Galvanic reaction based generation of electronically transparent corrugated Ag–Au nanoparticle thin films†
Sonit Kumar
Gogoi‡
a,
Anumita
Paul
*a and
Arun
Chattopadhyay
*ab
aDepartment of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781 039, India. E-mail: anumita@iitg.ernet.in; arun@iitg.ernet.in.; Fax: +91 361 2690762; Tel: +91 361 258 2308
bCentre for Nanotechnology, Indian Institute of Technology Guwahati, Guwahati 781 039, India. E-mail: arun@iitg.ernet.in; Fax: +91(361) 258 2349; Tel: +91 361 258 2304
First published on 28th February 2012
Abstract
We report the fabrication of thin corrugated films consisting of Ag and Au nanoparticles. The films were generated by the reaction of Ag containing foils of compact discs (CDs) and digital versatile discs (DVDs) with HAuCl4 in the presence of cetyl trimethyl ammonium bromide.
Organized assemblies of nanoparticles (NPs) offer additional properties which are not available with individual NPs. For example, two-dimensional arrays of Au and Ag NPs have been demonstrated to exhibit separation-dependent Fano-type resonance.1 Additionally, one and two dimensional assemblies of Ag NPs provide ‘hot spots’, which enhance the signals for surface enhanced Raman spectroscopy.2 In this regard, bimetallic NPs and their assemblies exhibit properties which are unique, and do not arise with monometallic NPs.3 In addition, they offer dual control for tuning the properties based on the changes in composition. On the other hand, thin films composed of monometallic4,5 or bimetallic5,6 NPs are of special importance for applications in optoelectronic nanodevices,7 catalysis,5 molecular recognition,8 and chemical and biological sensing.7,9
There are several methods, such as chemical and physical vapour depositions, electrochemical and Langmuir–Blodgett film deposition techniques,10 which are available for the generation of supported thin films. In addition, evaporation induced formation of an assembly of NPs on a substrate surface, with the separation between the NPs being controllable by the dimensions of the stabilizing molecules, has been reported.11 On the other hand, methods for the formation of free-standing thin films with component NPs are few. For example, free-standing polymer–NP or ligand–NP films have been generated at fluid interfaces or at the air–water interface, based on self-assembly or by chemical reaction, along with the option for lithographic patterning.12–14 The film could have porous structures incorporated into it. It has also been reported that the films could be used for enzyme immobilization15 or growth of inorganic crystals.16 Although the above methods address the issue of formation of film constituting NPs and encompassing the advantage of lithography, they fall short of the need to have structured films where the NPs not only constitute the film, but are also part of the secondary and tertiary structures of the film. In other words, it would be important to have thin films of NPs ‘synthesized’ in such a way that any pattern formation would involve the NPs directly, rather than using indirect means. For example, patterned thin films of a polymer–NP composite could be developed using lithographic techniques where the pattern generation would be due to the etching of the polymer by an electron or ion beam. On the other hand, if the NPs constitute the patterns, then not only would the continuity be maintained, but the other advantages of a structured film could also be harnessed. In this regard, if the patterned thin film is made up of two metals then the advantages would be even higher. Interestingly, to the best of our knowledge there is no report on the generation of a patterned thin film constituting NPs of a single metal or two metals. Further, versatility of the principles of chemistry could be incorporated if the film is generated by chemical means or by a combination of ‘top-down’ and ‘bottom-up’ approaches. Thus a chemical approach for the generation of patterned films may not only provide alternative ways, but could also help incorporate interesting structures that may otherwise not be possible using the top-down approach only.
Herein we report the formation of thin corrugated films of Ag–Au composite NPs starting with commercially available metallic silver containing compact discs (CDs) and digital versatile discs (DVDs). The films, which were nanoporous, consisted of interconnected networks of fused NPs of Ag and Au. The corrugated films, which could be generated by galvanic replacement reactions of patterned metallic Ag foils by HAuCl4, in the presence of cetyltrimethylammonium bromide (CTAB), were amenable to manipulations such as floating on water and the transfer to glass slides. The corrugations present in the films had the motifs of the parent CD and DVD, which were engraved on the polycarbonate plates of the discs. Also, the films were partially transparent to low energy electrons, as observed using a scanning electron microscope (SEM). The current method utilizes the advantages of the combination of “top-down” and the “bottom-up”17 fabrication strategies, along with templated growth, in order to produce the thin films. The top-down generated structures were bulk Ag foils with corrugated structures present in the CDs and DVDs, which were to be galvanically replaced partially by the oxidizing agent HAuCl4. On the other hand, HAuCl4 reacted with the bulk Ag and was reduced and deposited as NPs providing bimetallic thin films with sub-100 nm thickness, thus constituting the bottom-up part of the approach. The presence of the surfactant, CTAB, was critical not only for the formation of the film based on the original template, but also in removing the film so formed from the template (CD/DVD substrate). Interestingly, films generated from previously ‘written’ CDs retained the patterns of the original imprints thus providing a way of generating ‘written’ films with corrugated structures. It may be mentioned here that we had used a similar galvanic replacement reaction between the Ag foil of CDs/DVDs and HAuCl4 to generate arrays of nanoparticles (NPs) consisting of Ag and Au, and in addition AgCl microparticles.18 However, the reaction conditions reported here are different along with the use of CTAB for the formation of the film. In addition, the current method is a new way of generating thin metallic films consisting of Ag and Au NPs and CTAB. Further, the presence of corrugation and the ability to have ‘written’ metallic films in the form of tertiary patterns make it unique with respect to the ease of reproduction worldwide.
When the Ag foil parts (while being attached to the lacquer) of the CD and DVD were immersed in aqueous solutions of HAuCl4, in the presence of CTAB, a golden coloration of the surface of the foils could be observed in about 12 h. The foils were then taken out and washed with milli-Q grade water followed by placing the same into a petri-dish full of water. Upon gentle shaking the films could be observed to have come out and float on the water’s surface. The films were then ready for transfer. Typically, 1.55 cm × 2.6 cm pieces of Ag foils were immersed in 10 mL water containing 2.0 − 5.0 × 10−4 M HAuCl4 and 0.1 M CTAB, in order obtain the best films. The films generated by the reaction of HAuCl4 with Ag foils, which were obtained from CDs and DVDs, appeared to be similar. For example, a photograph of a film, originating from a CD—with illumination from the same side—appeared golden in color as shown in Fig. 1a. On the other hand, when the same film was photographed with illumination from the opposite direction it appeared blue (Fig. 1b). Also, the presence of interference fringes in the photograph is apparent in Fig. 1a. The corresponding images of films from DVDs are shown in Fig. S1 in the ESI.† The appearance of different colors in reflected and transmission modes indicate the presence of Au NPs, the color of which are known to appear differently depending on the view, especially blue in the transmittance mode of observation. Also, the blue colors of the films indicated either the formation of larger sized particles of Au NPs or the presence of assemblies of smaller particles.19 The UV-visible spectrum of the films revealed characteristics of Ag–Au NPs as shown in Fig. 1c (a similar spectrum for a film from a DVD is included in Fig. S1C in the ESI†).20 The peak at 464 nm indicated the possible presence of Au core–Ag shell (Ag@Au) NPs. Also, the strong background in the higher wavelength region is indicative of the presence of large NPs or assemblies of NPs. Further, it can also be argued that the peak could also be indicative of the formation of Ag–Au alloy NPs. Thus overall the core–shell and alloy NPs may be present across the film. SEM images of the films obtained from CDs and DVDs are shown in Fig. 1d–f. As is clear from Fig. 1d, a thin film was formed on top of the substrate (lacquer) of the CD, with a clear indication of the presence of serrated lines in the film. It is interesting to note that the particles (crystals) which were present on the surface of the substrate could be observed through the film. Considering that the accelerating voltage of electrons (in SEM) was 20 kV the film must be sufficiently thin to help observe the particles present underneath the films, generated from the CD. Importantly, the particles could also be observed underneath the part of the film that was folded, i.e. through two layers of the film, as can be seen in Fig. 1d. This is indicative of the partial transparent nature of the films to the impinging electrons. A higher resolution image, shown in Fig. 1e, indicates that the primary structural motif of the original CD was maintained in the generated film. The crest–crest pitch was found to be 1.6 μm. This matched well with the pitch of the original CD film (refer to ESI,† Fig. S2). An SEM image of a film generated from a DVD is shown in Fig. 1f. The image not only shows the partial transparent nature of the film to the impinging electrons, but also the presence of corrugation in the structure. The pattern of lines is similar to that of the original DVD film with a crest–crest pitch of approximately 900 nm (refer to ESI,† Fig. S2). This also matched well with the pitch of the original film. It may be mentioned here that the overall integrity of the film formed was substantial, with a minimum presence of discernible deformation or damage in both cases, although the film from the DVD was easier to handle. The observed cracks or damage probably occurred during handling and transfer (using forceps) for recording the images.
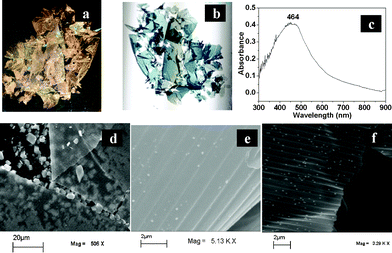 | ||
| Fig. 1 (a) and (b) are photographs of thin films recorded after they were transferred to a glass cover slip. The films were obtained by the reaction of HAuCl4 with the Ag foil of a CD. The photographs were taken by a digital camera in reflected and transmitted light, respectively. (c) UV-Visible spectrum of the film from a CD in transmittance mode. (d) Representative SEM images of the thin films generated from the silver foil of a CD as prepared on its original substrate; (e) the same at a higher magnification showing the CD tracks. (f) A SEM image of a film obtained by the reaction of HAuCl4 with Ag foil from a DVD. | ||
Energy dispersive X-ray (EDX) spectroscopic analyses of the films indicate the presence of Ag and Au, in addition to the presence of Br and C (refer to ESI,† Fig. S3). Also, FTIR (Fourier transform infrared) spectroscopic measurements of the films indicate the possible presence of CTAB (refer to ESI,† Fig. S4). X-Ray diffraction (XRD) measurements of the as-prepared films from CDs and DVDs consisted of peaks at 38°, 44°, 64.5°, which were assigned to diffractions from (111), (200) and (220) planes, respectively, of Ag or Au20 (refer to ESI,† Fig. S5). Further, treatment of the film with a saturated solution of NaCl not only improved the quality of the peaks due to Ag/Au, but also gave rise to the occurrence of peaks at 31° and 55°, which could be due to the presence of AgBr crystals.21 It is plausible that Br− ions present as counter ions in CTAB, which were adsorbed to the film, were replaced by the Cl− ions from NaCl as Cl− is harder than Br−. Thus the released Br− reacted with adsorbed Ag+ ions to form the less soluble AgBr in the form of crystals. The details of the XRD results are included in the ESI† (Fig. S5). Thus the EDX and FTIR spectroscopy and XRD studies indicate that the reaction of HAuCl4 with Ag films of CDs and DVDs led to the formation of films consisting of Ag, Au and CTAB. It may be mentioned here that in absence of CTAB there was no formation of a film either from the CD or DVD Ag foil.
Transmission electron microscopic (TEM) images of Ag–Au films generated from the CDs and DVDs are shown in Fig. 2. The films were washed with water and then transferred to copper grids before recording the images. A low magnification image, as shown in Fig. 2a, of the film generated from the CD, apparently appears to be devoid of the structures present in the original CD film. However, a careful observation shows that there are parallel tracks as marked by black lines. These lines are approximately 2 μm apart, which is larger than the distance from centre of the crest–crest or trough–trough in the original CD (1.8 ± 0.1 μm). It is possible that a higher value of centre–centre distance than that measured by TEM was due to the expansion of the corrugated film upon transfer to the TEM grid. The higher magnification image in Fig. 2b shows the porous nature of the film. The NPs constituting the film and the formation of the film as a result of fusion of the NPs can be observed in Fig. 2c, as marked by black circles. Selected area electron diffraction (SAED) patterns of the film (refer to ESI,† Fig. S6) indicate the metallic nature of the film with the presence of polycrystalline particles. Similarly, the TEM image of a section of the film generated from the DVD is shown in Fig. 2d. The image indicates that the film was rather thin, so as to be able to record the TEM, and the transparency of the film is further supported by the clarity of the image. The image also indicates a rather continuous film that grew well according to the template of the original DVD. The lines are distinct with the pitch length being 0.7 ± 0.1 μm, and further supports the results obtained from SEM studies. Interestingly, Fig. 2e shows the presence of systematic perforation in the film. A careful examination reveals that the perforation largely occurred at the crests–troughs of the film although there were discernible perforations in the troughs–crests of the film. It can be speculated that since the galvanic reaction at the crests of the original films would be faster due to the availability of the reactant (HAuCl4) in comparison to that at the troughs, the so generated film would be more porous in the crests vis-à-vis the original template. Imaging at a higher magnification (Fig. 2f) indicated the presence of distinct holes in the film with sizes on the order of 50–100 nm. The holes did not have any distinct shapes in particular. In addition, discrete networks of nanoscale structures surrounding the holes could be observed. The presence of dark and lighter regions in the network possibly indicates merged particles of varying thickness with the darker ones representing thicker particles. SAED results of the film (refer to ESI,† Fig. S6B) indicate the metallic nature of the film containing polycrystalline particles, which formed the network structures.
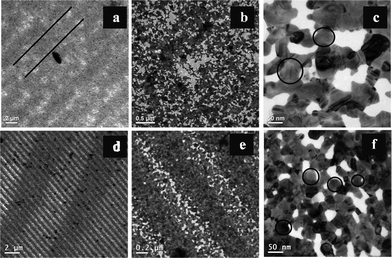 | ||
| Fig. 2 Transmission electron microscopic images of the films at different magnifications. Images in (a), (b) and (c) are from the film generated from the CD; whereas the images in (d), (e) and (f) correspond to the film generated from the DVD. | ||
Atomic force microscopy (AFM) investigations of the films revealed their three-dimensional (3D) nature. For example, images in Fig. 3a and 3b show the 3D nature of the thin films generated from the CD and DVD, respectively. The corresponding height profiles are shown in Fig. 3c and 3d, respectively. The typical features of ‘hills’ and ‘valleys’ of the original metallic film present in the CD and DVD are shown in Fig. S2 (refer to ESI†). It is clear from the figures that the general undulation patterns present in the original film were retained in the product films. For the films generated from the CD, the original pattern of the CD tracks appears somewhat distorted, although the tracks can be seen as marked by black lines in Fig. 3a. The crest–crest pitch was observed to be 1.5 ± 0.2 μm. The thickness of a film from the CD was measured to be 31 ± 7 nm. This was pursued by placing the film on a glass slide and measuring its height profile with respect to the glass substrate at several points (refer to ESI,† Fig. S7A and C). It may be that, since the films obtained from the CD were considerably thin, and thus during transfer to the glass slides they were vulnerable to stretching or squeezing. Hence the value of the pitches found may be higher/lower than the original film. On the other hand, the initial DVD pattern was retained better in the thin films generated from the DVD, as is clear from Fig. 3b. Also, the general structure of the film from the DVD appeared to be more robust than that from the CD. The height profile of the film from the DVD, as shown in Fig. 3d, clearly indicates distinct crests and troughs with the crest–crest pitch being 1.1 ± 0.2 μm. This is very close to the value of the original metallic film (1.0 ± 0.1) μm. Further, the thickness of the film from the DVD was measured to be 68 ± 3 nm (refer to ESI,† Fig. S7B and D), which is twice as thick as the film from the CD. Thus the film from the DVD being thicker, it was easier to transfer and was less prone to damage or distortion than a film from the CD. Further investigations of the AFM topographic images indicate the nanoparticulate nature of the constituting blocks of the films. Typical particle sizes were measured to vary from 20–200 nm for both the films (refer to ESI,† Fig. S8). The fusion of the particles constituting the film was also apparent from the images. The results are consistent with the TEM studies. A CD or DVD metallic film typically contains Ag. Thus the galvanic replacement reaction of the original Ag foil with HAuCl4 would lead to the formation of the structure consisting of fused Ag and Au NPs. On the other hand, the presence of CTAB helped form the film structure. Further, since the Ag foil in the original CD is typically thinner than that in the DVD, it could be that the film generated by the reaction of the Ag foil in the CD with HAuCl4 would result in a thinner film than that from the DVD. Thus a film from the DVD was more robust and easier to handle. This may be the reason for the observed expanded TEM image of the film from the CD, while the same was not observed for the film from the DVD. Notwithstanding that it is interesting to observe that a metallic film with corrugation would retain its surface and height profile upon reaction with HAuCl4, wherein CTAB would play the crucial role in retaining the template and mechanical strength required for handling a free standing film. At lower concentrations of CTAB no film formation was observed. On the other hand at higher concentrations the reaction was very slow and film formation, if at all, took a very long time and was thus not pursued further. Further, it is interesting to note that the film formation took place in the presence of CTAB (at its appropriate concentrations). It has been established that the presence of CTAB in the medium facilitated self-assembly of Au nanorods.22 On the other hand, the evaporation of the solvent containing CTAB and Au NPs also led to self-assembly.23 In the present situation, the presence of CTAB may also have helped form the assembly of NPs consisting of Au and Ag, resulting from the galvanic replacement reaction. That the original template of the film was retained in the product film indicates the formation of the assembly immediately followed the reaction and the NPs did not diffuse out into the medium.
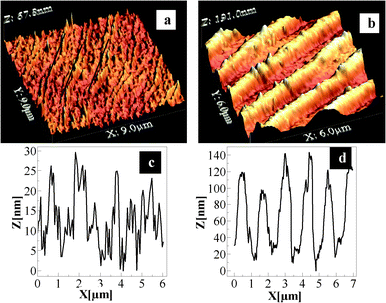 | ||
| Fig. 3 Representative AFM images of the films generated from the CD and DVD. Images in (a) and (b) represent three-dimensional views; whereas (c) and (d) are height profiles of the films generated from the CD and DVD, respectively. The images were recorded after the films were transferred to microscope cover slips. | ||
We have also tried to incorporate tertiary structures in addition to the undulation present in the thin films. For that, a CD was first ‘written’ optically by copying an ordinary data file. This was followed by removing the Ag foil (as before) and then treatment with HAuCl4, in the presence of 0.1 M CTAB. SEM images of a patterned original foil and that of a typical film that were so developed are shown in Fig. 4a and b, respectively. A more prominent film with a clear presence of imprints is shown in Fig. 4c, where the original film was displaced from the substrate during handling. As is clear from the figures, the tertiary patterns present in the original substrate were transferred to the film with one-to-one transfer of the imprints. The results clearly indicate that not only was the motif of the original structures present in the CD substrate transferred, but also the tertiary imprints incorporated in the form of writing using the dye present in the substrate were transferred. It may be mentioned that a CD-R generally contains phthalocyanine dyes for optical writing of data. Thus, when the Ag containing foil was dismantled from the original CD-R the dye present was at least partially transferred to the foil, which when reacted with the HAuCl4 gave rise to a film with imprints of the original data storage being transferred accurately. Thus, the role of the dye present in the film may also be important in the generation of the final imprinted films.
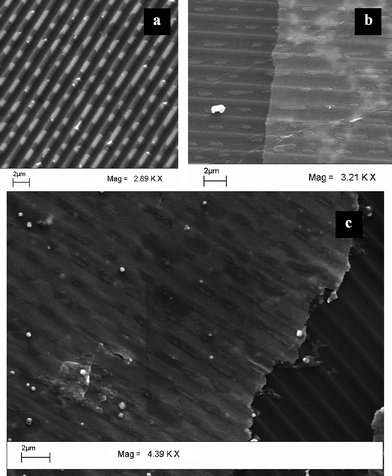 | ||
| Fig. 4 SEM images of the Ag foil of (a) a written CD, (b) thin film generated from silver foil of a written CD, (c) a large area image showing the writing pattern more distinctly. | ||
Conclusions
In this work we have demonstrated a novel method of generating free-standing and corrugated bimetallic NP (of Au and Ag) thin films by using galvanic replacement reactions in the presence of a surfactant (CTAB). This was achieved by a reaction of the metallic Ag foil present in CDs and DVDs with HAuCl4. The free standing thin films retained the corrugated pattern of the substrates and could easily be transferred to substrates following their formation. We have also demonstrated that tertiary patterns or marks generated on the original CD (or DVD) could be transferred to the final Ag–Au thin films. The three-dimensional nature of the films provides additional advantages for the future generation of thin NP based films with well-defined morphology in terms of surface undulation. This can potentially be an important method for generating thin films consisting of NPs, especially for surfaces where direct deposition is not possible. These corrugated porous films with the imprints of tertiary patterns could not only be excellent materials for photonic and optoelectronic applications, but could also be used as porous membranes.Acknowledgements
We thank the Department of Science and Technology, Govt. of India (DST SR/S1/PC-30/2008 and SR/S5/NM-108/2006) for funds. SKG thanks CSIR, New Delhi, for fellowships (09/731(0043)/2006-EMR-I.References
- B. Luk'yanchuk, N. I. Zheludev, S. A. Maier, N. J. Halas, P. Nordlander, H. Giessen and C. T Chong, Nat. Mater., 2010, 9, 707 CrossRef CAS.
- A. J. Haes, C. L. Haynes, A. D. McFarland, G. C. Schatz, R. P. Van Duyne and S. Zou, MRS Bull., 2005, 30, 368 CrossRef CAS.
- N. Toshima and T. Yonezawa, New J. Chem., 1998, 22, 1179 RSC.
- L. Chai and J. Klein, Langmuir, 2007, 23, 7777 CrossRef CAS.
- M. Ou, G. Lu, H. Shen, A. Descamps, C. A. Marquette, L. J. Blum, G. Ledoux, S. Roux, O. Tillement, B. Cheng and P. Perriat, Adv. Funct. Mater., 2007, 17, 1903 CrossRef CAS.
- L. Lu, A. Eychmuller, A. Kobayashi, Y. Hirano, K. Yoshida, Y. Kikkawa, K. Tawa and Y. Ozaki, Langmuir, 2006, 22, 2605 CrossRef CAS.
- A. N. Shipway, E. Katz and I. Willner, ChemPhysChem, 2000, 1, 18 CrossRef CAS.
- L. Han, D. R. Daniel, M. M. Maye and C. J. Zhong, Anal. Chem., 2001, 73, 4441 CrossRef CAS.
- J. Homola, Chem. Rev., 2008, 108, 462 CrossRef CAS.
- G. Cao, Nanostructures and Nanomaterials, Imperial College Press, London, 2004, 173 Search PubMed.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M.Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef CAS.
- J. Pang, S. Xiong, F. Jaeckel, Z. Sun, D. Dunphy and C. J. J. Brinker, J. Am. Chem. Soc., 2008, 130, 3284 CrossRef CAS.
- M. H. Lim and D. G. Ast, Adv. Mater., 2001, 13, 718 CrossRef CAS.
- H. Xia and D. Wang, Adv. Mater., 2008, 20, 4253 CrossRef CAS.
- S. Phadtare, V. P. Vinod, P. P. Wadgaonkar, M. Rao and M. Sastry, Langmuir, 2004, 20, 3717 CrossRef CAS.
- D. Rautaray, P. S. Kumar, P. P. Wadgaonkar and M. Sastry, Chem. Mater., 2004, 16, 988 CrossRef CAS.
- B. D. Gates, Q. Xu, M. Stewart, D. Ryan, C. G. Willson and G. M. Whitesides, Chem. Rev., 2005, 105, 1171 CrossRef CAS.
- S. K. Gogoi, S. M. Borah, K. K. Dey, A. Paul and A. Chattopadhyay, Langmuir, 2011, 27, 12263 CrossRef CAS.
- T. Ung and L. M. Liz-Marzan, Colloids Surf., A, 2002, 202, 119 CrossRef CAS.
- D. H. Chen and C. J. Chen, J. Mater. Chem., 2002, 12, 1557 RSC.
- V. Sambhy, M. M. MacBride, B. R. Peterson and A. Sen, J. Am. Chem. Soc., 2006, 128, 9798 CrossRef CAS.
- B. Nikoobakht, Z. L. Wang and M. A. El-Sayed, J. Phys. Chem. B, 2000, 104, 8635 CrossRef CAS.
- T. K. Sau and C. J. Murphy, Langmuir, 2005, 21, 2923 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Experimental section, additional SEM images, AFM images, TEM image, EDX spectroscopy results and XRD patterns are available. See DOI: 10.1039/c2ra20211e |
| ‡ Current address: Department of Chemistry, Gauhati University, Guwahati – 781 014, India. |
| This journal is © The Royal Society of Chemistry 2012 |
