealing over 200 °C of these deposits drives Ag2O decomposition to form AgNPs in a distribution pattern (Fig. 4c, Z-contrast) similar to that of the original Ag2O deposit (Fig. 4a). AgNPs produced by the Ag2O decomposition have sizes that scale up to 200 nm and are mainly of near spheroidal shapes (Fig. 4d). These drastic morphological differences allow Ag2O particles and AgNPs to be clearly distinguished.
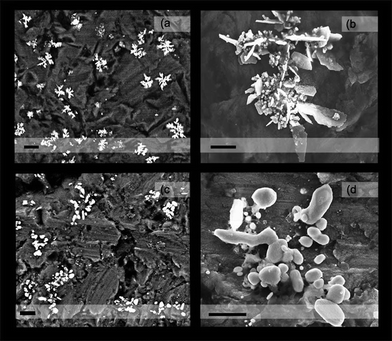 | ||
| Fig. 4 SEM photographs of deposits obtained at 2 °C: (a) Z-contrast (backscattered electrons). Scale bar 2 μm. (b) Topographical contrast (secondary electron scattering). Scale bar 500 nm. Photographs of deposits post-annealing: (c) Z-contrast. Scale bar 1 μm. (d) Topographical contrast. Scale bar 500 nm. | ||
Extinction spectra calculations performed by assuming Ag2O deposits (prepared at 2 °C) as a thin slab produce the shape of smooth decreasing profiles like those obtained experimentally (Fig. 3, black line), only for slab thickness below 50 nm and for very small values of Ag2O volume fraction (<0.01) (more details in ESI-B†). A Ag2O volume fraction at such a low value indicates that the slab dielectric properties are mainly determined by those corresponding to the host material (glass). This low value is also consistent with SEM images where flower-like cluster structures of Ag2O can be seen sparsely distributed all over the substrate surface (Fig. 4a). Beyond the simplicity of the thin slab model, an accurate qualitative description for Ag2O deposits is obtained.
Deposits prepared at 25 °C exhibit the unmistakable trace of the thermal decomposition, that is the AgNPs SPR peak (Fig. 5a, black solid line), which takes place along the drying process. Such a distinctive extinction feature is absent in Ag2O deposits prepared at 2 °C (Fig. 5a, black dashed line) but can be easily generated by their thermal annealing at 200 °C (Fig. 5a, red line). The SPR peak obtained under such conditions is red-shifted in comparison to that of the deposits prepared at room temperature (Fig. 5a, black solid line), indicating the presence of AgNPs with larger sizes. Since it is well known that silver oxidation occurs at room temperature when the metal is exposed to atmospheric oxygen, AgNPs formed by thermal annealing of Ag2O deposits (Fig. 5b, red line) were expected to undergo oxidation, too. Such a process can be followed with the changes in the shape of the deposit extinction profile. As time elapses, the SPR peak extinction of the AgNPs decreases as the metal oxidation progresses to gradually re-shape the profile into that characteristic of Ag2O (Fig. 5b, black dashed line). Thus, the conclusions obtained so far can be summarized, in addition to previous knowledge, according to the following reaction scheme:
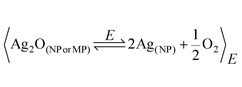 | (4) |
 | ||
| Fig. 5 (a) UV-Vis extinction profile for deposits on glass obtained from silver (I)/alkali solutions drying: (---) at 2 °C and (black) at 25 °C. (red) deposit prepared at 2 °C after 2 min. annealing at 200 °C. (b) Evolution of the extinction profile of an annealed deposit during its re-oxidation with atmospheric oxygen at room-temperature: (red) 0 (blue) 1, (pink) 24, (green) 36, (cyan) 52 and (---) 63 days. | ||
Although the forward reaction of eqn (4) might involve the generation of hydrogen peroxide (H2O2) as an intermediary species, since H2O2 is thermodynamically unstable regarding the decomposition reaction to give molecular oxygen,17 its omission in eqn (4) is associated only with the purpose of presenting a simple reaction scheme rather than discussing a detailed reaction mechanism.
3.3 Synthesis application
The thermal instability of aqueous Ag2O colloids does not depend on either the silver salt (AgNO3, AgClO4) or the alkali (NaOH, KOH) used to drive the precipitation. The increase of temperature remarkably accelerates the Ag2O decomposition as it can be concluded from the results displayed in Fig. 6a.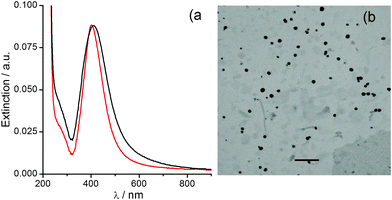 | ||
| Fig. 6 a) UV-Vis extinction spectra for AgNPs produced by thermal decomposition of silver (I)/alkali solutions (0.1 mM AgNO3, 1 mM NaOH): (black) 10 days at 25 °C, and (red) 1 h at 98 °C. b) TEM image of the AgNPs produced by the thermal decomposition of a 0.1 mM AgNO3, 0.2 mM NaOH, 1 mM NH3 solution at 98 °C for one hour. Scale bar = 200 nm. | ||
The decomposition of silver (I)/alkali solutions at room temperature over ten days and at 98 °C for one hour are compared (Fig. 6a, black and red lines, respectively). Both extinction profiles exhibit similar shape and SPR peak wavelength, although the smaller peak half-height width observed for the sample “aged” at 98 °C indicates a narrower size distribution.16 Since the height of both SPR peaks is almost identical, then the yields of AgNPs obtained are comparable. This is clearly indicating that the decomposition reaction is sped up by the temperature increase. The activation of Ag2O decomposition, however, can be alternatively performed by using microwave as it has been already proved effective in producing nanoparticles and nanostructures.18 In addition, the morphological control of AgNPs is noticeably improved by the use of NH3 as a co-reactant species during the oxide decomposition (Fig. 6b), conditions under which size distribution is narrowed down (ESI-Fig. 3†) to be centred around 25 nm average diameter, as DLS results indicate (ESI-Fig. 4†). It is worth noting that AgNPs produced by Ag2O decomposition are electrostatically stabilized by hydroxyl anions only, an aspect that makes further functionalization easy. Since the OH− adsorption is relatively weak, it can be considered that clean-surface AgNPs are produced with this reaction, an important aspect in catalysis9 as well as in SERS.8 The AgNPs are easily obtained with minor by-products generation and are stable over long periods of time. It is well known that control over NPs' morphology is more difficult to achieve for silver than for gold, even under similar reaction conditions.19 In such regard, the fact that AgNPs size distribution is improved only by rising the temperature and by addition of ammonia constitutes a very important result from the synthetic viewpoint since the surface properties of AgNPs are preserved.
Experiments performed in mixtures of water/acetone indicate that the dielectric properties of the environment constitute another important synthetic variable. Fig. 7 shows the spectral evolution for silver (I)/alkali solutions prepared with different volume relations between water and acetone. The spectral evolution obtained for a water/acetone mixture containing only 50% of volume of water (Fig. 7a) exhibits singly peaked extinction profiles, whose SPR peak extinction increases gradually as the aging time elapses, indicating that the amount of small AgNPs (diameter < 50 nm) increases as the reaction advances. Since the SPR peak wavelength values remain almost constant, this evolution corresponds to a reaction where the continuous nucleation is the most relevant process. Interestingly, a decrease of the water volume down to 25% of the total mixture volume produces remarkable changes in the reaction (Fig. 7b). During the first five days, the spectral evolution is similar to that of Fig. 7a and a continuous nucleation is the main process, producing AgNPs with sizes below 50 nm (Fig. 7b, green line). For longer times, a new SPR is defined, first as a shoulder and, later, resolved as a second peak at longer wavelength values (Fig. 7b, red, blue, and black lines). This second SPR peak also exhibits a gradual red shift as the reaction advances. Whereas the SPR peak around 430 nm is associated with small AgNPs (below 50 nm); the second SPR peak is associated to the presence of AgNPs with anisotropic shapes and/or with sizes over 50 nm and, certainly, constitutes a clear evidence of the influence of growth process on the shape/size of the AgNPs. It is also important to note that the rise and red shift of the second SPR peak take place simultaneously with the decrease of the SPR at ca. 430 nm. These spectral changes are interpreted as the overall effect of the interplay between a growth process that enlarges small AgNPs (below 50 nm) and a slowed-down nucleation which does not produce enough amounts of new AgNPs to restore those which have grown. Of course for such long times the reaction is mainly governed by the growth process. It is also important to highlight that AgNPs' SPR extinction values obtained in acetone/water 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (Fig. 7a) are between 5 and 10 times higher than those obtained in aqueous solutions (Fig. 6a), a feature indicative of higher reaction yields.
50 (Fig. 7a) are between 5 and 10 times higher than those obtained in aqueous solutions (Fig. 6a), a feature indicative of higher reaction yields.
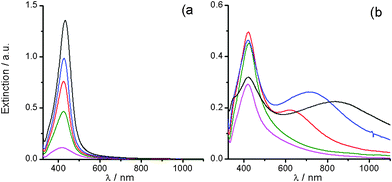 | ||
| Fig. 7 UV-Vis extinction spectra evolution during the aging at 25 °C of silver (I)/alkali solutions prepared with water/acetone mixtures having water volume percent fraction of: (a) 50% and (b) 25%. Reaction time: (pink) 1, (green) 5, (red) 15, (blue) 26, and (black) 102 days. Silver (I)/alkali solution 0.1 mM AgNO3, 0.2 mM NaOH, 1 mM NH3. | ||
In water/acetone, silver oxide colloids are unstable, as similarly occurs in aqueous environment at room temperature, where it was proved that they undergo thermal decomposition. It sounds reasonable to expect acetone to play no role as a reducing agent, given the inability of [Ag(NH3)2]+ to oxidise alcohol and ketone groups (negative Tollens' test). Therefore, evidence recently reported on the AgNPs production with silver oxide in acetone20 might have, by considering the silver oxide instability, an alternative explanation more consistent with the aqueous silver (I) chemical knowledge.
In light of some important synthetic aspects, like control over reaction rate, size, and shape of AgNPs produced; the reaction of thermal decomposition of silver oxide colloids was analysed and proved suitable as a base reaction to develop new synthetic routes in the production of AgNPs.
4 Conclusions
We report here conclusive evidence that Ag2O colloids in aqueous/water-enriched environments thermally decompose into metallic AgNPs and O2 at room temperature. Stability of Ag2O particles and AgNPs is presented in terms of a general scheme where Ag2O decomposition and Ag oxidation can be seen as environment sensitive processes at room temperature. The thermal instability of Ag2O at room temperature constitutes a strong factual base to expect the formation of oxide layers as a very unlikely process when nanostructured silver substrates are kept in alkaline aqueous/water-enriched environments. This knowledge should be very helpful in maintaining the Raman enhancing properties of naostructured-silver substrates. The decomposition of aqueous Ag2O colloids at room temperature as a fact has direct applications in synthesis, in spite of the limitations discussed. In brief, the suitability of the thermal decomposition of Ag2O in aqueous/water-enriched environments as a base reaction to produce AgNPs was shown. Synthesis routes using thermal decomposition of Ag2O in aqueous/water-enriched environments allow AgNPs production with low cost starting materials, low energy consumption, easy and safe procedures, easy purifying products, and low production of by-products and pollutants.Acknowledgements
We thank Claudia Nome from IFFIVE for her technical assistance in TEM, to the personnel of LAMARX-FaMAF from the Universidad Nacional de Córdoba for their assistance in SEM characterization, to Mathias Douglas for his advice in graphic design, and to Martin Harvey for his proof reading. This research has been supported by the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina and the Secretaría de Ciencia y Tecnología (SECYT-UNC).References
- (a) Y. Chiu, U. Rambabu, M.-H. Hsu and H.-P. D. Shieh, J. Appl. Phys., 2003, 94, 1996 CrossRef; (b) D. M. Newman and P. Panchmatia, IEE Proc.: Sci., Meas. Technol., 2003, 150, 214 Search PubMed; (c) F. Derikvand, F. Bigi, R. Maggi, C. G. Piscopo and G. J. Sartori, J. Catal., 2010, 271, 99 Search PubMed; (d) W. Wang, Q. Zhao, J. Dong and J. Li, Int. J. Hydrogen Energy, 2011, 36, 7374 Search PubMed.
- (a) N. Yamamoto, S. Tonomura, T. Matsuoka and H. Tsubomura, Jpn. J. Appl. Phys., 1981, 20, 721 CrossRef CAS; (b) V. V. Petrov, T. N. Nazarova, A. N. Korolev and N. F. Kopilova, Sens. Actuators, B, 2008, 133, 291 CrossRef.
- E. Sanli, B. Z. Uysal and M. L. Aksu, Int. J. Hydrogen Energy, 2008, 33, 2097 CrossRef CAS.
- Y. Ida, S. Watase, T. Shinagawa, M. Watanabe, M. Chigane, M. Inaba, A. Tasaka and M. Izaki, Chem. Mater., 2008, 20, 1254 CrossRef CAS.
- W.-X. Li, C. Stampfl and M. Scheffler, Phys. Rev. B: Condens. Matter, 2003, 68, 165412 CrossRef.
- (a) A. F. Benton and L. C. Drake, J. Am. Chem. Soc., 1924, 56, 255 Search PubMed; (b) P. J. Herley and E. G. Prout, J. Am. Chem. Soc., 1960, 82, 1540 CrossRef CAS; (c) B. V. L'vov, Thermochim. Acta, 1999, 333, 13 CrossRef CAS and references herein.
- A. B. Moshe and G. Markovich, Chem. Mater., 2011, 23, 1239 CrossRef.
- (a) M. Erol, Y. Han, S. K. Stanley, C. M. Stafford, H. Du and S. Sukhishvili, J. Am. Chem. Soc., 2009, 131, 7480 CrossRef CAS; (b) S. D'Agostino and F. Della Sala, ACS Nano, 2010, 4, 4117 Search PubMed.
- (a) D. D. Evanoff and G. Chumanov, J. Phys. Chem. B, 2004, 108, 13948 CrossRef; (b) D. D. Evanoff and G. Chumanov, J. Phys. Chem. B, 2004, 108, 13957 CrossRef CAS; (c) G. Merga, R. Wilson, G. Lynn, B. H. Milosavljevic and D. Meisel, J. Phys. Chem. C, 2007, 111, 12220 CrossRef CAS.
- (a) S. J. Lee, Z. Guan, H. Xu and M. Moskovits, J. Phys. Chem. C, 2007, 111, 17985 CrossRef CAS; (b) P. L. Stiles, J. A. Dieringer, N. C. Shah and R. P. Van Duyne, Annu. Rev. Anal. Chem., 2008, 1, 601 Search PubMed; (c) F. Le, D. W. Brandl, Y. A. Urzhumov, H. Wang, J. Kundu, N. J. Halas and P. Nordlander, ACS Nano, 2008, 2, 707 CrossRef CAS; (d) D. L. Deldheim, C. A. Foss and M. Dekker, Metal Nanoparticles-Synthesis Characterization and Applications, New York, 2000 Search PubMed; (e) M.-K. Kwon, J.-Y. Kim, B.-H. Kim, I.-K. Park, C.-Y. Cho, C. C. Byeon and S.-J. Park, Adv. Mater., 2008, 20, 1253 CrossRef CAS.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940 CrossRef CAS.
- K sp (½Ag2O → Ag+ + OH−) = 1.99 × 10−8, from S. Kotrlý, L. Šůcha, Handbook of Chemical Equilibria in Analytical Chemistry, Halsted Press, Prage, 1985 Search PubMed.
- (a) C. F. Bohren and D. R. Huffman, Absorption and Scattering of Light by Small Particles; Wiley Interscience: New York, 1983 Search PubMed; (b) K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668 CrossRef CAS.
- (a) E. D. Palik, Handbook of Optical Constant of Solids; Academic Press: New York, 1985 Search PubMed; (b) L. A. A. Pettersson and P. G. Snyder, Thin Solid Films, 1995, 270, 69 CrossRef CAS; (c) B. N. De and J. A. Woollam, J. Appl. Phys., 1989, 66, 5602 Search PubMed; (d) X.-Y. Gao, H.-L. Feng, J.-M. Ma, Z.-Y. Zhang, J.-X. Lu, S.-E. Yang, Y.-S. Chen and J.-H. Gu, Phys. B, 2010, 405, 1922 Search PubMed.
- E. A. Coronado and G. C. Schatz, J. Chem. Phys., 2003, 119, 3926 CrossRef CAS.
- M. A. Pérez, R. Moiraghi, E. A. Coronado and V. A. Macagno, Cryst. Growth Des., 2008, 8, 1377 CrossRef CAS.
- D. He, A. M. Jones, S. Garg, A. Ninh Pham and D. T. Waite, J. Phys. Chem. C, 2011, 111, 5461 Search PubMed.
- (a) P. C. Sherrell, J. Chen, J. M. Razal, I. P. Nevirkovets, C. Crean, G. G. Wallace and A. I. Minett, Energy Environ. Sci., 2010, 3, 1979 RSC; (b) L. Dennany, P. Sherrell, J. Chen, P. C. Innis, G. G. Wallace and A. I. Minett, Phys. Chem. Chem. Phys., 2010, 12, 4135 RSC; (c) M. N. Nadagouda, T. F. Speth and R. S. Varma, Acc. Chem. Res., 2011, 44, 469 CrossRef CAS.
- Z. S. Pillai and P. V. Kamat, J. Phys. Chem. B, 2004, 108, 945 CrossRef CAS.
- I. Halaciuga, S. LaPlante and D. V. Goia, J. Colloid Interface Sci., 2011, 354, 620 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: A) Ag2O-MPs: DLS and UV-Visible spectrum of aqueous Ag2O colloids containing microparticles (ESI-Fig. 1). B) Characterisation of Ag2O–MPs and films: about the “actual” optical properties of Ag2O (ESI-Fig. 2). C) Ag Nanoparticles: AgNPs produced by thermal decomposition of a 0.1 mM AgNO3, 0.2 mM NaOH, 1 mM NH3 solution at 98 °C for one hour (ESI-Fig. 3, ESI-Fig. 4). See DOI: 10.1039/c2ra01044e/ |
| This journal is © The Royal Society of Chemistry 2012 |
