A free-standing, hybrid TiO2/K-OMS-2 hierarchical nanofibrous membrane with high photocatalytic activity for concurrent membrane filtration applications†
Tong
Zhang
,
Yinjie
Wang
,
Jiawei
Ng
and
Darren D.
Sun
*
School of Civil and Environmental Engineering, Nanyang Technological University, Nanyang Avenue, Singapore 639798. E-mail: DDSun@ntu.edu.sg; Fax: +65-6791-0676; Tel: +65-6790-6273
First published on 16th March 2012
Abstract
A novel microfiltration nanofibrous membrane assembled with a hierarchical TiO2/K-OMS-2 nanowire structure was fabricated and used for concurrent filtration, adsorption and photocatalytic oxidation in water treatment process.
Inorganic membranes are of considerable importance in water treatment due to their chemical, thermal and mechanical resistance and ease of thermal regeneration.1–5 Research progress nowadays has enabled one dimensional (1D) nanostructures, such as nanofibers and nanotubes, to be assembled into nanofibrous membranes that are free-standing, flexible and mechanically stable.6–12 Recently, self-cleaning TiO2 nanofibrous membranes are increasingly drawing attention in environmental research due to their concurrent filtration and photocatalytic oxidation (PCO) capabilities.12,13 However, the separation ability of inorganic membranes is mainly dependant on the pore size,14 and reports have shown that organic pollutants with small particle sizes and low affinity towards TiO2 nanowires are not efficiently rejected by these membranes.15 To address this issue, an adsorption technique can be adopted in the filtration process. It is predictable that membranes with higher organic adsorption capacity would enhance rejection of organic molecules. Various strategies such as increasing the specific surface area and ionic-doping of TiO2 have been proposed to improve adsorption ability of the material.16–18 Apart from being an efficient and economical method, loading TiO2 on various support materials, such as glass beads, silica gels and activated carbon,15,17,19,20 can enhance the organic adsorption efficacy of TiO2 by reconditioning its surrounding environment.21 Furthermore, the support material can act as a dispersing template in controlling the morphology of TiO2; downsizing its particulate size and in the process increasing the specific surface area to volume ratio.16,22,23 Hence, it is desirable to synthesize hybrid TiO2 nanomaterials with efficient adsorption and PCO properties. As a suitable adsorbent for the removal of organic pollutants, cryptomelane-type manganese oxide (K-OMS-2) nanowires would be a good candidate to pair up with TiO2 nanostructures.9,10,24–27 Compared with other support materials, one dimensional K-OMS-2 nanowires possess more adsorption sites with increased BET surface area. Moreover, the hydrophobic property of the synthesized K-OMS-2 plays a crucial role in the surface reactions of the synthesized materials. Supports with hydrophobic surface are suitable for the efficient photocatalytic degradation of organics in water, noting that most organic compounds are hydrophobic in nature.15,20 The synthesized material can be further developed into a free-standing, nanofibrous membrane that incorporates filtration, adsorption, and self-cleaning properties.
In this work, we report the fabrication of a novel free-standing nanofibrous microfiltration membrane, assembled using hierarchical TiO2/K-OMS-2 nanowires. Organic pollutants can be rejected by the membrane via a combination effect of interception and adsorption. Furthermore, a PCO process can be carried out concurrently, making it possible to degrade organic pollutants and alleviate membrane fouling at the same time. An illustration of the concurrent filtration, adsorption and PCO in water treatment process is shown in Scheme 1. In a typical process, the organic contaminated water was filtrated through the membrane under an external pressure. The big organic particles will be intercepted directly by membrane pores, and the smaller ones would be adsorbed onto the nanowires via an adsorption process that is greatly enhanced by the hydrophobic nature of the supporting K-OMS-2 nanowires, as well as large surface area of the hierarchical nanowires. The organic pollutants on the photocatalytic membrane were simultaneously degraded by the PCO process under a UV light, alleviating membrane pore blocking by small organic molecules, thus maintaining a constant permeate flux. In addition, the PCO process helps to free up adsorption sites occupied by organic pollutants, contributing to the regeneration of the membrane.
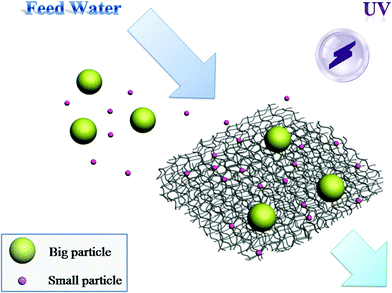 | ||
| Scheme 1 Scheme of the concurrent filtration, adsorption and PCO in water treatment process using the synthesized membrane. | ||
A Field Emission SEM (FESEM) image of the synthesized K-OMS-2 nanowires is shown in Fig. 1A. The K-OMS-2 nanowires have diameters of about 40–80 nm and are over several hundreds of microns long. Several nanowires readily combine to form larger bundles of nanowires with diameters over 100 nm. As shown in Fig. 1B, TiO2 nanostructures were successfully grafted onto the surfaces of the K-OMS-2 nanowires, constructing a hierarchical heterostructure. It can be found that the K-OMS-2 nanowires were uniformly coated. TEM images of K-OMS-2 nanowire and TiO2/K-OMS-2 hierarchical nanowire are shown in Fig. 1D and 1E respectively, which confirms the high density secondary TiO2 hair-like structures anchored on surfaces of the primary underlying K-OMS-2 nanowires. In addition, film-like TiO2 structures may also exist on the surface of K-OMS-2 nanowires, which may cause the loss of hydrophobic sites on the surface of K-OMS-2. It can be seen from Fig. 1E that the TiO2 nanostructures are of lengths ranging from 20–40 nm and have diameters of about 3 nm. Fig. 1C and 1F reveal that the calcination at 550 °C for 1 h did not change the apparent morphology of the synthesized material. As observed from Fig. S1, ESI,† the N2 adsorption isotherm of the hierarchical TiO2/K-OMS-2 exhibits mesoporosity in the form of a Type IV nitrogen isotherm with a Type H3 hysteresis loop, which does not exhibit any limiting adsorption at high relative pressures. According to the IUPAC classification, mesoporous materials characterizing the Type H3 loop are commonly observed with aggregates of plate-like particles, leading to the formation of slit-shaped pores.28 This is in good agreement with the long slit pores possibly formed by the interweaving of long nanowires, while the plate particles may be induced by the mesh-like hierarchical structure of overlaying nanowires of the 2 hybrid compounds in K-OMS-2 and TiO2. In addition, the mesoporosity of the TiO2/K-OMS-2 membrane is well-justified based on the inset of Fig. S1,† where the pore size distribution curve indicates a sharp peak centred at the pore diameter of 35 nm, providing evidence that the majority of the pores in the sample are mesoporous (2 nm < pore size < 50 nm). The BET surface area of the synthesized K-OMS-2, TiO2 and TiO2/K-OMS-2 (after calcination at 550 °C) are recorded at 25.08 m2 g−1, 72.74 m2 g−1 and 111.58 m2 g−1, respectively. The mesoporous structure and high BET surface area of TiO2/K-OMS-2 are expected to enhance adsorption of organic compounds onto the membrane. It is noteworthy that the loading of TiO2 nanostructures on the K-OMS-2 nanowires has greatly increased the specific area of the material. This phenomenon can be attributed to the presence of the K-OMS-2 scaffold, which acts as a dispersing template to downsize the TiO2 nanostructures during the synthesis process.16 Therefore, the adsorption capacity and photocatalytic activity of the synthesized membrane can be enhanced since adsorption and PCO processes are predominantly driven by surface reactions on the material. Moreover, it has been reported that K-OMS-2 doped TiO2 materials possess good PCO efficiency due to the presence of OH bonded groups on the catalyst surface.29
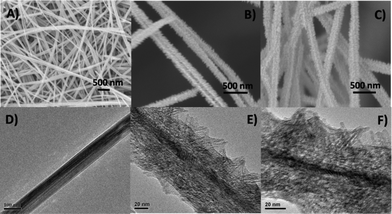 | ||
| Fig. 1 A) FESEM image of the K-OMS-2 nanowires. B) FESEM image of the hierarchical TiO2/K-OMS-2 nanowires before calcination. C) FESEM image of the hierarchical TiO2/K-OMS-2 nanowires after calcination at 550 °C for 1 h. D) TEM image of the K-OMS-2 nanowire. E) TEM image of the hierarchical TiO2/K-OMS-2 nanowire before calcination. F) TEM image of the hierarchical TiO2/K-OMS-2 nanowire after calcination at 550 °C for 1 h. | ||
Subsequently, X-ray diffraction (XRD) analysis was employed to investigate the crystal phase of K-OMS-2 nanowires and TiO2/K-OMS-2 nanostructures. All diffraction peaks of the upper curve in Fig. 2 can be perfectly indexed to the K-OMS-2 crystalline phase (JCPDS 44-1386). The XRD pattern of synthesized TiO2/K-OMS-2 nanostructures after calcination showed much more intense and sharper peaks compared with the sample before calcination. The diffraction peaks at 27°, 36° and 55° indicate that the rutile phase of TiO2 (JCPDS 21-1276) is present in the synthesized material, while the diffraction peaks at 25° and 48° evidenced the co-existence of anatase TiO2 (JCPDS 21-1272) in the final TiO2/K-OMS-2 membrane. SEM-EDX analysis further confirmed that the external surface of the membrane is comprised of the elements K, Mn, Ti and O, as shown in Fig. 3D. High-resolution XPS spectra of the Mn 2p taken on the TiO2/K-OMS-2 (Fig. S2, ESI†) revealed that Mn was also presented on the surface of the material, indicating that the K-OMS-2 nanowires were partially covered by TiO2.
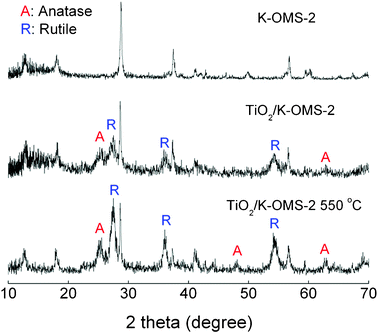 | ||
| Fig. 2 XRD patterns of the synthesized K-OMS-2, TiO2/K-OMS-2 before calcination and TiO2/K-OMS-2 after calcination at 550 °C. | ||
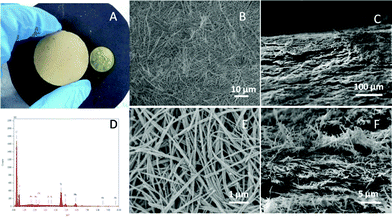 | ||
| Fig. 3 A) Digital photo of the TiO2/K-OMS-2 membrane. B) Top view FESEM image of the TiO2/K-OMS-2 membrane. C) FESEM image of the cross-section. D) EDX spectrum of the TiO2/K-OMS-2 membrane. E) High resolution top view FESEM image of the TiO2/K-OMS-2 membrane. F) High resolution FESEM image of the cross-section. | ||
The digital photo of the as-synthesized free-standing membrane, fabricated by a combination of filtration and hot press processes, is shown in Fig. 3A. Fig. 3B shows the top view FESEM image of the membrane, which reveals a relatively flat topology with no observed cracks or pinholes. From the high-resolution FESEM image (Fig. 3E), it can be deciphered that the open porous network was formed by overlapping and interweaving of the ultra long hierarchical TiO2/K-OMS-2 nanowires, which improves the permeability of water during the filtration process. Based on the FESEM image, pore size of the synthesized membrane ranges approximately from 0.05 to 0.3 μm, classifying it under the microfiltration membrane category. To further determine the membrane pore size, polystyrene (PS) microspheres were used in a filtration process, and the rejection rate for 0.1 μm and 0.05 μm microspheres were 93.9% and 85.8% respectively. (Table S1, ESI†) Since the pore size of a membrane can be defined as the diameter of latex microspheres which are 90% retained by the membrane,30 the pore size of the synthesized membrane can be characterized at 0.1 μm. In addition, cross-sectional images (Fig. 3C and 3F) of the synthesized membrane further reveal that the membrane consists of nanowires assembled over multiple length scales, and the tightly interwoven nanowires can endow the membrane with a compact functional layer.
To evaluate the adsorption capacity of the hierarchical TiO2/K-OMS-2 nanowires for organic pollutant removal, an adsorption kinetics study of acid orange 7 (AO 7) was conducted. From the results (Fig. 4), the synthesized TiO2/K-OMS-2 exhibited much higher adsorption capacity than pristine TiO2 which was prepared using an identical procedure to that of TiO2/K-OMS-2. For example, using TiO2/K-OMS-2 over pristine TiO2 led to an increase in removal efficiency of AO 7 from 8.8% to 36.5% in 360 min. The increased BET surface area of TiO2/K-OMS-2 may partly explain the enhanced adsorption capacity. However, the adsorbed amount of AO 7 on TiO2/K-OMS-2 is around 4 times greater than that of pristine TiO2, while the BET surface area of TiO2/K-OMS-2 (111.58 m2 g−1) is only about 1.5 times larger than that of pristine TiO2 (72.74 m2 g−1). K-OMS-2 has been found to possess excellent hydrophobicity and strong affinity towards organic compounds,24,26 which in turn affects the surrounding environment of the constructed TiO2 nanostructures. Fig. 4 shows that AO 7 can be efficiently removed by K-OMS-2 nanowires, which indicates that the hydrophobic nature of K-OMS-2 nanowires may also account for the high adsorption capacity of the synthesized TiO2/K-OMS-2.
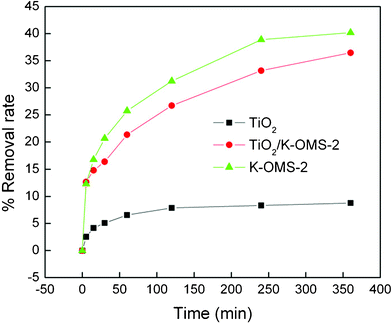 | ||
| Fig. 4 Adsorption kinetics of AO 7 onto the prepared K-OMS-2, TiO2 and TiO2/K-OMS-2 at pH 5 and 20 °C. | ||
The performance of the synthesized membrane was evaluated using a dead-end filtration cell (Fig. S3, ESI†). Like other types of membrane, organic pollutants of large particle sizes can be rejected directly by the smaller membrane pore size. For example, 0.5 μm PS microspheres can be retained and subsequently accumulated to form a cake layer on the surface of the membrane (Figure S4A and S4B†). To investigate membrane performance on the degradation of smaller organic pollutants, aqueous AO 7, which exhibits high solubility in water, was used as a model organic pollutant. Experimental results indicate that only 8.7% of AO 7 was removed using the as-synthesized membrane alone, which can be attributed to sole adsorption capability of the membrane. When UV irradiation was concurrently applied to the membrane, an AO 7 removal rate of 96.3% was achieved, owing to the combined adsorption and PCO effects. However, only 54.1% of total organic carbon (TOC) was removed in this process due to the incomplete photocatalytic mineralization of the AO 7 molecules. In addition, the AO 7 and TOC removal rates were also measured under different permeate flux. As shown in Fig. 5, the AO 7 and TOC removal rates generally decreased with increase of permeate flux, and they stayed relatively stable as long as the preset flux did not exceed 60 L m−2 h−1. When the controlled flux was greater than 60 L m−2 h−1, the removal rates of AO 7 and TOC began to decrease significantly, indicating that a maximum flux of 60 L m−2 h−1 was the critical point in establishing an optimal balance between the photocatalytic degradation of AO 7 and the flux under the stated experimental conditions.
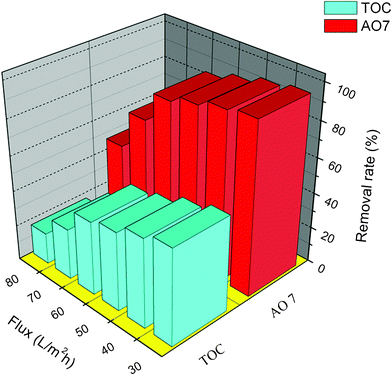 | ||
| Fig. 5 Effect of the permeate flux on the performance of the synthesized membrane on AO 7 and TOC removal. | ||
In conclusion, we have successfully synthesized a novel microfiltration nanofibrous membrane assembled with a hierarchical TiO2/K-OMS-2 nanowire structure. A concurrent filtration, adsorption, and photocatalytic oxidation module was developed to study the removal of organic pollutants by the membrane. The membrane exhibited excellent performance in the removal of organic pollutants because of its unique features, which are as follows: 1) The K-OMS-2 nanowires can act as a dispersing template to control the morphology of TiO2 and hence increase the specific surface area, reaction sites and UV light adsorption efficiency of the membrane. 2) Since the synthesized material is mesoporous in nature, organic pollutants have higher affinity towards the hydrophobic K-OMS-2 nanowires, resulting in greater adsorption efficiency. 3) The doping of cryptomelane can enhance the photocatalytic activity of TiO2.29 We believe that the synthesized hierarchical TiO2/K-OMS-2 nanofibrous membrane will have great potential in extensive applications of membrane filtration and water treatment technologies.
Acknowledgements
We are grateful for the financial support received from the Prime Minister's Office of Singapore via an initiative called The Enterprise Challenge under award number P00579/1273, Singapore Environment & Water Industry (EWI) Development Council under award number MEWR 621/06/166 and the Public Utilities Board of Singapore.References
- W. F. Maier, I. C. Tilgner, M. Wiedorn, H. C. Ko, A. Ziehfreund and R. Sell, Adv. Mater., 1993, 5, 730–735 CrossRef CAS.
- X. B. Ke, H. Y. Zhu, X. P. Gao, J. W. Liu and Z. F. Zheng, Adv. Mater., 2007, 19, 785–790 CrossRef CAS.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS.
- D. S. Sholl and J. K. Johnson, Science, 2006, 312, 1003–1004 CrossRef CAS.
- S. P. Albu, A. Ghicov, S. Aldabergenova, P. Drechsel, D. LeClere, G. E. Thompson, J. M. Macak and P. Schmuki, Adv. Mater., 2008, 20, 4135–4139 CAS.
- A. Srivastava, O. N. Srivastava, S. Talapatra, R. Vajtai and P. M. Ajayan, Nat. Mater., 2004, 3, 610–614 CrossRef CAS.
- H. W. Liang, L. Wang, P. Y. Chen, H. T. Lin, L. F. Chen, D. He and S. H. Yu, Adv. Mater., 2010, 22, 4691–4695 CrossRef CAS.
- W. Dong, A. Cogbill, T. Zhang, S. Ghosh and Z. R. Tian, J. Phys. Chem. B, 2006, 110, 16819–16822 CrossRef CAS.
- J. Yuan, K. Laubernds, J. Villegas, S. Gomez and S. L. Suib, Advanced Materials, 2004, 16, 1729–1732 CrossRef CAS.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332–336 CrossRef CAS.
- X. Zhang, A. J. Du, P. Lee, D. D. Sun and J. O. Leckie, J. Membrane Sci., 2008, 313, 44–51 CrossRef CAS.
- X. Zhang, T. Zhang, J. Ng and D. D. Sun, Adv. Funct. Mater., 2009, 19, 3731–3736 CrossRef CAS.
- S. P. Albu, A. Ghicov, J. M. Macak, R. Hahn and P. Schmuki, Nano Lett., 2007, 7, 1286–1289 CrossRef CAS.
- K. Keizer, R. J. R. Uhlhorn, R. J. Van vuren and A. J. Burggraaf, J. Membrane Sci, 1988, 39, 285–300 CrossRef CAS.
- Y. Kuwahara, T. Kamegawa, K. Mori and H. Yamashita, Curr. Org. Chem., 2010, 14, 616–629 CrossRef CAS.
- Y. J. Xu, Y. Zhuang and X. Fu, J. Phys. Chem. C, 2010, 114, 2669–2676 CAS.
- G. Balasubramanian, D. D. Dionysiou, M. T. Suidan, I. Baudin and J. M. Laìné, Appl. Catal., B, 2004, 47, 73–84 CrossRef CAS.
- C. Ooka, H. Yoshida, K. Suzuki and T. Hattori, Micropor. Mesopor. Mater., 2004, 67, 143–150 CrossRef CAS.
- K. Kobayakawa, C. Sato, Y. Sato and A. Fujishima, J. Photoch. Photobio. A, 1998, 118, 65–69 CrossRef CAS.
- K. Maekawa, O. Chiyoda, S. Ohshiro, S. Okada, M. Anpo and H. Yamashita, C. R. Chim., 2006, 9, 817–821 CrossRef CAS.
- K. Ikeue, H. Yamashita, M. Anpo and T. Takewaki, J. Phys. Chem. B, 2001, 105, 8350–8355 CrossRef CAS.
- R. T. Koodali and D. Zhao, Energy Environ. Sci., 2010, 3, 608–614 CAS.
- S. L. Suib, C. H. Chen, L. Jin, A. E. Espinal, B. T. Firliet, L. Xu, M. Aindow and R. Joesten, Small, 6, 988–992 Search PubMed.
- B. Hu, C. H. Chen, S. J. Frueh, L. Jin, R. Joesten and S. L. Suib, J. Phys. Chem. C, 2010, 114, 9835–9844 CAS.
- J. Lahann, Nat. Nanotechnol., 2008, 3, 320–321 CrossRef CAS.
- J. Luo, Q. Zhang, J. Garcia-Martinez and S. L. Suib, J. Am. Chem. Soc., 2008, 130, 3198–3207 CrossRef CAS.
- J. Luo, Q. Zhang, A. Huang and S. L. Suib, Micropor. and Mesopor. Mater., 2000, 35-36, 209–217 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- R. Jothiramalingam and M. K. Wang, J. Hazard. Mater., 2007, 147, 562–569 CrossRef CAS.
- S. Nakao, J. Membrane Sci., 1994, 96, 131–165 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: details of experimental information available. See DOI: 10.1039/c2ra00908k/ |
| This journal is © The Royal Society of Chemistry 2012 |
