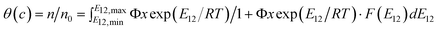DOI:
10.1039/C2RA01218A
(Paper)
RSC Adv., 2012,
2, 3034-3048
Enhanced photovoltaic properties of TiO2 film prepared by polycondensation in sol reaction†
Received
1st December 2011
, Accepted 22nd January 2012
First published on 17th February 2012
Abstract
A TiO2 film of dye-sensitized solar cells was fabricated using a TiO2 sol prepared from titanium isopropoxide as a starting material. The effect of polycondensation time and temperature on physical and chemical properties of TiO2 film shows that the crystallinity, particle size, roughness, and surface area of TiO2 film can be successfully controlled by adjusting the polycondensation time and temperature in the synthesis process of the TiO2 sol. Adsorption properties (adsorption equilibrium, isosteric enthalpies of adsorption, and adsorption energy distributions) of N719 dye molecules on the prepared TiO2 film revealed that the short circuit current (Isc) and overall conversion efficiency (ηeff) are highly dependent on the crystallite size and the N719 dye adsorption properties. The TiO2 film has a pure anatase phase, high surface area including pore volume, and large anatase crystallite size and is capable of enhancing the photovoltaic performance. The energy conversion efficiency of the dye sensitized solar cells is highly dependent on the physical and chemical properties of TiO2 films.
Introduction
Dye-sensitized solar cells (DSSC) based on mesoporous oxide such as nanocrystalline TiO2 have been attracting much attention recently for their high efficiency and low cost in comparison to conventional silicon solar cells.1,2 A typical sandwich DSSC consists of a transparent conducting oxide (TCO) glasses covered with TiO2 film, dye molecules attached to the surface of TiO2, a redox couple electrolyte such as iodide/triiodide, and a counter electrode such as a Pt deposited on TCO glass substrate by a simple thermal decomposition process. The light absorption is performed by a chemical adsorption of ruthenium complex dye on the surface of the nanocrystalline TiO2 particles. The absorbed light is excited on the dye molecules, and the electrons are transferred into the TiO2 conduction band. The oxidized dye is subsequently reduced by electron donation from an electrolyte containing the iodide/triiodide redox system. The injected electron flows through the semiconductor network to arrive at the back contact and then through the external load to the counter electrode coated with Pt on FTO (F-doped thin oxide) glass. At the counter electrode, the iodide is in turn regenerated by the reduction of triiodide, with the electrical circuit completed via electron migration through the external load.3,4 Currently, most research is being conducted mainly on materials such as TiO2 with high specific area, highly ordered morphology and porosity, highly efficient dye molecules and various counter electrodes. Research on electrolytes is also focused on the development of high efficiency solid materials.5,6
Designing TiO2 particles with a gel-network structure is of the utmost importance for highly efficient DSSCs because nanostructured particles are used as an electron transferring medium. For photovoltaic cells in DSSCs, ruthenium(II) complexes containing polypyridyl ligand dyes adsorbed on TiO2 porous film are excited by absorbing the visible light. Efficiency of the electron injection to the conduction band of TiO2 is highly dependent on the structural properties of the semiconductor TiO2 film.7 In addition, the electron transfer in DSSCs is strongly influenced by the physical and chemical properties of TiO2.8 For this reason, TiO2 particles have received great attention for use as a photoelectrode in DSSC systems. In general, the TiO2 film consists of nanosized TiO2 that is sintered on a transparent conducting substrate, thus has a porous geometry.9,10 Various preparation methods such as inert gas condensation, hydrothermal, flame spray pyrolysis, and the sol–gel process have been suggested to synthesize the nanosized TiO2, each with different advantages and disadvantages.11,12 Among the methods that have been introduced, the sol–gel method, which is based on the hydrolysis and polycondensation of metal (i.e., silicon and aluminum) oxides, provides the most effective route for the controlling the physical and chemical properties of TiO2.13,14 Compared to the other methods, this method has a specific advantage in producing different types of coating material such as TiO2 sol, paste and calcined particles. Here, titanium tetra-isopropoxide (Ti(OR)4) has been popularly used as a representative starting material for that purpose.
The sol–gel process has many advantages for the fabrication of TiO2 film in DSSCs. The function of nanocrystalline TiO2 films fabricated from TiO2 sol is strongly dependent on the TiO2 sol preparation process. These synthetic procedures have been shown to properly control a wide range of material properties of the resulting TiO2 films, including the particle size and crystallo-graphic phase, film porosity, surface structure, film roughness, electron transport properties, and optical light scattering.15,16 Recently, the synthesis of TiO2 colloidal paste has drawn much attention for making the photoelectrode in DSSCs.17–20 However, little has been reported on the systematic control of microstructured TiO2 film to induce high efficiency in DSSCs. In particular, few systematic studies have reported on the influence of the adsorption properties between N719 molecules and the TiO2 film surface on the energy conversion efficiency of DSSCs.21
This paper examines the relationship between the photovoltaic performance and the morphological characteristics of TiO2 film. The different textural and crystalline structures of TiO2 films were fabricated using TiO2 sol prepared from titanium isopropoxide, they were used as a starting material by applying the sol–gel method under different polycondensation conditions.22 The adsorption properties of N719 dye on TiO2 films were investigated by adsorption equilibrium isotherm, isosteric enthalpies of dye adsorption,23 and adsorption energy distributions.24–26 We found that the polycondensation process among the sol preparation steps is the most influential step in controlling the physical and chemical properties of the TiO2 film on the photovoltaic performance of the DSSC.
Results and discussion
1. Characterization of TiO2 sol particles by polycondensation
A particle size analysis was performed to investigate the influence of polycondensation conditions on the particle sizes of the TiO2 sol. Fig. 1 shows the particle size distribution curves and their average particle size values of TiO2 sol prepared at different polycondensation times (0, 24, and 72 h) at two temperatures (313.15 and 363.15 K). In this work, the TiO2 sol particles prepared under different conditions were denoted as KYJ-X. Namely, the samples were coded according to their polycondensation temperature and polycondensation time. Six sets of samples were named as KYJ-1 (313.15 K, 0 h), KYJ-2 (313.15 K, 24 h), KYJ-3 (313.15 K, 72 h), KYJ-4 (363.15 K, 0 h), KYJ-5 (363.15 K, 24 h) and KYJ-6 (363.15 K, 72 h), respectively. These results clearly show that the particle size distribution can be substantially altered by adjusting the polycondensation conditions. In other words, the polycondensation time and polycondensation temperature play the key role in controlling the particle size of TiO2. On the whole, the distribution curves are gradually shifted towards the larger particle size as the polycondensation temperature and time increased. The range of particle sizes and average particle sizes of TiO2 sol prepared at a temperature of 363.15 K become broader (50–500 nm) and larger (90–323 nm) than those of the TiO2 sol prepared at a temperature of 313.15 K (10–150 nm and 19–78 nm). We also found that the longer the polycondensation time at the same temperature, the larger the average particle size. For example, the average particle sizes of the TiO2 sol prepared at temperatures of 313.15 and 363.15 K for 72 h are 323 and 78 nm, respectively, which means that they are about 3.6 and 2.7 times larger than those of the TiO2 sol prepared at temperatures of 313.15 and 363.15 K for 0 h. The chemical formation mechanism of the TiO2 sol particles according to polycondensation conditions has been systematically described in our previous works.9,27 These results clearly indicate that the polycondensation time and temperature are important process variables in controlling the particle size.27–29
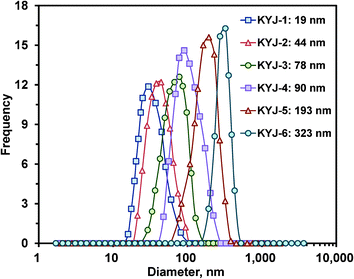 |
| | Fig. 1 Particle size distributions of TiO2 sol particles according to the different polycondensation conditions. | |
2. Characterization of TiO2 film by polycondensation
An XRD analysis was conducted to investigate the effect of polycondensation conditions on the crystallinity of the prepared samples. As shown in Fig. 2, the XRD patterns of the TiO2 films prepared under different polycondensation conditions reveal that the main crystal phase of all the prepared samples is anatase (d101, d004, d200, d105, and d211) although they have also rutile (d110, d111, and d210) and brookite (d121) crystalline phases to some extent. In addition, TiO2 films having a partly rutile crystalline phase are clearly changed into a pure anatase crystalline phase with increasing polycondensation times irrespective of their polycondensation temperature. The anatase crystalline phase as (d101) is a very important adsorption site between TiO2 and N719 dye molecules.19,21 The approximate anatase crystallite size of the TiO2 film samples was calculated using Scherrer's equation. In addition, the anatase constant equation was used to determine the ratio of anatase crystalline phase. Table 1 lists the physical properties obtained from XRD characterization of the TiO2 films prepared at different polycondensation conditions. The crystallinity and crystallite size of the anatase phase as (d101) continually increased with polycondensation. The crystallite size, which varied between 11 and 20 nm as listed in Table 1, is also dependent on the polycondensation temperature. The TiO2 films prepared at 363.15 K have relatively larger sizes compared to those of the TiO2 films prepared at 313.15 K. Except for KYJ-1F and KYJ-4F, no rutile phase peak was observed. This result indicates that the polycondensation time as well as the polycondensation temperature is a critical factor affecting the crystal phase of TiO2 film.
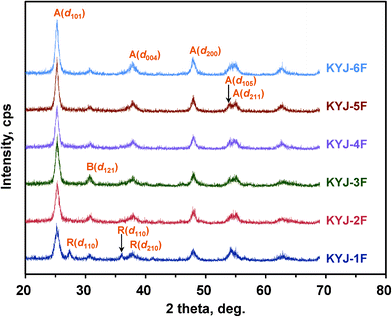 |
| | Fig. 2 X-Ray diffraction patterns of prepared TiO2 film from TiO2 sol according to the different polycondensation conditions. | |
Table 1 Physical properties of TiO2 film with different polycondensation conditions
| Sample No.a |
Size of anatase crystalline phase as (d101), nm |
Anatase ratio (%) |
Fractal dimension, Db |
|
Sample number: KYJ-1F, KYJ-2F, KYJ-3F, KYJ-44F, KYJ-5F, and KYJ-6F are prepared TiO2 film from TiO2 sol according to different polycondensation conditions (313.15 K, 0 h, 313.15 K, 24 h, 313.15 K, 72 h, 313.15 K, 0 h, 363.15 K, 24 h, and 363.15 K, 72 h) in this work.
Fractal dimension was calculated by the Avnir–Jaroniec equation.
|
|
KYJ-1F
|
11 |
71 |
2.489 |
|
KYJ-2F
|
14 |
100 |
2.514 |
|
KYJ-3F
|
15 |
100 |
2.526 |
|
KYJ-4F
|
17 |
91 |
2.490 |
|
KYJ-5F
|
18 |
100 |
2.519 |
|
KYJ-6F
|
20 |
100 |
2.514 |
In order to quantitatively characterize the degree of geometrical nonuniformity (or surface irregularities) of the prepared TiO2 particles, fractal dimension (or fractal geometry) D was used, which shows values varying between 2 and 3. For a perfectly smooth surface, the value is D = 2 while the totally irregular (or rough) surface has a fractal dimension of 3. In this study, we used Avnir and Jaroniec’s equation based on the Frenkel–Halsey–Hill model and the nitrogen adsorption isotherm data to calculate the surface fractal dimension of the prepared TiO2 particles. The Avnir–Jaroniec equation has the following form: 30–33
| | | ln(x) = K − (3 − D)ln(A) | (1) |
where,
Here, x is the amount adsorbed, K is a constant, and A corresponds to the adsorption potential. R is the universal gas constant, T is the absolute temperature, and P0 and P are the saturation and equilibrium pressures during the gas adsorption. Table 1 lists the calculated results of the surface fractal dimensions of the prepared TiO2 films from the TiO2 sol. As shown in Table 1, the prepared films have fractal dimensions ranging from 2.489 to 2.526, which suggest the existence of an irregular surface. In addition, the fractal dimensions are reasonably correlated with the polycondensation conditions. In other words, the surface roughness (or irregularity) of TiO2 films increase with the polycondensation time and the anatase ratio. We also found that the size of anatase crystalline phase and the ratio of anatase increase as the surface geometry of TiO2 film becomes more roughen (or irregular). The 3-dimensional AFM images are also used to get further insight into the morphological changes brought about by polycondensation time and polycondensation temperature on the surface of TiO2 film. The images are recorded at the scan rate of 0.3 Hz on 3 μm × 3 μm planar in non-contact mode as shown in Fig. 3.
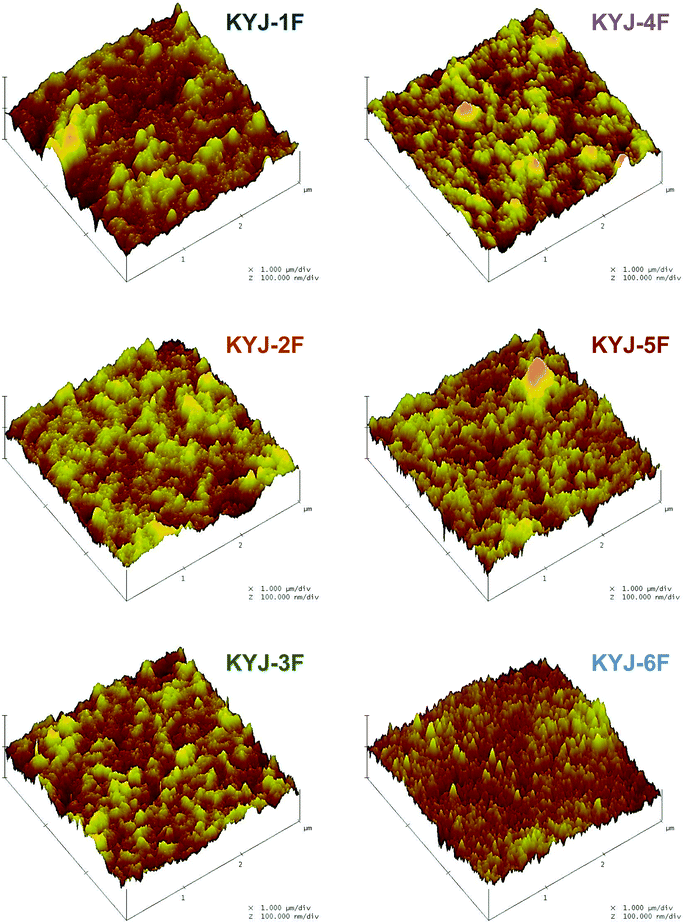 |
| | Fig. 3 Three-dimensional atomic force microscopy images of TiO2 film according to different polycondensation conditions. | |
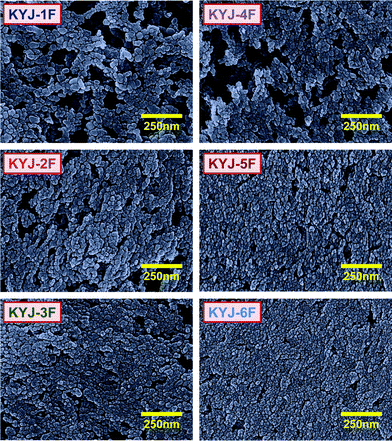
The surface of the prepared films becomes rougher as the polycondensation time and polycondensation temperature increase. The AFM images also clearly show that the KYJ-6F has a more uniformly coated surface without voids and aggregated particles than those of other samples. Table 2 also lists the root mean square (Rms) (Rq) and mean (Ra) roughness value of the TiO2 film. The surface roughness values of TiO2 film are observed to decrease in the sequence of KYJ-6F > KYJ-5F > KYJ-3F > KYJ-2F > KYJ-4F > KYJ-1F, which is the same as that of the size of the anatase crystalline phase. The TiO2 films having a rutile phase (KYJ-1F and KYJ-4F) represent relatively lower surface roughness values compared to that of the films having a pure anatase phase. This result can be explained by the transition degree of the TiO2 crystalline phase. Our previous study has shown that the phase transformation from anatase to rutile is partially dependent on the particle size.9
Table 2 Physical properties of TiO2 film with different polycondensation conditions
| Sample No. |
Root mean square roughness (Rq), nm |
Mean roughness (Ra), nm |
|
KYJ-1F
|
9.36 |
7.18 |
|
KYJ-2F
|
10.21 |
7.97 |
|
KYJ-3F
|
11.16 |
8.93 |
|
KYJ-4F
|
9.65 |
7.72 |
|
KYJ-5F
|
12.78 |
9.73 |
|
KYJ-6F
|
14.26 |
11.03 |
In other words, the TiO2 sols with larger particle size represent a relatively slower transition of the crystalline phase. Therefore, the TiO2 film samples with some rutile phase (KYJ-1F and KYJ-4F) have low surface roughness. In general, if the TiO2 film has a high surface roughness, the amount of the N719 dye adsorbed is drastically increased.34
Fig. 4 shows the FE-SEM surface images of TiO2 film. Some of the rutile phase with larger particle sizes (>50 nm) is observed after the fabrication of TiO2 film in the case of the samples prepared at polycondensation temperatures of 313.15 and 363.15 K for 0 h (KYJ-1F and KYJ-4F) although many aggregated TiO2 particles are also observed. However, the TiO2 particles prepared at a polycondensation time of 24 h (KYJ-2F and KYJ-5F) and 72 h (KYJ-3F and KYJ-6F) exhibit the predominantly anatase phase with small particle size (average: 20 nm). Especially in the case of KYJ-5F (24 h) and KYJ-6F (72 h) prepared at polycondensation temperature of 363.15 K, a well-ordered surface and highly dispersive pore structures are observed. The particles of the fabricated TiO2 showed higher adsorption capacity of N719 dye on TiO2 film as the anatase crystalline phase increased. This phenomenon is attributed to the fact that TiO2 particles in the crystalline phase are smaller than those in the rutile crystalline phase.35
In general, N719 dye adsorption is substantially dependent on the specific surface area of TiO2 film. It has been reported that the higher the TiO2 film surface area which supports the larger available adsorption site, the larger the N719 dye adsorption onto TiO2 film.34–36Table 3 lists the BET specific surface area, average pore size and total pore volume of TiO2 films prepared at different polycondensation conditions. The direct correlations between the textural properties and the polycondensation conditions are observed. As listed in Table 3, the surface area and total pore volume of TiO2 particles are increased with increasing polycondensation time and temperature, while the average pore size is decreased. In other words, the longer polycondensation time at the same polycondensation temperature resulted in the higher surface area, total pore volume and narrower average pore size. The TiO2 films having the pure (100%) anatase phase have a relatively higher BET surface area and pore volume than that of the TiO2 films consisting of both anatase and rutile structures (i.e., KYJ-1F and KYJ-3F). Moreover, in the case of the films representing the same structure, the larger the crystallite size is, the greater the BET surface area and the pore volume are (see Table 1 and 3). These findings led us to conclude that the textural and crystalline properties of TiO2 film are highly dependent on the polycondensation time and temperature.
Table 3 Textural properties of TiO2 film prepared according to the different polycondensation conditions
| Sample No. |
Surface areaa, m2 g−1 |
Total pore volumeb, cm3 g−1 |
Average pore sizec, nm |
|
Specific BET surface area (P/P0 = 0.1 − 0.2).
Average pore size and total pore volume (P/P0 = 0.99) calculated by the BJH method.
Average pore size and total pore volume (P/P0 = 0.99) calculated by the BJH method.
|
|
KYJ-1F
|
41.2 |
0.103 |
8.57 |
|
KYJ-2F
|
46.7 |
0.118 |
6.55 |
|
KYJ-3F
|
54.6 |
0.125 |
7.66 |
|
KYJ-4F
|
44.2 |
0.111 |
8.18 |
|
KYJ-5F
|
63.4 |
0.133 |
6.35 |
|
KYJ-6F
|
69.5 |
0.138 |
6.24 |
The performance of DSSCs is closely related with the adsorption equilibrium amount of dye on TiO2. Thus it is appropriate to investigate the adsorption conditions optimized for improving their cell performance. In particular, the adsorption equilibrium amount of N719 dye on TiO2 film is highly dependent on the BET surface area, the crystalline size and the phase. In the current work, the N719 dye adsorption isotherms are obtained at different temperatures and different pHs. To analyse the experimental isotherm data, we used Langmuir and Languir–Freundlich isotherm equations, which are summarized in Table 4.37
Table 4 Langmuir and Langmuir-Freundlich isotherm equation
| Isotherm equation |
| Langmuir |
|
| Langmuir–Freundlich isotherm equation |
| | |
q = qm(Kc)n/1 + (Kc)n
| (4) |
|
| Where q is the adsorbed amount, qm is the monolayer adsorption capacity, K is the equilibrium constant, c is the solute equilibrium concentration and n is the system heterogeneity parameter. |
As our previous work has shown, the photovoltaic performance of dye-sensitized solar cells (DSSCs) is closely related with the textural properties of TiO2 and its adsorption equilibrium amount of dye as well as the coordination of dye adsorbed in TiO2 film.21,36 Thus, it is necessary to systematically examine the effect of polycondensation conditions on the adsorption characteristics (i.e., equilibrium and kinetics) of the dye in the TiO2 film. In this study, dye N719, which has two bipyridyl ligands with two carboxyl groups at the 4 and 4′ position of the bipyridyl group, is used as a model adsorbate. The adsorption equilibrium isotherm data (symbols) of dye N719 in six different TiO2 film samples at three different temperatures (303.15, 318.15 and 333.15 K) are shown in Fig. 5, and their calculated Langmuir and Langmuir–Freundlich (LF) isotherm parameters are listed in the ESI,† Table S1 and S2, respectively. We also found a clear correlation between the adsorption equilibrium amount and textural properties of TiO2 film. The obtained adsorption equilibrium amounts of N719 dye in the TiO2 film samples are in the order KYJ-6F (363.15 K, 72 h) > KYJ-5F (363.15 K, 24 h) > KYJ-3F (313.15 K, 72 h) > KYJ-2F (313.15 K, 24 h) > KYJ-4F (363.15 K, 0 h) > KYJ-1F (313.15 K, 0 h), which is identical to that of the BET surface area and the total pore volume. In addition, the adsorption capacities of N719 dye are closely related with the anatase ratio, the surface roughness, the fractal dimension and the size of anatase crystalline phase. As shown in Fig. 2 and Table 1, the higher the anatase ratio and the larger the anatase crystalline phase size, the greater the adsorption equilibrium amount. In other words, TiO2 films having an anatase ratio of 100% exhibit a higher adsorption equilibrium amount of N719 dye than that of TiO2 films having an anatase ratio of 91 and 71%. In the case of TiO2 films having the same anatase ratio (100%), the adsorption equilibrium amount increases with the increasing anatase crystalline phase size. The values of the surface roughness (or fractal dimension) of TiO2 film increased also with the increasing polycondensation time irrespective of the polycondensation temperature (Table 2).
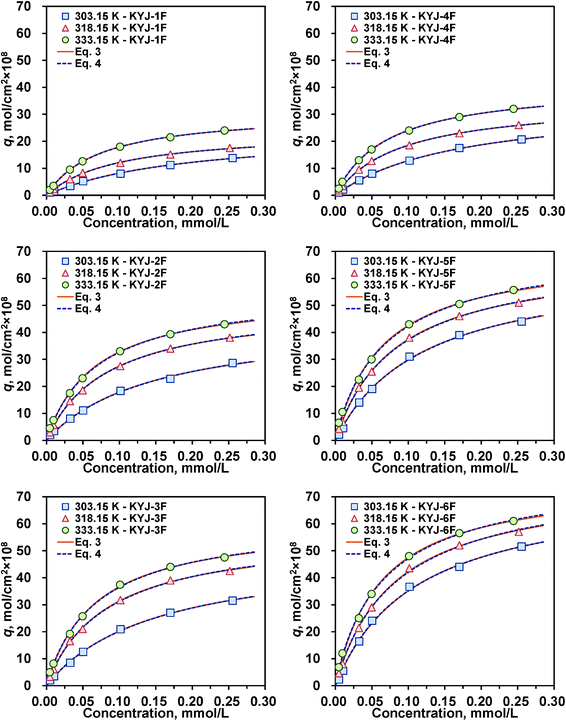 |
| | Fig. 5 Adsorption isotherms of N719 on KYJ-TiO2 film series at three different temperatures (Langmuir and LF equation). | |
On the other hand, the solid lines in Fig. 5 and 6 are the fitting results. As can be seen, the Langmuir and LF isotherms are found to adequately represent the N719 adsorption isotherm data. We found that these isotherm parameters qm and b increase with increasing temperature, and they increase in the order of KYJ-1F < KYJ-4F < KYJ-2F < KYJ-3F < KYJ-5F < KYJ-6F, which is exactly identical to that of the BET surface area and pore volume. Moreover, the heterogeneity parameter n (LF isotherm) decreases with increasing temperature in all samples, implying a greater surface heterogeneity at higher temperatures (see ESI,† Table S2). The heterogeneity parameter is also in the order KYJ-1F > KYJ-4F > KYJ-2F > KYJ-3F > KYJ-5F > KYJ-6F, which is the same as that of the BET surface area and pore volume. These results indicate that the TiO2 film having a higher BET surface area and pore volume represents the stronger adsorption interaction between N719 dye and these textural properties. Furthermore, they indicate that the crystallinity of the TiO2 film also plays a key role in N719 dye adsorption. In general, the initial pH of the solution plays an important role in the adsorption process, since it affects the surface charge of the adsorbent and the ionic and structural properties of adsorbate (or N719 dye) as well as the interaction between the adsorbate and the adsorbent.
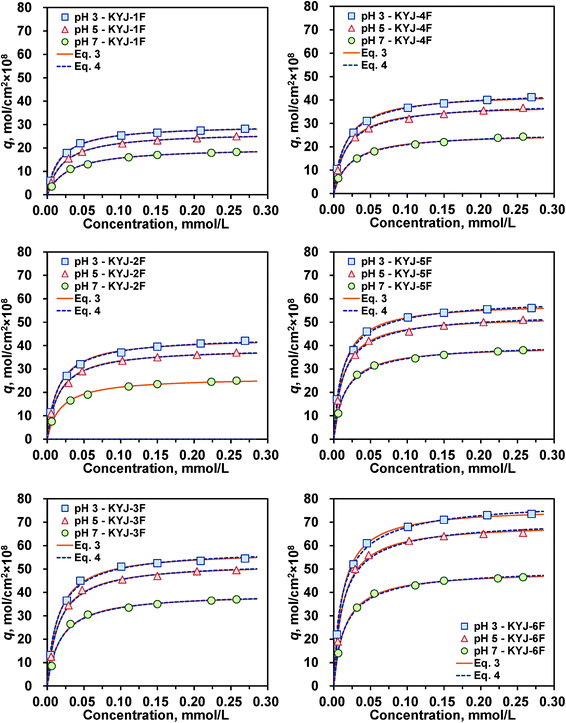 |
| | Fig. 6 Adsorption isotherms of N719 on KYJ-TiO2 film series at three different pH values (Langmuir and LF equation). | |
To examine the effect of solution pH on the dye adsorption, the adsorption equilibrium isotherms of dye N719 are also obtained for six different TiO2 films at three different pH values (pH = 3, 5, and 7) under the same condition of 303.15 K. Fig. 6 shows their adsorption isotherms plotted as adsorbed equilibrium amount versus aqueous phase equilibrium concentration. This result also proves that the N719 dye adsorption is highly dependent on the solution pH, the textural and crystalline properties of TiO2 films and the system temperature. The adsorption equilibrium amount increases with decreasing initial pH in all the samples studied, suggesting that the N719 dye adsorption favours acidic conditions. This result is related to the electrostatic interactions between the N719 dye exhibiting a negative charge in ethanol solution and the surface of TiO2 film representing a positive charge in acidic condition. Thus the higher N719 dye adsorption is achieved at lower pHs. Langmuir and LF isotherms were also used to correlate the N719 dye adsorption equilibrium isotherm data and their determined parameters. They are listed in the ESI,† Table S3 and S4, respectively. As shown in these figures and tables, the adsorption of N719 dye in TiO2 films is well represented by the Langmuir and LF isotherm model equations. In addition, the isotherm parameters show the similar tendencies observed from the temperature effect on the N719 dye adsorption. In other words, the isotherm parameters qm and b decrease with increasing pHs irrespective of the model equation, but the heterogeneity parameter n increases with pH. This result reveals that the degree of heterogeneity increased at lower pHs and larger BET surface area and pore volume.
To further investigate the adsorption processes and the adsorbed state of N719 dye in TiO2 films prepared at different conditions, several thermodynamic functions (i.e., the adsorption free energy (ΔGo), enthalpy (ΔHo) and entropy (ΔSo)) were calculated using the Langmuir isotherm constant and the Van't Hoff equation. Table 5 lists the model equations used in this study. These parameters give valuable information about the adsorption mechanism that exists between the N719 (adsorbate) and the TiO2 film (adsorbent). Table 6 shows the thermodynamic parameters determined for N719 dye adsorption on the six different TiO2 films. The thermodynamic values obtained can be classified into two main groups: (1) negative values of adsorption free energy (ΔGo) and (2) positive values of enthalpy (ΔHo) and entropy (ΔSo). As shown in Table 6, the adsorption free energy values (ΔGo) are negative for all cases and they become gradually lower (or smaller) as the temperature increases. These findings led us to suggest that the N719 dye adsorption in TiO2 film is a spontaneous process which is closer to the physisorption region of −20 to 0 kJ mol−1 rather than the chemisorption region of −80 to −400 kJ mol−1. It is also a thermodynamically favourable process that demonstrates the increase in the adsorption equilibrium corresponding to temperature increases.
Table 5 Thermodynamic parameters used in this study
|
(a) Adsorption free energy (ΔGo)
|
|
|
| where b is the Langmuir equation constant, R is the gas constant and T is the temperature. |
|
(b) Van't Hoff equation
|
|
|
| where b is the Langmuir equation constant, R is the gas constant, T is the temperature, ΔH° is the adsorption enthalpy and ΔS° is the adsorption entropy. |
Table 6 Thermodynamic parameters of N719 dye (Gibbs energy, enthalpy and entropy)
| Sample No. |
ΔGo |
ΔHo, KJ/mol K |
ΔSo, J mol−1 K |
| 303.15 K |
318.15 K |
333.15 K |
|
KYJ-1F
|
−4.162 |
−6.156 |
−7.256 |
27.25 |
104.06 |
|
KYJ-2F
|
−4.800 |
−6.595 |
−7.517 |
22.80 |
91.49 |
|
KYJ-3F
|
−4.814 |
−6.657 |
−7.536 |
22.55 |
90.79 |
|
KYJ-4F
|
−4.485 |
−6.419 |
−7.354 |
24.68 |
96.70 |
|
KYJ-5F
|
−5.364 |
−6.664 |
−7.625 |
17.54 |
75.72 |
|
KYJ-6F
|
−5.571 |
−6.710 |
−7.760 |
16.56 |
73.04 |
In addition, the adsorption enthalpy (ΔHo) and entropy (ΔSo) values were positive in all the adsorption tests, reflecting the endothermic process of N719 dye adsorption and the increased degree of freedom (or randomness) between the N719 dye and TiO2 film. It is also noted that the thermodynamic values are gradually increased (or decreased) as the polycondensation time increased under the same polycondensation temperature. Moreover, the TiO2 films prepared at 363.15 K have relatively lower (or higher for ΔGo) thermodynamic values than that of the TiO2 films prepared at 313.15 K. The calculated values show that the larger the ΔHo, the greater are the entropy (ΔSo) values. The magnitudes of adsorption enthalpy and entropy are in the order of KYJ-1F > KYJ-4F > KYJ-2F > KYJ-3F > KYJ-5F > KYJ-6F, which is identical to that of the BET surface area and the pore volume. On the whole, the TiO2 films having the pure anatase phase have lower adsorption enthalpy and entropy values than that of the TiO2 films composed of both anatase and rutile phase. It is also interesting to note that the larger the anatase crystalline phase size, the smaller the adsorption enthalpy and entropy are. This result implies that the interaction between N719 dye and TiO2 film is highly dependent on the textural and anatase crystalline phase. An adsorption energy distribution (AED) function has been also used to characterize porous solid surfaces having complex porous structure and chemical composition. This function can provide valuable information on the interactions between the probe molecule and the solid surface. In this work, we used LF equation and the generalized nonlinear regularization method to calculate the AED function, which is summarized in Table 7.24 In particular, the AED functions are calculated using the data points below the monolayer coverage which were determined from the LF equation. Detailed explanation for calculating the AED function can be found elsewhere.25,38
Table 7 Adsorption energy distribution function used in this study
| Adsorption energy distribution function |
| |
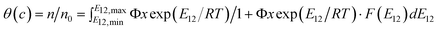
| (7) |
|
| where θ(c) is the total fraction coverage of solute, Φ = Φ(c,θ) is a model dependent function, E12 = E1 − E2 is the energy difference between the solute and water, F(E12) is the energy distribution function, T is the absolute temperature, R is the gas constant; x is c/csol, where csol is the solubility of the solute in water. |
Fig. 7 and 8 show the variation of the AED curves for N719 dye on TiO2 films prepared at different poly condensation conditions in different pH values. The corresponding AED parameters are listed in the ESI,† Table S5. The calculated AED functions represent only one peak for all the TiO2 films, supporting the presence of one type of surface energy on TiO2 samples. Furthermore, the peak height and the shape of AED curves became gradually lower and broader and the peak location was also shifted toward the lower energy when the pH decreased from 7 to 3 except for the AED pattern of KYJ-1F. For example, the pronounced peak maximums (or the peak width) of the AED function for KYJ-6F sample were about 9.45 (10.3), 10.1 (10.3), and 10.3 (13.0) kJ mol−1, respectively, for pH 7, pH 5, and pH 3. In addition, the peak height values of the same sample were 0.211, 0.207 and 0.169 mol kJ−1, respectively, for the same pHs. The peak maximum of the AED function shifted about 0.8 kJ mol−1 to the higher energy as the pH values increased. These results clearly indicate that the adsorption interactions between the N719 dye and the surface of TiO2 films are enhanced with decreasing pHs. The characteristics of the AED function are also well correlated with the degree of surface heterogeneity of the TiO2 films. As the LF parameter (n) value increased, the peak width and the peak height of AED curves became narrow and high, respectively. The shifting of the curves toward the relatively lower energies with increasing the heterogeneity parameter n is also observed. This result also implies that the interaction between the N179 dye molecules and the TiO2 film increased with the surface heterogeneity. Fig. 7 (lower right side) and Fig. 8 (lower right side) compare the calculated AED curves for N719 dye on different TiO2 films in the same value of pH (pH 7). Contrary to our expectations, there is no clear correlation between the AED peaks and the TiO2 films. With the increase of BET surface area and pore volume, the peak maximum values and the peak width of AED curves are increased and narrowed, respectively.
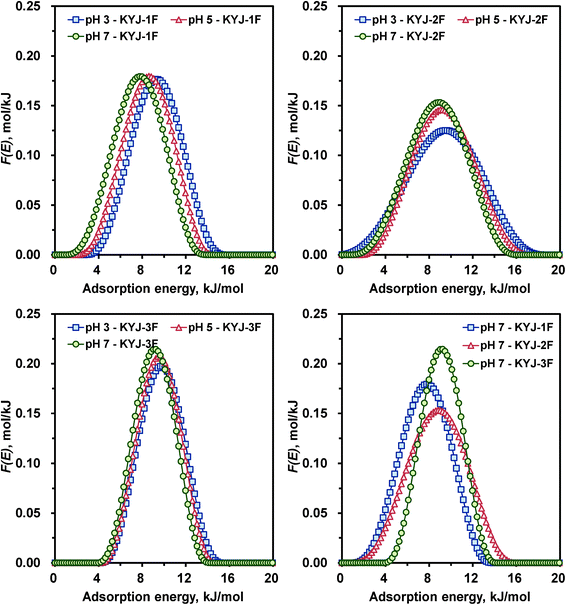 |
| | Fig. 7 Calculated adsorption energy distribution curves for KYJ-1F, KYJ-2F and KYJ-3F at three different pH values. | |
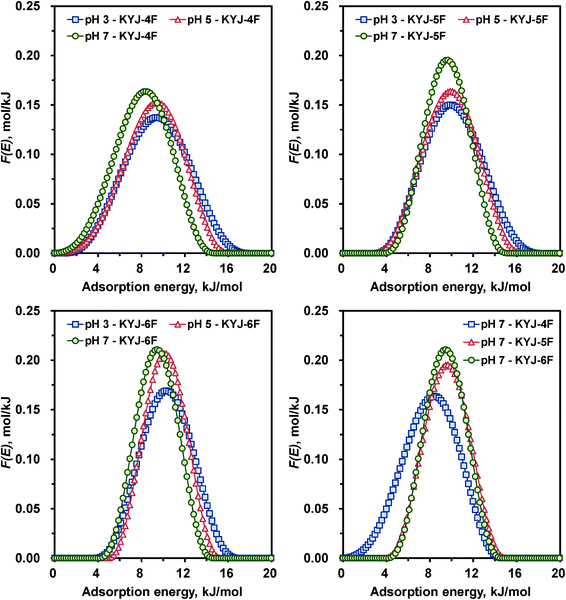 |
| | Fig. 8 Calculated adsorption energy distribution curves for KYJ-4F, KYJ-5F and KYJ-6F at three different pH values. | |
The variations of the AED curves for N719 dye on the same TiO2 films investigated at three different temperatures are also shown in Fig. 9 and 10. The corresponding AED parameters are also listed in the ESI,† Table S6. The AED peak shape and patterns are generally similar to that of the AED curves obtained at three different pH. The higher the system temperature, the broader and lower the AED curve. In addition, a gradual shift of the distribution peak location toward higher adsorption energies was observed as the system temperature increased. In other words, by increasing the system temperature from 303.15 to 333.15 K, the AED peak height of KYJ-6F for N719 dye decreased from 0.237 to 0.188 mol kJ−1; conversely, the AED peak width increased from 8.19 to 11.55 kJ mol−1.
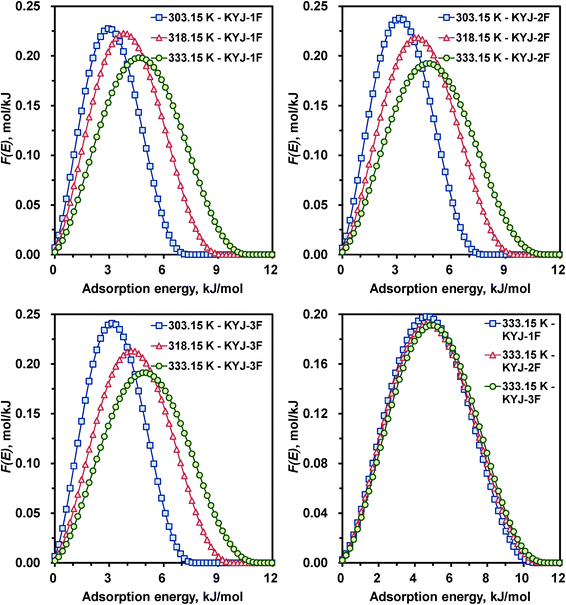 |
| | Fig. 9 Calculated adsorption energy distribution curves for KYJ-1F, KYJ-2F and KYJ-3F at three different temperatures. | |
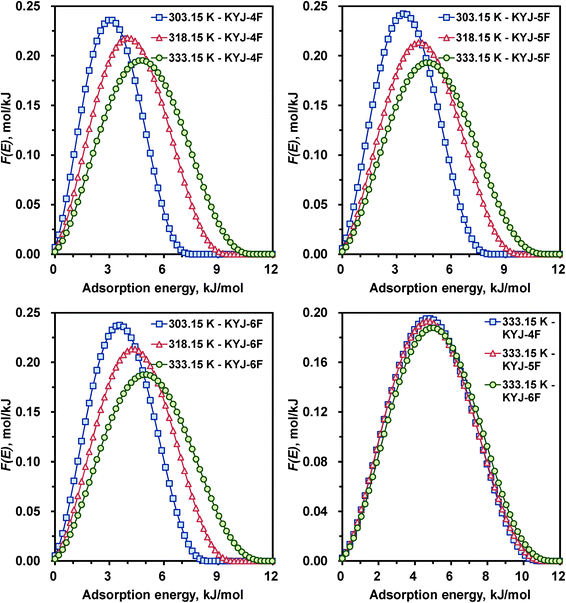 |
| | Fig. 10 Calculated adsorption energy distribution curves for KYJ-4F, KYJ-5F and KYJ-6F at three different temperatures. | |
In addition, the pronounced maximum of the AED curve of KYJ-6F sample are 3.78 (303.15 K), 4.62 (318.15 K), and 5.04 (333.15 K) kJ mol−1, respectively. On the other hand, Fig. 9 (lower right side) and 10 (lower right side) compare the calculated AED functions of N719 dye on TiO2 films at the same of polycondensation temperature (333.15 K). As can be seen, the AED curves have a close relation with the textural properties of TiO2 films, although the difference between the peak width and the peak height of the AED curves is small. It is evident that the increase in adsorption energy may be mainly attributed to both the increase of surface area and pore volume and the difference in the size of anatase crystalline phase and the ratio of anatase–rutile. The larger the BET surface area (or pore volume), the lower and broader are the AED curves. The peak maxima also are shifted toward higher adsorption energy when those values increased. Moreover, a similar tendency is also observed in the heterogeneity parameter n. The AED curves become gradually lower and broader with decreasing the parameter n. In addition, the peak maximum value of AED curves was increased with decreasing the parameter n.
4. Photovoltaic performance of TiO2 film by polycondensation
To investigate the influence of polycondensation conditions on the photovoltaic performance of TiO2 thin films, several key factors such as the current density (Isc), open circuit voltage (Voc), fill factor (FF), and energy conversion efficiency (ηeff) are measured and compared in Fig. 11 and Table 8. Based on the adsorption isotherm result, the TiO2 thin films were immersed in a solution containing 0.3 mM N719 dye at pH 3 and 333.15 K for 12 h to fabricate the whole DSSC. As can be seen, the photovoltaic performances are closely related with the textural properties and the crystallinity of TiO2 films. On the whole the TiO2 films prepared at 363.15 K (KYJ-4F-6F) have relatively higher energy conversion efficiency and the current density than that of the TiO2 films prepared at 313.15 K (KYJ-1F-3F). In addition, the longer polycondensation time at the same polycondensation temperature, the higher photovoltaic the performance is. It is also found that the TiO2 films exhibiting 100% anatase phase have relatively higher photovoltaic performance than that of the TiO2 films consisting of both anatase and rutile phase. Moreover, in the case of the TiO2 films exhibiting the same crystallite phase, the larger crystallite size, the surface area, the pore volume, the higher the photovoltaic performance is.
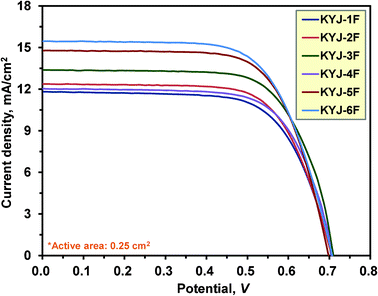 |
| | Fig. 11
I–V curves of KYJ-TiO2 film series under different polycondensation conditions. | |
Table 8 Photovoltaic performance of DSSC for KYJ-TiO2 film series under different aging conditions
| Sample No. |
Film thickness, μm |
I
sc
, mA cm−2 |
V
oc
, V |
Fill factor, FF |
η
eff
(%) |
|
KYJ-1F
|
7.0 |
11.8 |
0.71 |
0.67 |
5.6 |
|
KYJ-2F
|
7.0 |
12.9 |
0.71 |
0.68 |
6.0 |
|
KYJ-3F
|
7.0 |
13.4 |
0.70 |
0.69 |
6.6 |
|
KYJ-4F
|
7.0 |
12.0 |
0.71 |
0.69 |
5.9 |
|
KYJ-5F
|
7.0 |
14.8 |
0.71 |
0.68 |
7.1 |
|
KYJ-6F
|
7.0 |
15.6 |
0.71 |
0.66 |
7.3 |
Fig. 12 shows the Nyquist plots of TiO2 thin film based DSSCs which are prepared at different polycondensation temperatures and polycondensation times. As can be seen, a large semicircle at low frequencies and a small one at high frequencies are observed for all solar cells. In general, the frequency responses can be classified into three regions: (i) high frequency range (103–106 Hz) corresponding to charge transfer processes occurring at the Pt/electrolyte interface, (ii) intermediate frequency range (1–103 Hz) representing the resistance occurred at the TiO2/dye/electrolyte interface, and (iii) low frequency range (0.1–1 Hz) relating to the Nernst diffusion within the electrolyte. As shown in Fig. 12, the resistance occurred at the TiO2/dye/electrolyte interface decreased with increasing polycondensation time and temperature, implying that the electron transport in the rutile layer (KYJ-1F and KYJ-1F) is slower than in the anatase layer (KYJ-2F, KYJ-3F, KYJ-5F and KYJ-6F).31 In addition, the larger the anatase crystallite size and the surface area and pore volume, the lower the resistance is. The KYJ-4F-6F thin film based DSSCs which are prepared at higher temperature (363.15 K) represent relatively lower resistance compared to that of the KYJ-1F-3F thin film based DSSC (see Fig. 12a).
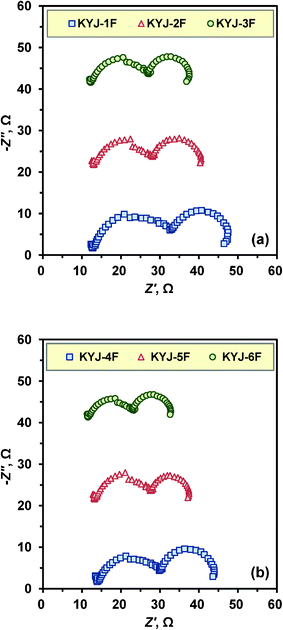 |
| | Fig. 12 Nyquist plots of KYJ-TiO2 film series with various polycondensation times at (a) 313.15 K (b) 363.15 K. | |
Fig. 13 clearly demonstrates that the photovoltaic performance has a close connection with the anatase crystallite size, N719 dye adsorption amount (Q) and thermodynamic parameters (ΔHo and ΔSo). It is evident that larger crystallite sizes are more efficient in enhancing the photovoltaic properties (ηeff and Isc) and the N719 dye adsorption amount irrespective of their composition ratio. On the whole, the ηeff and the Isc of the TiO2 film increased linearly with the crystallite size although the TiO2 film consisting of both anatase and rutile phase gives slightly lower ηeff and the Isc than that of the TiO2 film having a pure anatase phase. In addition, the TiO2 film (KYJ-4F) having relatively higher anatase phase (91%) exhibit slightly higher photovoltaic performance than that of the film (KYJ-1F) representing lower anatase phase (71%), which is attributed to its higher N719 dye adsorption amount and lower ΔHo and ΔSo (see Fig. 13a dotted square). Moreover, the pure anatase (100%) phase films exhibit much higher photovoltaic performance compared to that of the anatase–rutile mixture phase films, which is closely related with the N719 dye adsorption characteristics. In other words, the greater the N719 dye adsorption amount and the lower the adsorption enthalpy and the adsorption entropy, the higher the ηeff and the Isc.
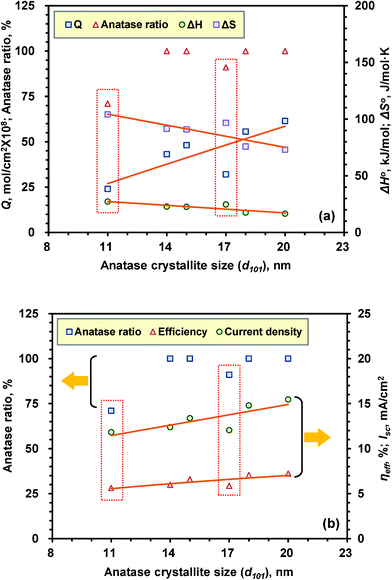 |
| | Fig. 13 (a) Correlation plot between crystallite size-adsorption (b) crystallite size-photovoltaic properties. | |
Conclusions
This paper considers the effects of polycondensation conditions (time and temperature) on the performance of DSSCs. More importantly, we demonstrate the relationship between the crystallite size, crystalline phase (anatase and rutile), N719 dye adsorption properties and photovoltaic performance. Our results reveal that the polycondensation time and polycondensation temperature are two important factors dominating the textural and crystal structure and crystallinity of TiO2 particles. The BET surface area, the pore volume and the crystallite size increase with polycondensation time and polycondensation temperature. The N719 adsorption equilibrium amount increases with increasing temperature and conversely with decreasing pH, which indicates that the endothermic and acidic condition is more effective in enhancing the N719 adsorption capacity. In addition, the greater the surface area, pore volume and crystallite size, the larger the N719 dye adsorption is. The TiO2 films having pure anatase phase represent a higher N719 dye adsorption amount than that of the TiO2 films composed of both anatase and rutile phases. The Langmuir and LF isotherm equation models are found to successfully describe the adsorption isotherm data. Moreover, the AED curves become relatively lower and broader, moving towards higher energy as the heterogeneity parameter n decreases and conversely increases the surface area and pore volume. A direct correlation between the crystallite size, the N719 dye adsorption amount and the photovoltaic performance (Isc and ηeff) was established. The short circuit current (Isc) and the overall conversion efficiency (ηeff) are highly dependent on the crystallite size and the N719 dye adsorption properties (i.e., adsorption equilibrium amount, adsorption enthalpy and entropy). We further demonstrate that the TiO2 film having a pure anatase phase, a high surface area including pore volume, and large anatase crystallite size is effective in enhancing the photovoltaic performance.
Experimental section
1. Synthesis of TiO2 sol
According to the previous woks,27 TiO2 films were fabricated using a synthesized TiO2 sol. For the control of nanostructured TiO2 films, the TiO2 sol was prepared through the poly-condensation process accompanied by hydrolysis using titanium-tetra-isopropoxide (TTIP, Junsei Chemical Co. > 98%) as a starting material. The reaction between TTIP and H2O was performed under the condition of 1000 rpm and temperatures of 313.15 K and 363.15 K. The amount of water was fixed at 100 H2O/Ti molar ratio.21 The first chemical reaction was a hydrolysis between TTIP and water. Although the hydrolysis reaction rate is known to be too fast in the TiO2 sol reaction, the reaction was easily controlled after the initial hydrolysis because of the slow rate. The characteristics of TiO2 sol particles were controlled by optimizing the time and temperature of polycondensation. The initial fast reaction between the water and TTIP reached the equilibrium temperature within 30 min. The reaction after 30 min was named “polycondensation”. The effect of polycondensation was investigated according to the variation of polycondensation times (0, 24, 72 h) under the condition of two different polycondensation temperatures of 313.15 K and 363.15 K. After the polycondensation, TiO2 solution was transformed into a solution phase like paste, and the peptizing agent needed acidic or basic electrolyte to peptize the solution effectively. Although various electrolytes such as HCl, HNO3 and NH4OH were available for peptizing agent, this experiment was performed under the condition of nitric acid (HNO3; Aldrich Co. 60%) as an inorganic acid to derive the ionic repulsive force required at the condition of low concentration. An acidic electrolyte was added to the solution with TiO2 aggregated particles (determined by polycondensation) to induce the repulsive force between inter particles and to prevent continuous aggregation. At this point, the acidic electrolyte was selected using nitric acid (HNO3, Aldrich Co. 60%) as an inorganic acid. The amount of the HNO3 was optimized at 0.25 HNO3/Ti molar ratio condition determined by the preliminary experiment.27
2. Preparation of TiO2 film
As mentioned before, TiO2 sols were obtained under the variation of polycondensation times (0, 24, 72 h) at two different polycondensation temperatures (313.15 K and 363.15 K). For the preparation of TiO2 film, TiO2 paste was prepared by adding 2.6 g TiO2 sol solutions, 0.7 ml 10% [v/v] acetyl acetone, 1.9 g hydroxypropyl cellulose (Mw. 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aldrich), and 10.8 ml water for 20 min at 1350 rpm using a paste mixer (PDM-300, Korea mixing technology Co.). Then, a TiO2 film was fabricated by coating a precursor paste onto the fluorine-doped SnO2 conducting glass plates (FTO, 8 Ω/cm2, Pilkington Co.) using the screen printing method. The TiO2 film was treated by heating at 773.15 K for 2 h. The TiO2 film formed thus on the FTO glass is 7 μm in thickness and 0.5 × 0.5 cm in size.
000, Aldrich), and 10.8 ml water for 20 min at 1350 rpm using a paste mixer (PDM-300, Korea mixing technology Co.). Then, a TiO2 film was fabricated by coating a precursor paste onto the fluorine-doped SnO2 conducting glass plates (FTO, 8 Ω/cm2, Pilkington Co.) using the screen printing method. The TiO2 film was treated by heating at 773.15 K for 2 h. The TiO2 film formed thus on the FTO glass is 7 μm in thickness and 0.5 × 0.5 cm in size.
3. Fabrication of DSSC
To fabricate the DSSCs, the prepared TiO2 film electrode was immersed in the N719 dye (Solaronix Co.) solution of 0.3 mM at 333.15 K for 12 h, rinsed with anhydrous ethanol and dried. A Pt coated glass-SnO2: F (FTO, 8 Ω/cm2, Pilkington Co.) electrode was prepared as a counter electrode with an active area of 0.25 cm2. The Pt electrode was placed over the dye adsorbed TiO2 film electrode, and the edges of the cell were sealed with 1 mm strips of 60 μm thick sealing sheet (SX 1170-60, Solaronix). Sealing was accomplished by hot-pressing the two electrodes together at 393.15 K. The redox electrolyte was injected into the cell through the small holes and sealed with a small square of sealing sheet. The redox electrolyte consists of 0.3 M 1,2-dimethyl-3-propylimidazolium iodide (Solaronix), 0.5 M LiI (Aldrich), 0.05 M I2 (Aldrich), and 0.5 M 4-tert-butylpyridine (4-TBP, Aldrich) and 3-metoxypropionitrile nitrile (3-MPN, Fluka) as a solvent.
4. Characterization of TiO2 sol and TiO2 film
The particle size distributions of TiO2 sol were measured by a particle size analyser (ELS-8000, OTSUKA Electronics Co.). The crystallinity of the manufactured TiO2 sol particles and TiO2 film was characterized by an X-ray diffraction (XRD; D/MAX-1200, Rigaku Co.) using a Cu-Kα X-ray and Ni filter at 35 kV and 15 mA. The surface morphology of the TiO2 film was also examined on an atomic force microscope (AFM; XE-100, Park Systems Co.) in the non-contact mode. The structural properties of the TiO2 film prepared from TiO2 sol were measured by field-emission scanning electron microscopy (FE-SEM; S-4700, Hitachi Co.). The nitrogen adsorption isotherms on TiO2 film were measured at 77 K using an automatic analyser (nanoPOROSITY, MiraeSI Co.). Before the measurements, the samples were outgassed for 2 h in the degas port of the adsorption apparatus. In addition, the pore size and pore volume were calculated from the adsorption branches of the isotherms by using the Barrett, Joyner, and Halenda (BJH) method.23 Adsorption equilibrium experiments were carried out by contacting a given amount of TiO2 film with N719 dye solution of 0.01–0.5 mM in a shaking incubator at 303.15–393.15 K for 12 h. The pH of N719 dye solution was adjusted by 0.1 M HCl solution and 0.1 M NaOH solution. The adsorption capacity of TiO2 film was measured by completely desorbing the adsorbed dye molecules from TiO2 film using 0.1 M NaOH solution/ethanol (50/50 vol%). The concentration of N719 dye solution was analyzed by a UV spectrophotometer (UV-160A, Shimadzu) at 522 nm. The adsorption properties of N719 dye on TiO2 films were calculated by adsorption equilibrium isotherm,26 isosteric enthalpies of adsorption,23 and adsorption energy distributions.24–26 The capacities of fabricated DSSCs and the current–voltage (I–V) curves were measured using a source measure unit under irradiation of white light from a 1000 W Xenon lamp (Thermo oriel instruments, USA). The incident light intensity and the active cell area were 100 mW cm−2 and 0.25 cm2, respectively. The I–V curves were used to calculate the short-circuit current (Isc), open-circuit voltage (Voc), fill factor (FF), and overall conversion efficiency (ηeff) of DSSCs. The electrochemical impedance spectroscopy (EIS) measurements were performed using the AC impedance (CHI 660A Electrochemical Work-station, USA) over the frequency ranging from 1 to 106 Hz with amplitudes of ± 5 mV over the Voc.
References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. Grätzel, J. Photochem.Photobiol. A: Chem., 2004, 164 Search PubMed.
-
M. Kaneko, I. Okura, Photocatalysis-Science and Technology, Springer: Kodansha, 2002 Search PubMed.
-
G. Hodes, Electrochemistry of Nanomaterials, Wiley-VCH: New York, 2001 Search PubMed.
- M. Biancardo, K. West and F. C. Krebs, J. Photochem. Photobiol., A, 2007, 187, 395 CrossRef CAS.
- M. Biancardo, K. West and C. F. Krebs, Sol. Energy Mater. Sol. Cells, 2006, 90, 2575 CrossRef CAS.
- H. Wang, Y. Liu, H. Xu, X. Dong, H. Shen, Y. Wang and H. Yang, Renewable Energy, 2009, 34, 1635 CrossRef CAS.
- B. C. O'Regan, K. Bakker, J. Kroeze, H. Smit, P. Sommeling and J. R. Durrant, J. Phys. Chem. B, 2006, 110, 17155 CrossRef CAS.
- K. J. Hwang, S. J. Yoo, S. H. Jung, D. W. Park, S. I. Kim and J. W. Lee, Bull. Korean Chem. Soc., 2009, 30(1), 172 CrossRef CAS.
- M. G. Kang, K. S. Ryu, S. H. Chang, N. G. Park, J. S. Hong and K. J. Kim, Bull. Korean Chem. Soc., 2004, 25(5), 742 CrossRef CAS.
- H. Yin, Y. Wada, T. Kitamura, T. Sumida, Y. Hasegawa and S. Yanagida, J. Mater. Chem., 2002, 12, 378 RSC.
- S. D. Park, Y. H. Cho, W. W. Kim and S. J. Kim, J. Solid State Chem., 1999, 146, 230 CrossRef CAS.
- C. C. Wang and J. Y. Ying, Chem. Mater., 1999, 11, 3113 CrossRef CAS.
- H. K. Shon, S. Vigneswaran, I. S. Kim, J. Cho, G. J. Kim, J. B. Kim and J. H. Kim, Environ. Sci. Technol., 2007, 41, 1372 CrossRef CAS.
- A. B. Kashout, M. Soliman and M. Fathy, Renewable Energy, 2010, 35, 2914 CrossRef.
- S. Lee, I. S. Cho, J. H. Lee, D. H. Kim, D. W. Kim, J. Y. Kim, H. Shin, J. K. Lee, H. S. Jung, N. K. Park, K. Kim, M. J. Ko and K. S. Hong, Chem. Mater., 2010, 22, 1958 CrossRef CAS.
- C. J. Barbe, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover and M. Grätzel, J. Am. Ceram. Soc., 1997, 80(12), 3157 CrossRef CAS.
- S. K. Dhungel and J. G. Park, Renewable Energy, 2010, 35(12), 2776 CrossRef CAS.
- F. Pichot, J. R. Pitts and B. A. Gregg, Langmuir, 2000, 16(13), 5626 CrossRef CAS.
- H. S. Jung, S. W. Lee, J. Y. Kim, K. S. Hung, Y. C. Lee and K. H. Ko, J. Colloid Interface Sci., 2004, 279, 479 CrossRef CAS.
- K. J. Hwang, S. H. Jung, D. W. Park, S. J. Yoo and J. W. Lee, Curr. Appl. Phys., 2010, 10, S184 CrossRef.
- B. E. Yoldas, Amer. Ceram. Soc. Bull., 1975, 54(3), 289 CAS.
-
W. Rudzinski and D. H. Everett, Adsorption of Gases on Heterogeneous Solid Surfaces, Academic Press: London, 1992 Search PubMed.
- W. G. Shim, H. C. Kang, C. Kim, J. W. Lee, S. C. Kim, C. J. Lee and H. Moon, J. Nanosci. Nanotechnol., 2006, 8, 4976 Search PubMed.
-
M. Jaroniec and R. Madey, Physical Adsorption on Heterogeneous Solids, Elsevier: Amsterdam. 1988 Search PubMed.
- M. V. Szombathely, P. Brauer and M. J. Jaroniec, J. Comput. Chem., 1992, 13, 17 CrossRef.
- K. J. Hwang, J. W. Lee, H. S. Yoon, H. D. Jang, J. G. Kim, J. S. Yang and S. J. Yoo, Bull. Korean Chem. Soc., 2009, 30(10), 2365 CrossRef CAS.
- S. J. Yoo, D. H. Kwak, H. K. Kim, U. Y. Hwang, H. S. Park, H. S. Yoon and H. D. Jang, Korean Chem. Eng. Res., 2008, 46(3), 545 CAS.
- S. J. Yoo, S. I. Lee, D. H. Kwak, K. G. Kim, K. J. Hwang, J. W. Lee, U. Y. Hwang, H. S. Park and J. O. Kim, Korean J. Chem. Eng., 2008, 25(5), 1232 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. J. Halenda, J. Am. Chem. Soc., 1951, 73, 373 CrossRef CAS.
- M. Jaroniec, Langmuir, 1995, 11, 2316 CrossRef CAS.
- A. Gil, G. Y. Cherkashinin and S. A. Korili, J. Chem. Eng. Data, 2004, 49, 639 CrossRef CAS.
- M. Jaroniec, K. P. Gadkaree and J. Choma, J. Colloid Surfaces A, 1996, 118, 203 CrossRef CAS.
-
A. Luque and S. Hegedus, Handbook of Photovoltaic Science and Engineering, John Wiley & Sons, Ltd: England, 2003 Search PubMed.
- N. G. Park, J. van de Lagemaat and A. J. Frank, J. Phys. Chem. B, 2000, 104, 8989 CrossRef CAS.
- K. J. Hwang, W. G. Shim, S. H. Jung, S. J. Yoo and J. W. Lee, Appl. Surf. Sci., 2010, 256, 5428 CrossRef CAS.
- R. J. Sips, Chem. Phys., 1948, 16, 490 CAS.
- J. Avom, J. K. Mbadcam, C. Noubactep and P. Germain, Carbon, 1997, 35(3), 365 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 











![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aldrich), and 10.8 ml water for 20 min at 1350 rpm using a paste mixer (PDM-300, Korea mixing technology Co.). Then, a TiO2 film was fabricated by coating a precursor paste onto the fluorine-doped SnO2 conducting glass plates (FTO, 8 Ω/cm2, Pilkington Co.) using the screen printing method. The TiO2 film was treated by heating at 773.15 K for 2 h. The TiO2 film formed thus on the FTO glass is 7 μm in thickness and 0.5 × 0.5 cm in size.
000, Aldrich), and 10.8 ml water for 20 min at 1350 rpm using a paste mixer (PDM-300, Korea mixing technology Co.). Then, a TiO2 film was fabricated by coating a precursor paste onto the fluorine-doped SnO2 conducting glass plates (FTO, 8 Ω/cm2, Pilkington Co.) using the screen printing method. The TiO2 film was treated by heating at 773.15 K for 2 h. The TiO2 film formed thus on the FTO glass is 7 μm in thickness and 0.5 × 0.5 cm in size.