Shape dependent peroxidase mimetic activity towards oxidation of pyrogallol by H2O2†
Nagaprasad
Puvvada
,
Pravas Kumar
Panigrahi
,
Dhritabrata
Mandal
and
Amita
Pathak
*
Department of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur 721302, India. E-mail: ami@chem.iitkgp.ernet.in
First published on 7th March 2012
Abstract
Truncated octahedron shaped magnetite nanoparticles have been prepared via a chemical method and subsequently their shape dependent peroxidase mimetic activity has been verified using pyrogallol substrate. Their peroxidase mimetic activity has been found to be superior to that of spherical-shaped nanoparticles.
Over the past few years, magnetic nanoparticles have attracted considerable interest due to their enormous promise for novel applications in diverse fields such as enhanced magnetic separation systems for biomolecules and cells,1,2 site specification,3 biosensors,4 hyperthermia agents for cancer therapy,5 magnetic resonance imaging (MRI) to diagnose tumours6–8 and cardiovascular disease,9 and data storage.10 These materials can also be used for homogeneous as well as heterogeneous catalysis reactions. Since the properties, including catalytic behavior, of nanomaterials are strongly associated with their size, shape and morphology,11 intense research is therefore in progress worldwide to fabricate magnetic nanomaterials not only with varied sizes, but also in a wide range of shapes, such as polyhedral,12 truncated octahedron (TO),13 cubic12etc. for achieving desired properties. For instance, polyhedral magmatite magnetic nanomaterials have shown highly enhanced catalytic behavior towards the conversion of styrene to benzaldehyde compared with their spherical shaped counterparts.14 The authors attributed this enhancement in the catalytic activity to the presence of the large number of surface reflection facets on the polyhedral magmatite nanoparticles. Similarly, Lee et al. observed excellent catalytic activity of TO nanoparticles of platinum for the methanation of carbon monoxide.15 Apart from their catalytic properties, magnetic nanoparticles must exhibit excellent biocompatibility for their usage as biological catalysts.
Compared to all other magnetic nanomaterials, magnetite (Fe3O4) nanoparticles (MNPs) are generally considered as non-toxic and highly biocompatible.16 Hence, the synthesis of MNPs and study of their catalytic properties in view of their application in biotechnology and medical science has currently received special attention. In order to use the magnetic properties of the nanoparticles for easy separation, MNPs have been coated with metal catalysts and enzymes. Although there are several reports available on biocatalysis using horseradish peroxidase (HRP)-entrapped MNPs, the intrinsic peroxidase-like activity of MNPs has been ignored until Gao et al. showed that these nanoparticles have intrinsic peroxide-like activity, analogous to natural peroxidases, and possess significant potential for novel applications.17 This discovery led to exploration of the possibility of a new potential application of MNPs as a dual functional catalyst having both catalytic properties as well as magnetic properties. During the past few years, the intrinsic peroxidase-like activity of MNPs has been studied extensively and a lot of work also performed to increase this peroxidase-like activity.17 However, all the MNPs that have been investigated so far for their peroxidase mimetic activity have had a spherical shape. The peroxidase mimetic activity of TO shaped MNPs has not been studied to date, though it has been predicted that TO MNPs are a special type of faceted nanomaterial that could exhibit unique properties associated with the facets.15,18 One of the basic challenges of studying and utilizing these types of MNPs is to fabricate them in large quantities to obtain well defined catalytic properties. This is obvious from the dearth of reports describing the fabrication of MNPs with TO morphology. Moreover, one of the intensively debated issues over the past few years has been centred on whether and how the shape of the magnetite nanoparticles influences the peroxidase-like activity of MNPs towards H2O2.
With these objectives, here, we have described the synthesis of TO shaped MNPs with an average size of 12 nm via a chemical method and the study of their shape dependent (spherical and TO shaped MNPs) catalytic activity towards the oxidation of a peroxidase substrate pyrogallol (PG) by H2O2. The catalytic activity has been determined by colorimetric assay.
The phase of the prepared samples was ascertained by X-ray diffraction (XRD) analysis (Fig. 1). The diffractogram shows peaks at 30.1, 35.6, 37, 43.19, 53.6, 57, 62.5°, which can be indexed to the reflections from crystal planes (220), (311), (222), (400), (422), (511) and (440) of the cubic phase of Fe3O4 according to the standard JCPDS data (card no. 19-0629). The absence of any unidentified peaks in Fig. 1 indicates that the synthesized nanoparticles are pure and crystalline. The crystallite size of the prepared sample, calculated by using the Scherrer equation, is around 9 nm. It can thus be inferred that the as-synthesized sample is composed of cubic structured Fe3O4. The magnetic properties of the prepared samples have been investigated using a vibration sample magnetometer (VSM) at room temperature. The variation of magnetization M with respect to applied magnetic field H is shown in Fig. 1. The M–H loop reveals that neither reminiscence nor hysteresis is observed. The high saturation magnetization of 74 and 66 emu g−1 along with the zero hysteresis hints at the superparamagnetic (SPM) behavior of the TO as well as the spherical shaped Fe3O4 nanoparticles. The SPM behavior was further confirmed by fitting the magnetization data with the Langevin function and the corresponding experimental data well matched with SPM behaviour.19,20 However, this behavior can be attributed to the very small size of the synthesized MNPs, as per the current study, which suggests that the magnetic properties of nanocrystalline MNPs are lower than that of its bulk counterpart (90 emu g−1).20
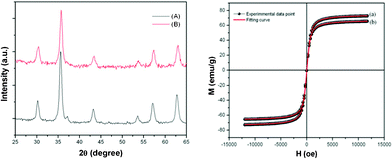 | ||
| Fig. 1 X-Ray diffraction patterns of as prepared TO (A) and spherical (B) shaped Fe3O4 and corresponding magnetization versus applied field curves at room temperature (a and b). | ||
It was further found from morphological analysis by TEM (Fig. 2) that the prepared sample is composed of TO shaped nanoparticles and the average particle size is 12 nm. The TO shape of the prepared nanoparticles is depicted by shape software analysis and also has very reasonable outlines, which can be seen from the higher magnification of the TEM image as shown in Fig. 2. The results indicate the presence of TO shaped MNPs with the surface reflection facets {100}, {110} and {111} of Fe3O4. Based on the reported methods, it can be predicted that chemical methods generally produce different shaped Fe3O4 nanocrystals. However, faceted Fe3O4 nanocrystals can be predominately fabricated, if the growth rate is lowered by the addition of some reducing agents.13,21,22 In the presence of a reducing agent, Fe2+ and Fe3+ undergo chemical and structural conversion.23 The surface energy density of different crystallographic planes is generally different and to decrease the surface energy, Fe3O4 crystals attain a truncated octahedral shape. As per previous literature reports concerning the cubic spinel structure, surface energies associated with the {100}, {110} and {111} planes are lower than those of the remaining planes21 and follow the decreasing order: {111} < {001} < {110}.21
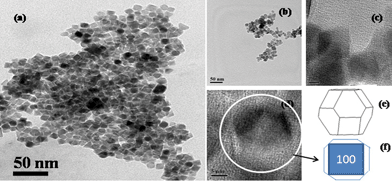 | ||
| Fig. 2 TEM images of truncated octahedron (TO) (a) and spherical shaped (b) MNPs; (c, d) higher magnification of TEM of TO shaped MNPs and TO single crystal (e) SHAPE (V7.2, Demo version) software analysis of MNPs and (f) TO nanocrystals sketched in two dimensional projection. | ||
The surface energies of these planes play a major role during the growth process leading to the evolution of various shapes. The facets of the lowest energy surface tend to be maximized, which results in the formation of polyhedral crystals. On the other hand, when the growth occurs homogeneously in all crystallographic directions, spherical particles are formed. It is generally accepted that oleic acid plays a major role in the formation of truncated octahedral Fe3O4 nanocrystals, but the detailed growth mechanism is not yet completely understood. Li et al.13 have found that oleic acid can be used as a reducing agent, which helps in the formation of nano-octahedra. Later, Hou et al.22 reported that oleic acid facilitates growth along the [100] direction over the [111] direction, which results in the formation of truncated octahedron FeO nanocrystals. Based on these reports, oleic acid has been used as a reducing agent in our synthesis to obtain TO shaped MNPs.13,22 But, our prepared TO MNPs, which involve the use of oleic acid as a reducing agent, are very small in size at around 12 nm. The small sized TO shaped MNPs motivated us to investigate their catalytic properties. We have also compared the results with those of spherical MNPs, synthesized through a well established route7 (the spherical morphology of this sample was confirmed by TEM analysis and its size is similar to that of the prepared TO shaped nanoparticles (Fig. 2)).
To investigate the peroxidase-like activity of the as-synthesized truncated octahedra as well as of the spherical MNPs, we have designed a set of experiments based on the oxidation of PG by H2O2 in the presence of the as-prepared MNPs. The experiments involved the addition of MNPs and H2O2 to solution of PG in phosphate buffer. After 12 min, the MNPs (either TO or spherical shaped MNPs) were removed from the solution by a magnet and the absorbance spectra of the supernatant solutions were taken. Upon addition of H2O2 and MNPs, the colourless PG solution became yellow and showed a new absorption peak at 420 nm (Fig. S1†). Throughout our experiment, we have considered this peak as the characteristic peak of the oxidized product of PG.24 It is well-known that a peroxidase can catalyze the oxidation of a peroxidase substrate, such as PG, to produce a change in colour from colourless to yellow, which is responsible for the appearance of the absorption band. A similar kind of colour change was also observed in the reaction of PG catalyzed by HRP.25,26 It can thus be inferred that the prepared sample has a biocatalytic activity similar to that of HRP.
It is well known that Fenton's reagent (i.e., Fe2+/Fe3+ ions in solution) can catalyze the breakdown of H2O2.27–28 To ensure that the catalytic activity originated from the intact MNPs themselves, and not from Fe2+ ions leached into the solution, the activity of the intact MNPs was compared with that of the leaching solution by UV-VIS spectroscopy under the same conditions (i.e., at same pH, temperature and concentrations) and the absorption at 420 nm was plotted against time (Fig. S2†). MNPs were dispersed in phosphate buffer and removed from the solution with a magnet. The supernatant solution was mixed with PG followed by the addition of H2O2 and the absorbance of the resultant solution was measured at 420 nm at different time intervals as shown in Fig. S2. The leaching solution showed much lower activity in comparison to the intact MNPs. This result demonstrated that the oxidation reaction of PG was mainly catalyzed by the MNPs. The amount of iron leached at different pH values was measured by AAS. Fig. S3 shows the amount of iron leached from the MNP sample into an acid and basic solution.† At pH ≤ 5, the amount of iron ions leached was higher and in basic medium as well as in neutral medium, iron leached was lower. This observation indicated that the dispersed samples are stable as shown in Fig. S3 and the prepared samples showed catalytic activity in acidic as well as in basic media. The absorbance of the reaction solution was measured at a wavelength of 420 nm with variation of the time interval in the presence and absence of MNPs. The catalytic reaction was completed after 12 min and the absorbance at 420 nm remained stable. At this time interval, the prepared sample showed maximum catalytic activity. This result indicated that the optimized time was 12 min.
The pH and temperature of the reaction mixture are important factors for the catalytic activity of the MNPs. So, the pH and temperature dependence of the catalytic activity of the prepared sample were investigated in the present study. Fig. S4 shows the effect of temperature and pH on the catalytic oxidation of PG with H2O2 in the presence as well as the absence of MNPs.† Absorption spectra were taken at 40 °C, both in the presence and in the absence of MNPs. These results were different from the literature data, where the absorbance was obtained at a pH of 8 and a temperature of 40 °C. Here, ΔA (A(MNP, 420 nm) − A(blank, 420 nm)), was plotted against pH and temperature for the determination of optimized pH and temperature. From Fig. S4, the optimized values of pH and temperature are found to be 8 and 40 °C respectively.
To find out the utility of TO MNPs as a catalyst in the breakdown of H2O2 in the presence of PG, we have further investigated the dependence of the reaction rate on the concentration of H2O2. It should be noted that PG can be oxidized by H2O2 in the absence of any catalyst under the present conditions. But upon adding MNPs as catalyst, the reaction rate increases drastically, which is demonstrated by the plot of absorbance against concentration of H2O2 (Fig. 3). This plot shows good response toward H2O2 detection with a linear range as shown in Fig. 3 (least concentrations measurable for TO and spherical are 2 × 10−6 and 5 × 10−5 M), which supports our work as a very efficient method for H2O2 detection in a very low concentration range. We have repeated this procedure taking spherical MNPs in place of our prepared TO MNPs and we have found that TO MNPs have higher catalytic activity than spherical MNPs.
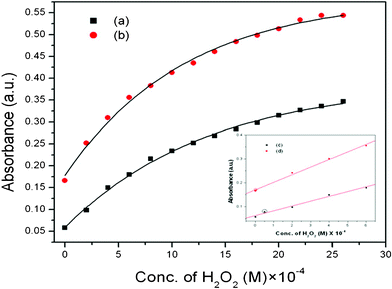 | ||
| Fig. 3 The variations of absorbance vs. concentration of H2O2 showing the catalytic responses of: (a) spherical, (b) TO magnetite nanoparticles. Inset: (c), (d) shows the linear range of the corresponding samples. | ||
With a view to obtain a better understanding of the catalytic aspects of the iron release mechanism, it is necessary to comprehend the enhanced PG oxidation on Fe3O4 nanocrystals. According to literature reports, it is generally known that the higher catalytic activities of the faceted nanocrystals of noble metals are dependent on high surface energy facets. As has been described previously, truncated octahedral magnetite nanoparticles have surface facets {100}, {220} and {111}, which are responsible for enhanced catalytic activity.23 Further, the facets have been clearly illustrated by HRTEM and the corresponding facets shown in Fig. 2 are in agreement with a recent study by Song et al.29 Song et al. reported TO Fe3O4 nanoparticles have four corner {220} facets along with {100} and {111} facets.29 These data are supported by the schematic illustration in Fig. S5 showing these Fe3O4 crystal planes, which reflect the Fe atoms present on surfaces.† The literature reported results confirm that {100} and {220} facets exhibited higher catalytic activities than the {111} facet.30 In our current study, we used literature, which reported that the {220} facet should be responsible for enhanced catalysis, because the {220} facet has more iron atoms than the {100} and {111}.14,21 The presence of more Fe atoms in the {220} facet that can readily undergo Fenton's mechanism (Fe3+/Fe2+ in the presence of H2O2) other than the {100} and {111} facets is responsible for the enhanced catalytic activity.
In summary, we have successfully synthesized high quality truncated octahedral Fe3O4 nanocrystals. A comparative study of their catalytic activity towards the oxidation of PG in the presence of H2O2 showed that our prepared sample (TO MNPs) is more active than that of the conventional nanomaterials (spherical MNPs). Surface facets like {220}, {100} and {111}, which are available in the truncated octahedral shaped MNPs, are responsible for their enhanced catalytic activity.
References
- H. H. Yang, S. Q. Zhang, X. L. Chen, Z. X. Zhuang, J. G. Xu and X. R. Wang, Anal. Chem., 2005, 77, 354–354 CrossRef CAS.
- H. H. Yang, S. Q. Zhang, X. L. Chen, Z. X. Zhuang, J. G. Xu and X. R. Wang, Anal. Chem., 2004, 76, 1316–1321 CrossRef CAS.
- H. L. Liu, M. Y. Hua, H. W. Yang, C. Y. Huang, P. C. Chu, J. S. Wu, I. C. Tseng, J. J. Wang, T. C. Yen, P. Y. Chen and K. C. Wei, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 15205–15210 CrossRef CAS.
- Y. R. Chemla, H. L. Grossman, Y. Poon, R. McDermott, R. Stevens, M. D. Alper and J. Clarke, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 14268–14272 CrossRef CAS.
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549–13554 CrossRef CAS.
- M. Brahler, R. Georgieva, N. Buske, A. Muller, S. Muller, J. Pinkernelle, U. Teichgraber, A. Voigt and H. Baumler, Nano Lett., 2006, 6, 2505–2509 CrossRef CAS.
- S. K. Sahoo, F. Dilnawaz, A. Singh and C. Mohanty, Biomaterials, 2010, 31, 3694–3706 CrossRef.
- I. Willner and E. Katz, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef.
- I. J. M. de Vries, W. J. Lesterhuis, J. O. Barentsz, P. Verdijk, J. H. van Krieken, O. C. Boerman, W. J. G. Oyen, J. J. Bonenkamp, J. B. Boezeman, G. J. Adema, J. W. M. Bulte, T. W. J. Scheenen, C. J. A. Punt, A. Heerschap and C. G. Figdor, Nat. Biotechnol., 2005, 23, 1407–1413 CrossRef CAS.
- G. Reiss and A. Hutten, Nat. Mater., 2005, 4, 725–726 CrossRef CAS.
- K. G. Stamplecoskie and J. C. Scaiano, J. Am. Chem. Soc., 2010, 132, 1825–1827 CrossRef CAS.
- H. Zeng, P. M. Rice, S. X. Wang and S. Sun, J. Am. Chem. Soc., 2004, 126, 11458–11459 CrossRef CAS.
- L. Li, Y. Yang, J. Ding and J. M. Xue, Chem. Mater., 2010, 22, 3183–3191 CrossRef CAS.
- N. Zhao, W. Ma, Z. M. Cui, W. G. Song, C. L. Xu and M. Y. Gao, ACS Nano, 2009, 3, 1775–1780 CrossRef CAS.
- S. W. Lee, S. Chen, W. Sheng, N. Yabuuchi, Y. T. Kim, T. Mitani, E. Vescovo and Y. Shao-Horn, J. Am. Chem. Soc., 2009, 131, 15669–15677 CrossRef CAS.
- O. Veiseh, J. W. Gunn and M. Zhang, Adv. Drug Delivery Rev., 2010, 62, 284–304 CrossRef CAS.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS.
- A. P. Alivisatos, Science, 1996, 271, 933–937 CAS.
- F. Dilnawaz, A. Singh, C. Mohanty and S. K. Sahoo, Biomaterials, 2010, 31, 3694–3706 CrossRef CAS.
- Y. W. Jun, J. W. Seo and J. Cheon, Acc. Chem. Res., 2008, 41, 179–189 CrossRef CAS.
- R. K. Zheng, H. W. Gu, B. Xu, K. K. Fung, X. X. Zhang and S. P. Ringer, Adv. Mater., 2006, 18, 2418–2421 CrossRef CAS.
- Y. Hou, Z. Xu and S. Sun, Angew. Chem., Int. Ed., 2007, 46, 6329–6332 CrossRef CAS.
- N. Mizutani, T. Iwasaki, S. Watano, T. Yanagida, H. Tanaka and T. Kawai, Bull. Mater. Sci., 2008, 31, 713–717 CrossRef CAS.
- D. A. Converso and M. E. Fernandez, Phytochemistry, 1995, 40, 1341–1345 CrossRef CAS.
- R. V. Rege, C. C. Webster and J. D. Ostrow, J. Lipid Res., 1987, 28, 673–683 CAS.
- Z. Zhao, Q. Chen and J.-i. Anzai, J. Environ. Sci., 2009, 21, S135–S138 CrossRef.
- P. Ciesla, P. Kocot, P. Mytych and Z. Stasicka, J. Mol. Catal. A: Chem., 2004, 224, 17–33 CrossRef CAS.
- N. Ding, N. Yan, C. L. Ren and X. G. Chen, Anal. Chem., 2010, 82, 5897–5899 CrossRef CAS.
- Q. Song, Y. Ding, Z. L. Wang and Z. J. Zhang, J. Phys. Chem. B, 2006, 110, 25547–25550 CrossRef CAS.
- J. Zheng, Y. H. Zhang, X. B. Song and X. G. Li, J. Nanopart. Res., 2011, 13, 4445–4450 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of magnetite nanoparticles. See DOI: 10.1039/c2ra01081j |
| This journal is © The Royal Society of Chemistry 2012 |
