Synthesis and gas-sensing properties of ZnO particles from an ionic liquid precursor
Rui
Li
a,
Yuxia
Luan
b,
Hua
Zou
a,
Jimin
Du
c,
Tiancheng
Mu
d and
Zhonghao
Li
*a
aKey laboratory for liquid-solid structural evolution and processing of materials, School of materials science and engineering, Shandong University, Jinan, Shandong Province 250061, P. R. China. E-mail: zhonghaoli@sdu.edu.cn; Tel: (86) 531-88399670
bSchool of pharmaceutical sciences, Shandong University, Jinan, 250012, P. R. China.
cSchool of Chemistry and Chemical Engineering, Anyang Normal University, Henan Province, P. R. China
dDepartment of chemistry, Renmin University of China, P. R. China
First published on 19th January 2012
Abstract
The synthesis and gas-sensing properties of ZnO particles from an ionic liquid precursor benzyltrimethylammonium hydroxide (BTMAH) is studied. The BTMAH, acting as reactant precursor and solvent, is demonstrated to be effective for synthesis of ZnO particles with controlled morphologies. The effect of the water and zinc salt concentration on the morphology of the synthesized particles is investigated. The gas-sensing tests shows that the sensors based on the synthesized ZnO nanoplates exhibit excellent ethanol-sensing properties.
Introduction
Zinc oxide is one of the most promising electronic and photonic materials because of its wide direct band gap of 3.37 eV and large exciton binding energy of 60 meV.1 It has attracted considerable interest in the past years because of its potential application in optical waveguides, surface acoustic wave transducers, blue light-emitting diodes, solar cells, chemical sensors and photocatalysts, etc.2–3 The interesting shape-dependent optoelectronic and gas sensing properties of ZnO make researchers explore facile methods to prepare ZnO particles with controlled structures. Until now, various ZnO nanostructures have been prepared by using the chemical vapor deposition method, thermal evaporating and pyrolysis protocols, hydrothermal and sonochemical route, etc.4Ionic liquids (ILs) are attractive environmentally benign solvents for organic chemical reactions, separations, and electrochemical applications.5–7 The advantages of ILs in inorganic nanomaterial synthetic processes have been gradually realized and have received more and more attention due to their unique physical and chemical properties, such as a large electrochemical window, low interface energies, high thermal stability, and extended hydrogen bond systems.8–21 Particularly, ionic liquids can act as solvents, reactants, and templates for the fabrication of inorganic materials.10 In some cases, ILs and the structurally related ionic liquid crystals combine these functions and serve as “all-in-one” solvent-reactant-templates, or ionic liquid (crystal) precursors (ILPs and ILCPs, respectively). For example, Taubert et al. have used ILCPs for the fabrication of CaF2 tubes, CuCl plates and Au plates.22–25 The ILCPs act as the precursor for the inorganic material, as the solvent for the reaction, and as the template over the final inorganic particle morphology as well. According to the original ILCPs approach,22 the concept for fabrication of inorganics from ILPs is developed.26–27
In our previous study, tetraethyl ammonium hydroxide (TEAH) and tetrabutylammonium hydroxide (TBAH) are demonstrated to be efficient ILPs for the fabrication of nanostructured inorganic particles.28–33 Inspired by this, we are interested in understanding the particle formation in other kinds of ionic liquid precursors which have a similar structure to TEAH and TBAH. Herein we use the ionic liquid benzyltrimethylammonium hydroxide (BTMAH), which has a similar structure to our previous studied ionic liquid precursors, as an example to demonstrate that ZnO particles with controlled morphologies can be obtained from this ionic liquid precursor. Our previous work concerning the synthesis of ZnO from ionic liquid precursors is mainly concentrated on their crystal growth aspects. Because of the important application of ZnO as gas sensing materials, the gas-sensing properties of the synthesized ZnO from the ionic liquid precursor should be highly desired. Therefore, in this present work, we systematically investigate the gas-sensing properties of our synthesized ZnO particles to reveal their potential applications. This work is an another contribution that demonstrates the inorganic materials synthesized in ionic liquid could have improved properties.
Experimental
Synthesis
In a typical synthesis, 40 mg of the zinc acetate dihydrate was dissolved in 0.5 g of benzyltrimethylammonium hydroxide methanol solution (BTMAH, 40% wt, 0.3% wt water) with 2 g water in a 10 ml tube. The tube with the solution was heated in oil bath at 80 °C for 3 h. The products were recovered by repeated centrifugation and washing with water and ethanol, and then dried. The procedure for the synthesis of ZnO particles at other conditions is similar to the above, although the zinc salt or the water amount is different.Characterization
X-ray diffraction was performed using a Rigaku Dmax-rc X-ray diffractometer with Ni-filtered Cu-Kα (λ = 1.5418 Å) radiation. Scanning electron microscopy (SEM) was performed using a Hitachi SU-70 FESEM operating at 10 kV. The specific surface area was measured according to the Brunauer–Emmett–Teller (BET) method with Gemini VII 2390.Gas-sensing measurements and fabrication of gas-sensor
The WS-30A static gas-sensing system (Weisheng Electronics Co. Ltd., China) was used to examine the sensing performance of the sensors. The procedure for the fabrication of a typical ZnO-based gas sensor is shown in Scheme 1. The as-prepared ZnO powder was mixed with ethanol to form a paste. The paste was directly coated on the ceramic tube with a pair of Pt wires covered on Au electrodes, and then dried in air. After that, the electrodes were joined on a support and a Ni–Cr heating wire was inserted into the ceramic tube. Finally, the as-prepared gas sensors were kept at 300 °C for 10 h in order to improve their stability. The working temperature can be controlled by adjusting the heating voltage (Vh) of the sensor. The gas-sensor performance was obtained from the Vout value of RL that cascades RS (the resistance of gas sensor). The RS can be calculated by the following equation: RS = RL (Vc − Vout)/Vout. In our experiment, the Vc = 5 V and RL = 1 MΩ. The sensor response was defined as S = Ra/Rg, where Ra is the resistance in dry air and Rg is that in the dry air mixed with detected gases. In addition, the response time was defined as the time required for the conductance to reach 90% of the equilibrium value after a test gas was injected, and the recovery time was the time necessary for a sensor to attain a conductance 10% above its original value in air.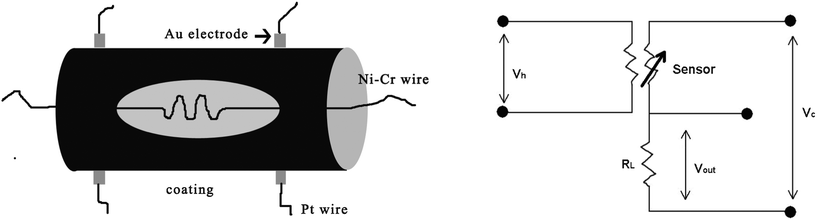 | ||
| Scheme 1 Schematic illustration of fabrication of the gas sensor. | ||
Results and discussion
The XRD pattern of the typical synthesized ZnO particles is presented in Fig. 1. All the diffraction peaks in the range 20° < 2θ < 80° can be indexed as the hexagonal ZnO, which are in good accordance with the values on the standard card (JCPDS 36-1451). The XRD results confirms the formation of pure ZnO powders and the product is well crystallized. According to the standard diffraction pattern, the intensity of the (002) peak is much lower than the (100) peak for the bulk ZnO. Here, the intensity of the (002) peak is higher than that of the (100) peak, which indicates that ZnO (001) planes are mainly oriented parallel to the substrate.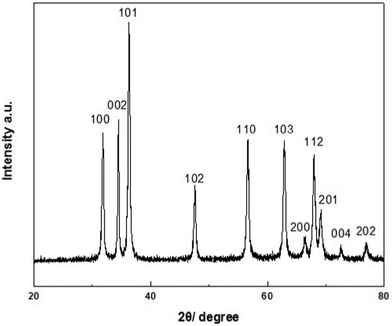 | ||
| Fig. 1 XRD pattern of the sample precipitated with 40 mg of zinc acetate dihydrate in 0.5 g BTMAH with 2 g water. | ||
Fig. 2 shows the low and high magnification SEM images of the ZnO products precipitated at 40 mg zinc acetate dihydrate in 0.5 g BTMAH with 2 g water. The SEM images manifest the formation of ZnO nanoplates. The length of the ZnO nanoplates is 243 ± 68 nm. The enlarged SEM image shown in Fig. 2b suggests that the thickness of the synthesized ZnO nanoplates is thin.
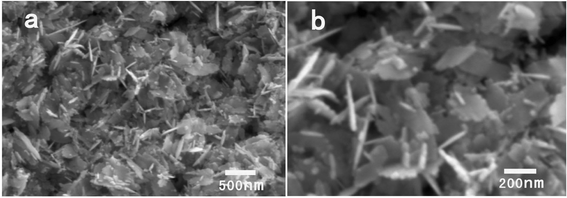 | ||
| Fig. 2 SEM images of the products synthesized with 40 mg zinc acetate dihydrate in 0.5 g BTMAH with 2 g water. | ||
To uncover the formation mechanism of these ZnO nanoplates, we recover the products at the initial stage of the reaction. Fig. 3a and Fig. 3b show the SEM images for the product obtained after reaction time of 10 s and 5 min. It is clear that nanoplate particles have already formed at the early stage. This morphology is similar to the products in Fig. 2. This indicates that the formation process of these nanoplate particles is fast. The present synthetic route offers a dramatic reduction of the reaction time in comparison with traditional methods which are usually time- and energy-consuming. The intrinsic electric fields of the polar ZnO lattice, where the crystallographic c-axis is polar, could be responsible for the formation of our observed nanoplate structures. Structurally, the ZnO crystal can be described as a number of alternating planes composed of four-fold tetrahedrally-coordinated O2− and Zn2+ ions stacked alternatively along the c-axis.32 The oppositely-charged ions produce positively-charged Zn (0001) and negatively-charged O (000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) surfaces, resulting in a normal dipole moment and spontaneous polarization along the c-axis, as well as a divergence in surface energy. It is well known that the polar surfaces usually appear as growing surfaces because of their high surface energy, and exhibit small facets or even disappear during the crystal growth. However, the surface energy of the polar planes can be reduced by compensating the surface charge with a passivating reagent. In our case, due to the strong electrostatic interactions between the benzyltrimethylammonium cation of the BTMAH and the polar surfaces, the surface energies of the basal polar {0001} planes decreases greatly in comparison to those of other crystal faces, resulting in a relatively slow growth rate for these polar planes. Therefore the {0001} planes appear as exposed surfaces, and the nanoplate structure forms.
) surfaces, resulting in a normal dipole moment and spontaneous polarization along the c-axis, as well as a divergence in surface energy. It is well known that the polar surfaces usually appear as growing surfaces because of their high surface energy, and exhibit small facets or even disappear during the crystal growth. However, the surface energy of the polar planes can be reduced by compensating the surface charge with a passivating reagent. In our case, due to the strong electrostatic interactions between the benzyltrimethylammonium cation of the BTMAH and the polar surfaces, the surface energies of the basal polar {0001} planes decreases greatly in comparison to those of other crystal faces, resulting in a relatively slow growth rate for these polar planes. Therefore the {0001} planes appear as exposed surfaces, and the nanoplate structure forms.
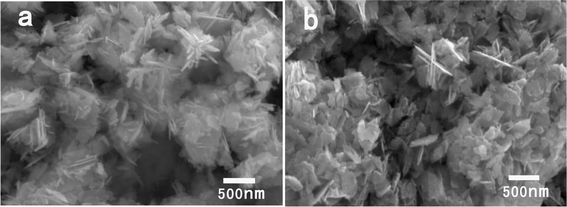 | ||
| Fig. 3 SEM images of the products synthesized with 40 mg zinc acetate dihydrate in 0.5 g BTMAH ionic liquid with 2 g water at different times (a) 10 s, (b) 5 min. | ||
In general, the reaction conditions have a dramatic effect on the mineralization of inorganic materials for a given synthetic route. This phenomenon has been observed numerous times before. In order to understand the influence of the amount of water on the final products, experiments were performed with different water amounts. Fig. 4 shows SEM images of the samples obtained at 1, 3, 5 and 8 g of water, respectively. At 1 g of water, similar to Fig. 2, the ZnO is in a nanoplate structure with a length of 240 ± 67 nm (Fig. 4a and 4b). With an increase of the water amount to 3 g, the ZnO nanoplates still form with a length of 222 ± 85 nm (Fig. 4c and 4d). When the water amount increases to 5 g, it shows that mainly 0D ZnO nanoparticles form with several large nanoplates coexisting in the sample (Fig. 4e and 4f). The size of the nanoparticles is 42 ± 10 nm. Further increasing the water to 8 g, the particles exhibit irregular shapes with a size of 95 ± 42 nm (Fig. 4g and 4h). Some of the irregular particles show shapes with a 2D nanostructure characteristic while others are 0D nanoparticles. The influence of the amount of water on the final products thus indicates that ZnO nanoplates form at low amounts of water while small 0D nanoparticles form at high amounts of water. This can be explained as follows. It is reported that the viscosity of ionic liquid and water mixtures decreases exponentially when the mole fraction of water increases based on the empirical equation.34–35 In principle, the diffusion of the component is retarded for the system with higher viscosity. In this case the nuclei preferred to grow into larger particles by consuming the reactant and/or the already formed nuclei nearby rather than forming new particles with a small size. That is, the particles formed at lower amount of water might have a bigger size than those formed at higher amounts of water. This is verified by the results that small 0D nanoparticles form at the higher amount of water. On the other hand, with the increase of the water amount, the concentration of the BTMAH ionic liquid decreases. In this case, the ionic liquid cation density adsorbing on the {0001} planes is low, which is not sufficient to efficiently decrease the surface energies of the basal polar {0001} planes. Thus, the preferred growth into plate particles is inhibited, which results in the additional formation of 0D nanoparticles in the products.
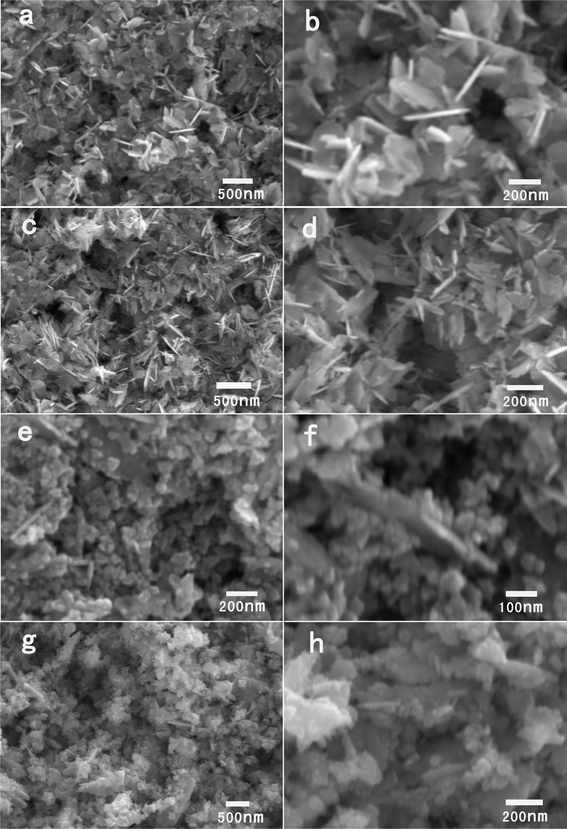 | ||
| Fig. 4 SEM images of the products synthesized with 40 mg of zinc acetate dihydrate in 0.5 g BTMAH ionic liquid with different amounts of water. (a, b) 1 g; (c, d) 3 g; (e, f) 5 g; (g, h) 8 g. | ||
In order to understand the influence of the zinc acetate dihydrate amount on the final products, we performed experiments with different amounts of zinc acetate dihydrate in 0.5 g of BTMAH ionic liquid with 2 g of water. Fig. 5 shows SEM images of samples obtained at 10 mg, 20 mg, 30 mg, 50 mg of zinc acetate dihydrate. At 10 mg of zinc acetate dihydrate, flower-like nanoparticles form which are constructed by rods with a length of 400 ± 58 nm. With the increase of the zinc acetate dihydrate to 20 mg, irregular ZnO particles with a size of 77 ± 42 nm form, which stack together to form aggregates. Further increasing the zinc acetate dihydrate amount to 30 mg and 50 mg, similar to the samples recovered at 40 mg zinc acetate dihydrate, ZnO nanoplates form with a length of 210 ± 82 and 226 ± 93 nm, respectively. The results thus show that there is a strong influence of the zinc acetate dihydrate amount on the final products. Overall, rod-constructed flower-like particles form at low amounts of zinc acetate dehydrate while ZnO nanoplates form at high amount of zinc acetate dihydrate. This can be explained as follows. At low amounts of zinc acetate dihydrate, there are plenty of ionic liquids existing in the solution compared with the small amount of ZnO primary particles formed at this condition. Therefore there is an excess of ionic liquid cations for utilization in the process of adsorbing onto the primary ZnO particles. In this case, the ionic liquid cations could also adsorb onto other planes of the primary particles, due to the ionic liquid being in excess, besides the preferred adsorbing onto the basal polar {0001} planes. Therefore not only do the surface energies of the basal polar {0001} planes decrease but also the surface energies of other planes are decreased together. That is, the order of magnitude for the surface energies of different planes might be unchanged or just changed a little. Therefore, the common rod-constructed flower-like ZnO particles would form, which have been well explained in the literature based on the intrinsic anisotropy in the growth rate of the ZnO crystals.36 At high amounts of zinc acetate dihydrate, there are plenty of ZnO primary particles formed. At this condition there is no excess ionic liquid to be utilized for the adsorbing process. In this case, the limited amount of the ionic liquid prefers to adsorb on the polar surfaces, and the surface energies of the basal polar {0001} planes decreases greatly in comparison to those of other crystal faces, resulting in a relatively slow growth rate for these polar planes. Therefore the {0001} planes appear as exposed surfaces, and the nanoplate structure forms.
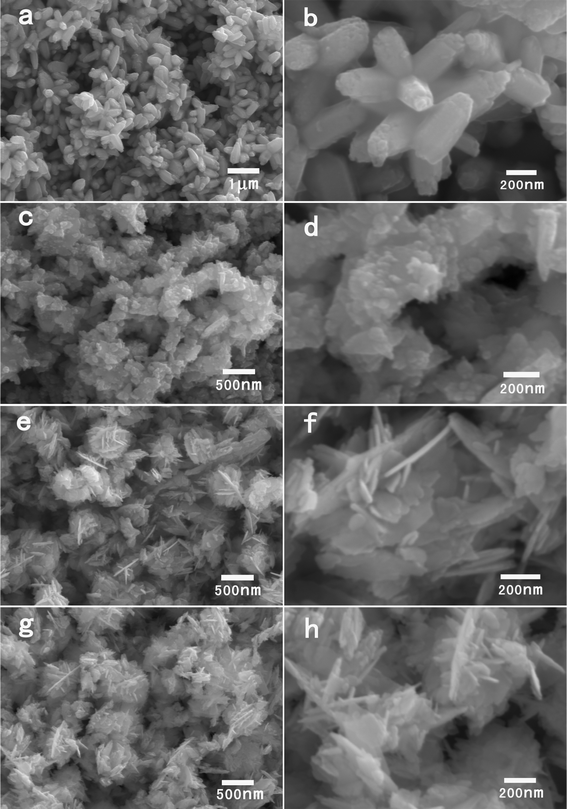 | ||
| Fig. 5 SEM images of the products synthesized with different amounts of zinc acetate dihydrate in 0.5 g BTMAH ionic liquid with 2 g water. (a, b) 10 mg, (c, d) 20 mg, (e, f) 30 mg, (g, h) 50 mg. | ||
The experiment results for the products recovered with different amounts of water and zinc acetate dihydrate in 0.5 g of BTMAH ionic liquid are summarized in Table 1.
| Sample | Zinc acetate dihydrate/mg | BTMAH/g | Added water amount/g | Morphology | Length/nm |
|---|---|---|---|---|---|
| 1 | 40 | 0.5 | 1 | Nanoplates | 240 ± 67 |
| 2 | 40 | 0.5 | 2 | Nanoplates | 243 ± 68 |
| 3 | 40 | 0.5 | 3 | Nanoplates | 222 ± 85 |
| 4 | 40 | 0.5 | 5 | Mainly nanoparticles | 42 ± 10 |
| 5 | 40 | 0.5 | 8 | Irregular nanoparticles | 95 ± 42 |
| 6 | 10 | 0.5 | 2 | Flower-like particles | 400 ± 58 |
| 7 | 20 | 0.5 | 2 | Nanoparticle aggregates | 77 ± 42 |
| 8 | 30 | 0.5 | 2 | Nanoplates | 210 ± 82 |
| 9 | 50 | 0.5 | 2 | Nanoplates | 226 ± 93 |
We have recently shown that the IL tetrabutylammonium hydroxide (TBAH) is an efficient ILPs for the fabrication of ZnO mesocrystals, including the hollow rod morphology via the reaction of zinc acetate with TBAH at the condition of reflux or at 80 °C.29,31 Subsequently, the hollow rods with smooth surfaces are formed from the ionic liquid tetraethylammonium hydroxide (TEAH) at 80 °C,33 which is different from the products obtained in TBAH. The mechanism for the hollow rods formed in TBAH and TEAH is different. In TBAH the formation of hollow rods is based on the assembly of the primary nanoparticle subunits while in TEAH the formation of hollow rods results from the selective dissolution of the initial rods. In the present study, nanoplates form from the ionic liquid BTMAH which is different from the products obtained in TBAH and TEAH at 80 °C. Here the formation of these nanoplates is due to the preferred adsorption of the benzyltrimethylammonium cation on the basal polar {0001} planes which decreases their surface energies greatly in comparison to those of other crystal faces, resulting in the nanoplate structure. The ZnO particles synthesized with different ionic liquid precursors thus show that the structures of the synthesized particle strongly depend on the kinds of the ionic liquid precursors. The difference of the particles synthesized in these ionic liquid precursors might be attributed to their different interactions with the ZnO crystal planes. For example the difference in Coulombic force interaction between the cation of the ionic liquid and the surface of the nuclei. Moreover the ionic liquid solution properties such as the viscosity might also influence the crystal growth process.
Fig. 6 shows the typical N2 adsorption–desorption isotherm for the ZnO nanoplates prepared with 40 mg of zinc acetate dihydrate in 0.5 g of BTMAH with 2 g of water. The N2 isotherm corresponds to a type II isotherm in the Brunauer classification. The BET surface area of the material is calculated to be 19.02 m2 g−1, which is a little higher than the ZnO nanoplates reported previously (15.9 m2 g−1).37 The higher BET surface area for our synthesized ZnO nanoplates could be attributed to their small size with thin thickness.
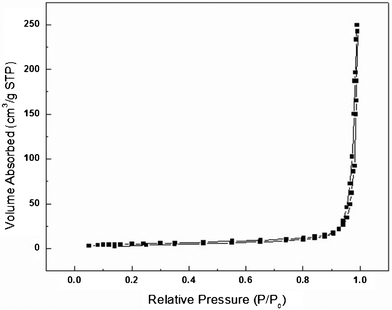 | ||
| Fig. 6 Typical nitrogen adsorption–desorption isotherm of the ZnO nanoplates. | ||
In order to understand the properties of our synthesized ZnO particles, the sensing experiments are performed. It is well known that the sensitivity of a semiconductor gas sensor is highly influenced by its operating temperature. To determine the optimum working temperature, the responses are examined to 100 ppm ethanol as a function of temperature for ZnO nanoplates synthesized with 40 mg zinc acetate dihydrate, as shown in Fig. 7, which presents the relationship between the sensor responses and the working temperature. In the range of 100 °C to 240 °C, the sensor response is sharply increased with increasing working temperature. Then, the sensor response is decreased with the further increase of working temperature. The results indicate that the optimum working temperature is 240 °C for further investigation of sensing performance of the as-fabricated gas sensors.
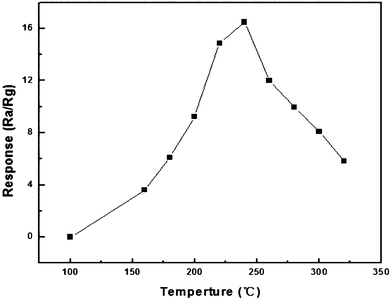 | ||
| Fig. 7 Sensor responses of the nanoplate-based sensor upon exposure to ethanol (100 ppm) at different working temperatures. | ||
The typical synthesized particles are nanoplates, nanoparticle aggregates and flower-like particles. For these particles, we studied their real-time response curves and sensor responses upon exposure to different concentrations of ethanol at a working temperature of 240 °C, respectively (Fig. 8). The response enhancement with the increase of ethanol concentration is observed in a range of 10–500 ppm for all the studied sensors. From Fig. 8d, it shows that the response increases rapidly with increasing ethanol concentration below 100 ppm. Above 100 ppm, the response increases slowly with increasing ethanol concentration. Moreover the response of the sensor from nanoplates is higher than those fabricated from nanoparticle aggregates and flower-like particles. For example, the response to 100 ppm ethanol is 16.12 , 11.4 and 7.08 for the sensors fabricated from nanoplates, nanoparticle aggregates and flower-like particles, respectively. At 100 ppm ethanol, the response and recovery times of the nanoplate-based sensor are 5 s and 15 s, respectively. It is obvious that our gas sensor exhibits rapid gas sensing behaviors when the target gas is injected or released.
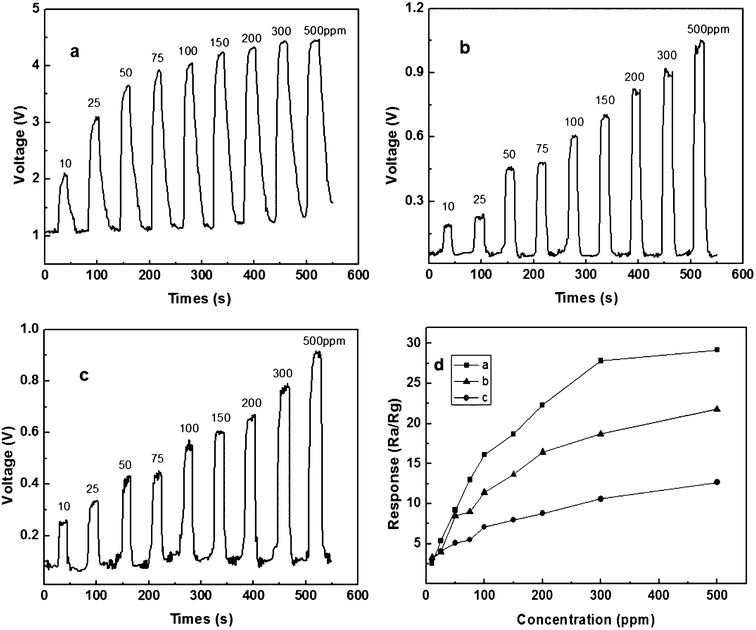 | ||
| Fig. 8 The real-time response curve (a, b, c) and the sensor responses (d) of sensor device upon exposure to different concentrations of ethanol at a working temperature of 240 °C. (a) nanoplates synthesized with 40 mg of zinc acetate dihydrate, (b) nanoparticle aggregates synthesized with 20 mg of zinc acetate dihydrate, (c) flower-like particles synthesized with 10 mg of zinc acetate dihydrate. | ||
Although there is a report showing the improved ethanol gas-sensing properties from ZnO based sensors,38 our experiments indicate that ZnO particles synthesized from an ionic liquid precursor could also have promising applications in sensing ethanol. For example, the responses of our nanoplate-based sensor to 100 ppm ethanol at 240 °C are 3 and 4 times higher than those of the ZnO nanowires synthesized by an oxidation reaction and the ZnO nanoparticles synthesized by a chemical precipitation method, respectively.39,40 The response to 100 ppm ethanol at 200 °C is higher than the porous ZnO nanoplates synthesized with a microwave heating method (less than 3)37 and the various ZnO thin films fabricated by a sol–gel method (3.68 to 8.11). This is obviously lower than 9.23 that we tested at 200 °C.41 Moreover, the response to 100 ppm ethanol at 300 °C is 2 times higher than the ZnO thin films prepared by the sol–gel method.42 The response (16.12) of our sensor to 100 ppm ethanol is comparable to the flower-like ZnO nanostructures synthesized by calcinations where the maximum response is about 15.43 Comparing with the literature, the sensor property studies clearly show that the inorganic particles synthesized with ionic liquid could also have improved properties.
Most of the semiconductor oxide gas sensors operate on the basis of the modification of the electrical properties of an active element, which is brought about by the adsorption of an analyte on the surface of the sensor.37 It is well-known that oxygen sorption plays an important role in electrical transport properties of ZnO nanostructures. When a ZnO sensor is exposed to air, oxygen molecules adsorb on the surface of materials to form O2−, O− and O2− ions by capturing electrons from the conductance band, and O− is believed to be dominant.44 The reaction could be described as follows:
| O2 (gas) → O2 (absorbed) + e− → O2− + e− → 2O− |
| CH3CH2OH (absorbed) + 6O− (absorbed) → 2CO2 + 3H2O + 6e− |
Conclusion
In conclusion, like the TBAH and TEAH ionic liquids, the present work shows that benzyltrimethylammonium hydroxide (BTMAH) is also an efficient ILPs for the controlled fabrication of zinc oxide particles with various shapes and sizes. The synthesis and gas-sensing properties of ZnO particles from the ionic liquid precursor benzyltrimethylammonium hydroxide (BTMAH) is systematically investigated in the present work. The effect of the water and zinc salt concentrations on the morphology of the synthesized particles is studied. The gas-sensing experiments show that sensors based on the ZnO nanoplates exhibit excellent ethanol-sensing properties. This work is another contribution that shows the ionic liquid precursor is effective for the synthesis of controlled inorganic particles with improved properties.Acknowledgements
This work is supported by National Natural Science Foundation of China (NSFC, No. 21173127, No.20803043, No.21003001), the Excellent Young Scientist Foundation of Shandong Province (BS2009CL002), the Natural Science Foundation of Shandong Province (ZR2011BQ003) and the Foundation of Key Laboratory of Colloid and Interface Chemistry (Shandong University), Ministry of Education.References
- Z. W. Pan, Z. R. Dai and L. Wang, Science, 2001, 291, 1947 CrossRef CAS.
- T. Siyama and A. Kato, Anal. Chem., 1962, 34, 1502 CrossRef.
- S. T. Aruna and A. S. Mukasyan, Curr. Opin. Solid State Mater. Sci., 2008, 12, 44 CrossRef CAS.
- S. Bhattacharyya and A. Gedanken, Microporous Mesoporous Mater., 2008, 110, 553 CrossRef CAS.
- R. Sheldon, Chem. Commun., 2001, 2339 Search PubMed.
- J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 1765 RSC.
- E. V. Dickinson, M. E. Williams, S. M. Hendrickson, H. Masui and R. W. Murray, J. Am. Chem. Soc., 1999, 121, 613 CrossRef CAS.
- M. Antonietti, D. Kuang, B. Smarsly and Y. Zhou, Angew. Chem., Int. Ed., 2004, 43, 4988 CrossRef CAS.
- A. Taubert, Acta Chim. Slov., 2005, 52, 183 CAS.
- A. Taubert and Z. Li, Dalton Trans., 2007,(7), 723 RSC.
- Z. Li, Z. Jia, Y. Luan and T. Mu, Curr. Opin. Solid State Mater. Sci., 2008, 12, 1 CrossRef CAS.
- Y. Zhu, W. Wang, R. Qi and X. Hu, Angew. Chem., Int. Ed., 2004, 43, 1410 CrossRef CAS.
- D. Batra, S. Seifert, L. M. Varela, A. C. Y. Liu and M. A. Firestone, Adv. Funct. Mater., 2007, 17, 1279 CrossRef CAS.
- Y. Wang and H. Yang, J. Am. Chem. Soc., 2005, 127, 5316 CrossRef CAS.
- K. Ding, Z. Miao, Z. Liu, Z. Zhang, B. Han, G. An, S. Miao and Y. Xie, J. Am. Chem. Soc., 2007, 129, 6362 CrossRef CAS.
- Z. Li, A. Friedrich and A. Taubert, J. Mater. Chem., 2008, 18, 1008 RSC.
- E. Redel, R. Thomann and C. Janiak, Chem. Commun., 2008, 1789 RSC.
- H. Kaper, F. Endres, I. Djerdj, M. Antonietti, B. Smarsly, J. Maier and Y. S. Hu, Small, 2007, 3, 1753 CrossRef CAS.
- K. Hala Farag and F. Endres, J. Mater. Chem., 2008, 18, 442 RSC.
- X. Liu, J. Ma and W. Zheng, Rev. Adv. Mater. Sci., 2011, 27, 43 Search PubMed.
- E. R. Parnham and R. E. Morris, Acc. Chem. Res., 2007, 40, 1005 CrossRef CAS.
- A. Taubert, Angew. Chem., Int. Ed., 2004, 43, 5380 CrossRef CAS.
- A. Taubert, Acta Chim. Slov., 2005, 52, 168 CAS.
- A. Taubert, P. Steiner and A. Mantion, J. Phys. Chem. B, 2005, 109, 15542 CrossRef CAS.
- A. Taubert, C. Palivan, O. Casse, F. Gozzo and B. Schmitt, J. Phys. Chem. C, 2007, 111, 4077 CAS.
- A. Taubert, I. Arbell, A. Mecke and P. Graf, Gold Bull., 2006, 39, 205 CrossRef CAS.
- H. Zhu, J. F. Huang, Z. Pan and S. Dai, Chem. Mater., 2006, 18, 4473 CrossRef CAS.
- K. S. Kim, S. Choi, J. H. Cha, S. H. Yeon and H. Lee, J. Mater. Chem., 2006, 16, 1315 RSC.
- Z. Li, A. Geβner, J. Richters, J. Kalden, T. Voss, C. Kubel and A. Taubert, Adv. Mater., 2008, 20, 1279 CrossRef CAS.
- Z. Li, P. Rabu, P. Strauch, A. Mantion and A. Taubert, Chem.–Eur. J., 2008, 14, 8409 CrossRef CAS.
- Z. Li, Y. Luan, T. Mu and G. Chen, Chem. Commun., 2009, 1258 RSC.
- Z. Li, Y. Luan, Q. Wang, G. Zhuang, Y. Qi, Y. Wang and C. Wang, Chem. Commun., 2009, 6273 RSC.
- Z. Li, Q. Yu, Y. Luan, G. Zhuang, R. Fan, R. Li and C. Wang, CrystEngComm, 2009, 11, 2683 RSC.
- C. Comminges, R. Barhdadi, M. Laurent and M. Troupel, J. Chem. Eng. Data, 2006, 51, 680 CrossRef CAS.
- J. Lian, X. Duan, J. Ma, P. Peng, T. Kim and W. Zheng, ACS Nano, 2009, 3, 3749 CrossRef CAS.
- H. Zhang, D. Yang, Y. Ji, X. Ma, J. Xu and D. Que, J. Phys. Chem. B, 2004, 108, 3955 CrossRef CAS.
- Z. Jing and J. Zhan, Adv. Mater., 2008, 20, 4547 CrossRef CAS.
- C. Ge, Z. Bai, M. Hu, D. Zeng, S. Cai and C. Xie, Mater. Lett., 2008, 62, 2307 CrossRef CAS.
- N. Hongsith, C. Viriyaworasakul and P. Mangkorntong, Ceram. Int., 2008, 34, 823 CrossRef CAS.
- J. Xu, J. Han, Y. Zhang and Y. Sun, Sens. Actuators, B, 2008, 132, 334 CrossRef.
- N. Kakati, S. H. Jee, S. H. Kim, J. Y. Oh and Y. S. Yoon, Thin Solid Films, 2010, 519, 494 CrossRef CAS.
- T. T. Trinh, N. H. Tuc, H. H. Lec and K. Y. Ryu, Sens. Actuators, B, 2011, 152, 73 CrossRef.
- Y. Zeng, T. Zhang, L. Wang, M. Kang and H. Fan, Sens. Actuators, B, 2009, 140, 73 CrossRef.
- X. H. Jia and H. Q. Fan, Mater. Lett., 2010, 64, 1574 CrossRef CAS.
- Z. Yang, L. M. Li, Q. Wan, Q. H. Liu and T. H. Wang, Sens. Actuators, B, 2008, 135, 57 CrossRef.
- J. Q. Xu, Y. P. Chen, D. Y. Chen and J. N. Shen, Sens. Actuators, B, 2006, 113, 526 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
