Green removal of aromatic organic pollutants from aqueous solutions with a zeolite–hemp composite
Xiaoqin
Zou
ab,
Jaâfar El
Fallah
a,
Jean-Michel
Goupil
a,
Guangshan
Zhu
b,
Valentin
Valtchev
a and
Svetlana
Mintova
*a
aLaboratoire Catalyse & Spectrochimie, ENSICAEN—Université de Caen—CNRS, 6, boulevard du Maréchal Juin, 14050 Caen, France. E-mail: svetlana.mintova@ensicaen.fr; Fax: +33 (0)2 31 45 28 22; Tel: +33 (0)2 31 45 27 37
bState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China
First published on 22nd February 2012
Abstract
A composite based on zeolite L nanocrystals (LTL-type structure) and hemp fibers was prepared. The intimate growth of zeolite nanocrystals in the hemp fibers is achieved by direct hydrothermal crystallization. The crystalline phase, particle size distribution, porosity and zeolite loading in the composite are measured by X-ray diffraction, dynamic light scattering, thermo-gravimetric analysis, and nitrogen sorption. Further, the composite is used for the removal of aqueous aromatic organic pollutants (benzene, toluene and chlorobenzene), and the sorption ability is studied by IR and UV spectroscopy. The zeolite–hemp composite exhibited a high removal degree (above 80%), and a high flux of 19.3 kg m−2 h−1, 20.9 kg m−2 h−1 and 19.9 kg m−2 h−1 for benzene, toluene and chlorobenzene, respectively. Also, the zeolite–hemp composite shows substantially higher water flux than that of pure zeolite. The better performance of the composite is explained by an improved absorption ability of the zeolite–hemp material coupled with higher dynamic separation efficiency. A kinetic model based on the sorption ability of the composite and solubility of the contaminants in water is proposed. Both the zeolite L nanocrystals and the natural hemp fibers are environmentally friendly compounds, which make the composite particularly appropriate for water purification.
Introduction
Organic contaminants in untreated domestic or industrial wastewater has been identified as serious pollutants in the water resources. Many of the organic substances, even in trace amounts, have been defined as neuro-toxic, carcinogenic, mutagenic,1,2 or causing abnormal sexual differentiation.3,4 Toxic mono-aromatic compounds which are produced in large quantities for use as fuels, solvents, and starting materials for chemical syntheses, are usually found in industrial water discharged by the petroleum and organic chemical industries.5 Many mono-aromatics are included in the lists of priority pollutants of the U. S. Environmental Protection Agency and European Union.6,7 Benzene, toluene and chlorobenzene are amongst the most often observed and dangerous pollutants. Also, the different contaminants in the water may also adversely affect the equipment leading to scaling and corrosion.8,9The major driving forces towards water recycling today are the growing demand (lifestyle changing, urbanization) for drinkable water of a constantly increasing population, diminishing natural water resources and avoiding negative impacts on aquatic organisms. In addition, more public awareness about environmental protection has provided further impetus for water recycling and more stringent wastewater quality discharge regulations. From these points of view, it is compulsory to recycle polluted water for agriculture and domestic needs.
Therefore, the development of safe processes and syntheses of new materials are of paramount importance in the removal of aromatic compounds for keeping up with global soaring water demand. In the past decades, a wide range of physicochemical techniques have been evaluated for removing these compounds, including adsorption,10–17 enzymatic oxidation combined with precipitation,18,19 heterogeneous photocatalysis20–22 and other advanced processes.23–25 Recent developments in filtration technology have led to the availability of these systems as an alternative to conventional treatment processes. Indeed, filtration technology shows great advantages in terms of appreciable energy savings, environmentally benign clean technology with easy operation and great design flexibility in comparison to the conventional energy costly processes. Consequently, various filters/membranes have been implemented in water recycling and purification units.26–35 Previous studies have confirmed the competency of artificial polymers or polymer hybrids for removal of dissolved organic compounds in water by means of reverse osmosis,36–39 nanofiltration,40–43 ultrafiltration,44–47 and microfiltration.48,49 Given the complementary treatment capacities of artificial polymer filters/membranes, natural fibers, such as hemp fibers, are alternatives based on an environmental sustainability perspective.50 Additionally, molecular sieves, and in particularly the zeolites are expected to achieve an overall enhanced performance by introducing them in composite materials. Zeolites are microporous crystalline materials that are widely used in adsorption due to their uniform micropores, mechanical, chemical, biological stabilities and unique surface properties.51–57
The goal of this study is to prepare a composite based on natural fibers and microporous aluminosilicate (zeolite) for further use in the separation and removal of benzene derivatives from simulated wastewater samples. Hemp fibers are chosen as the natural source for preparation of the composite (Table 1). Benzene, toluene and chlorobenzene aromatic compounds are selected among other contaminants in water due to their wide spreading from chemical and petroleum factories, and thus are commonly found in the discharged waste water. Among a variety of zeolite types, the zeolite L, with LTL-type structure possessing one-dimensional channels (7.1 × 7.1 Å), bigger than the size of the benzene mono-derivatives, is considered as appropriate to remove these pollutants. The size of the LTL crystals can be tailored in the range of 30 nm–3 μm via different synthesis approaches.58,59 Additionally, the LTL-type zeolite possesses high mechanical and chemical stabilities, and also it is an environmental benign material due to its synthesis from organic-free precursor suspensions. The zeolite–hemp composite is applied to remove benzene, toluene and chlorobenzene as model organic contaminants of wastewater (Table 2).
| Physical properties | Fiber type | Length/mm | Diameter/μm | Microfibril angle/degrees |
| Bast fibers | 5–60 | 20–40 | 4 | |
| Chemical composition/% | Cellulose | Hemi-cellulose | Lignin Pectin | Non-cell-wall materials |
| 54–64 | 10–15 | 3–13 | 12–19 | |
| Others | High strength and durability, softness, resistant to mold and ultraviolet light, porous nature, high productivity, insulation, biodegradable, renewable and ecologically friendly | |||
| Parameters | Benzene | Toluene | Chlorobenzene |
|---|---|---|---|
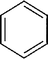
| Molecular formula | C6H6 | C7H8 | C6H5Cl |
| Chemical structure |
|
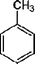
|
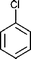
|
| Molecular size/nm | 0.55 × 0.55 | 0.55 × 0.6 | 0.55 × 0.55 |
| Physical state | Liquid | Liquid | Liquid |
| Water solubility (20 °C, g L−1) | 1.8 | 0.47 | 0.466 |
| Dipole moment (20 °C) | 0D | 0.31D | 1.54D |
| Contaminant sources | Petroleum and chemical factories | Petroleum and chemical factories | Agricultural and chemical factories |
| Health effect | Anemia or cancer risk | Nervous system problems | Liver or kidney problems |
Experimental
Treatment of hemp fibers
Prior to the preparation of the zeolite–hemp composite, 5 g of hemp fibers (La Chanvriere de L'aube, France) were treated with 0.1 M NaOH solution at 363 K for 24 h, followed by another treatment with 0.2 M NaOH at 363 K, in order to completely remove the wax. After this treatment, the hemp fibers were washed with water to neutral pH and dried at 363 K.Preparation of zeolite–hemp composite
The precursor suspension for zeolite L was prepared according to the synthesis procedure reported by Hölzl et al.60 A precursor suspension with the following molar composition was used: 5 K2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 Al2O3
0.5 Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 SiO2
10 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 H2O. Potassium hydroxide (KOH, Riedel de Haën, 85%) and aluminum hydroxide (Al(OH)3, Alfa Aesar, 76.5%) were dissolved in water, and mixed with colloidal silica (SiO2, Sigma-Aldrich, SM-30). This precursor suspension was aged for 16 h prior to use for preparation of the composite. For each experiment, 1 g treated hemp fibers were immersed in 40 mL precursor suspension and agitated for 16 h. Then a hydrothermal treatment of the suspension in a Teflon lined autoclave at 443 K for 24 h was carried out. The material obtained was thoroughly washed with water in order to remove any unbound zeolite nanocrystals. The purified as-prepared material (1 g) was dried at 363 K for 16 h and then pressed into a plate-like composite with a diameter of 35 mm and a thickness of 1 mm.
200 H2O. Potassium hydroxide (KOH, Riedel de Haën, 85%) and aluminum hydroxide (Al(OH)3, Alfa Aesar, 76.5%) were dissolved in water, and mixed with colloidal silica (SiO2, Sigma-Aldrich, SM-30). This precursor suspension was aged for 16 h prior to use for preparation of the composite. For each experiment, 1 g treated hemp fibers were immersed in 40 mL precursor suspension and agitated for 16 h. Then a hydrothermal treatment of the suspension in a Teflon lined autoclave at 443 K for 24 h was carried out. The material obtained was thoroughly washed with water in order to remove any unbound zeolite nanocrystals. The purified as-prepared material (1 g) was dried at 363 K for 16 h and then pressed into a plate-like composite with a diameter of 35 mm and a thickness of 1 mm.
Characterization
The crystalline phase and purity of the zeolite nanoparticles anchored on the hemp fibers were investigated by X-ray powder diffraction using a PANalytical X'Pert Pro diffractometer in Debye-Scherrer geometry with Cu-Kα radiation (λ = 1.5418 Å). Information about the size and morphology of the zeolite nanocrystals was obtained by dynamic light scattering (DLS) measurements of as-synthesized suspensions with a Malvern Zetasizer-Nano instrument and TESCAN Philips XL30 scanning electron microscopy (SEM), respectively. Nitrogen sorption experiments at 77 K were carried out with a Micromeritics ASAP 2020 instrument to determine the porosity of the samples (all samples were degassed at 443 K for 24 h).The quantity of zeolite L crystals embedded in the hemp fibers was determined by thermo-gravimetric analysis (TGA) using a SETSYS evolution instrument (SETARAM); the measurements are carried out in an air atmosphere with a heating ramp of 5 K min−1.
Absorption study of composite
The absorption ability of pure hemp fibers, pure zeolite L, and the zeolite–hemp composite for benzene, toluene and chlorobenzene was studied by IR spectroscopy. The up-taken quantity of pollutant by the zeolite–hemp composite (25 mg pressed into a self-supported wafer with a diameter of 16 mm) was monitored by IR with a static Nicolet Nexus FTIR spectrometer, equipped with MCT detectors. The composite was activated by heating at 443 K (heating rate of 1 K min−1) under vacuum and maintained for 24 h, followed by cooling to room temperature. Calibrated doses of benzene, toluene and chlorobenzene vapours were sent to the activated samples at room temperature. The change in the IR bands corresponding to benzene (1479 cm−1), toluene (1495 cm−1) and chlorobenzene (1477 cm−1) absorbed on the samples was measured.Separation study of composite
The separation experiments for benzene derivatives in model wastewater were carried out using the set up schematically shown in Fig. 1. The zeolite–hemp composite was sealed in the chamber with xylene resistant fluorocarbon isolation rings. The aqueous solution containing benzene (200 ppm), toluene (200 ppm), or chlorobenzene (200 ppm) was introduced in the mixing tank by one inlet and stirred continuously (33 rpm) in order to obtain a homogenous solution. The nitrogen was introduced from another inlet to keep a constant pressure of 0.2 bars. The permeated solution was collected in a trap at room temperature, and at regular intervals of 15 min, the amount of permeated solution was measured by weighing the trap. UV spectra of the permeated solutions were recorded with a UV-vis Hewlett Packard HP8453 spectrometer (detection limit is from several ppb to 1 ppm). These results can be compared with standard GC analysis with a detection limit less than 10 ppb.61–62 The composite was regenerated after the separation experiments by heating overnight in an oven at 393 K, and was cooled down in desiccators prior to further experiments.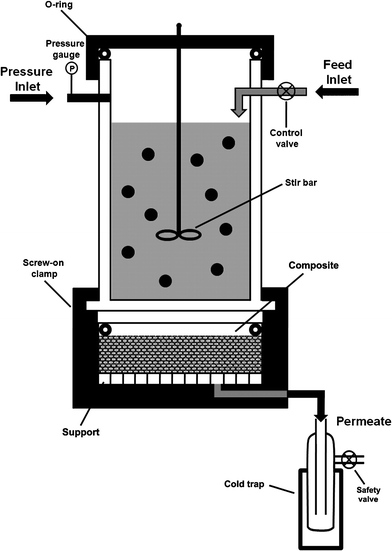 | ||
| Fig. 1 Schematic representation of the experimental setup used for separation of organic contaminants from water with the zeolite–hemp composite. | ||
The separation characteristics of the composite are defined in terms of flux (eqn (1)) and removal degree (eqn (2)) as follows:
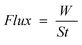 | (1) |
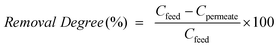 | (2) |
Results and discussion
General characterization of materials
The crystalline features of pure zeolite L nanocrystals, treated hemp fibers, and zeolite–hemp composite were investigated by XRD and the corresponding patterns are shown in Fig. 2. The appearance of Bragg peaks at 15°, 16.5°, and 22.7° 2θ confirms that the hemp fibers are semi-crystalline which is a base for moderate mechanical strength and stability. Therefore, these hemp fibers are considered as a good support for the preparation of the zeolite–hemp composite. The XRD pattern of pure zeolite L synthesized in the same batch is shown in Fig. 2b. The as-synthesized zeolite L crystals exhibit high crystallinity and high purity. The major peaks of zeolite L are observed in the zeolite–hemp composite, and the peak of the main crystalline plane (002) at 22.7° 2θ from hemp fibers is also present. This confirms that the crystallinity of hemp fibers is preserved after the growth of zeolite nanocrystals under hydrothermal conditions (Fig. 2c).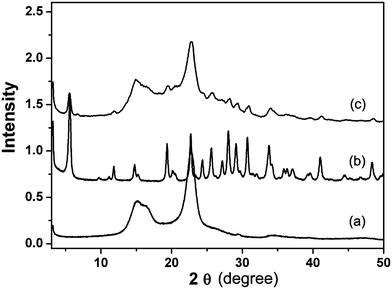 | ||
| Fig. 2 X-ray diffraction patterns of (a) pure hemp fibers (b) pure zeolite L nanocrystals, and (c) the zeolite–hemp composite. | ||
The surface features of the composite, morphology and size of hemp fibers, and zeolite L crystallites are studied by SEM. The SEM images of treated hemp fibers and the zeolite–hemp composite are shown in Fig. 3. The smooth surface of a single hemp fiber isolated from a bundle is shown in Fig. 3a. An individual fiber of size 40–50 μm is built from well integrated small fibrils (Fig. 3b). In contrast, the surface of the as-prepared zeolite–hemp composite is rough as shown in Fig. 3 c and d. Most of the zeolite L nanocrystals grow within the hemp fibrils while a small portion of the particles are strongly attached at the fibers' surface (Fig. 3d). The stability of the composite was confirmed by recording the XRD and SEM from the samples before and after use for water purification. No changes in the appearance of the samples was observed by SEM, and the XRD patterns are identical. The particle size distribution of the crystals grown in the suspension is shown as an insert in Fig. 3c, while the morphological appearance of the crystallites is shown as an insert of Fig. 3d.
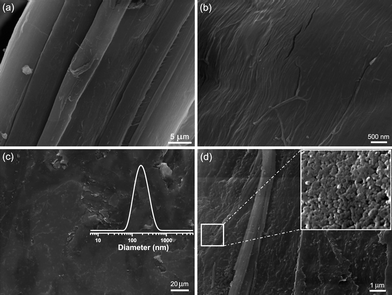 | ||
| Fig. 3 SEM images of (a, b) pure hemp fibers and (c, d) zeolite–hemp composite at different magnifications. Insert (c): DLS data of zeolite L nanocrystals in as-synthesized suspension, Insert (d): LTL nanocrystals on hemp fibers at high magnification. | ||
The amount of zeolite L crystallized in the hemp fibers was determined by TG analysis (Fig. 4). In the pure zeolite L sample, a weight loss of 8.8 wt% in the temperature range 298–473 K was measured (Fig. 4a), which is attributed to the water release. The TG analysis of the hemp fibers showed a weight loss of 2 wt% below 200 °C, and a two-step weight loss at 473–773 K, which corresponds to decomposition of organic components (Fig. 4c). The mass of the remaining inorganic residues of this sample was 5.5 wt%.
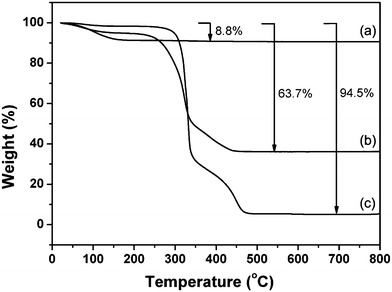 | ||
| Fig. 4 TG curves of (a) pure zeolite L nanocrystals, (b) zeolite–hemp composite, and (c) pure hemp fibers. | ||
On the basis of the weight losses of the zeolite and the hemp fibers, it was calculated that the zeolite–hemp composite contains 65 wt% of hemp and 35 wt% of zeolite (Fig. 4b).
Porosity and specific surface area of the zeolite crystals were measured by nitrogen sorption. A steep uptake at low relative pressures, followed by nearly horizontal absorption and desorption branches, typical for microporous materials, were recorded (Fig. 5). A distinct hysteresis at high relative pressure is characteristic for inter-particle voids formed by aggregation of similar in size nanoparticles. The specific Brunauer-Emmet-Teller surface area (SBET) and micropore volume of pure zeolite L is 409 m2 g−1 and 0.138 cm3 g−1, respectively. These values correspond to a highly crystalline material, which is in a good agreement with the SEM and X-ray diffraction data. The porosity of the zeolite–hemp composite is also characterized. The micropore volume of the composite (0.027 cm3 g−1) is in agreement with the amount of zeolite determined by TG analysis. This result shows that the access to the zeolite pores is slightly influenced by their integration in the hemp fibers. The decrease in surface area and micropore volume of the composite with respect to pure zeolite is reasonable, bearing in mind that about 35 wt% of crystals are embedded in the composite. Additionally, the hysteresis loop at high relative pressure reveals the presence of mesopores in the composite, thus an improved sorption ability of zeolite–hemp composite is expected, which would favour a high flux (Fig. 5). The pure hemp fibers were also characterized by N2 sorption and the results are summarized in Table 3.
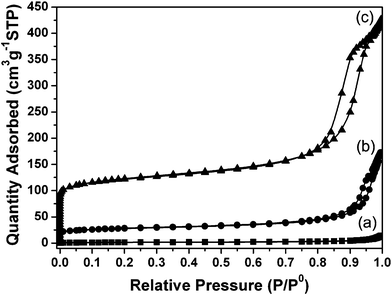 | ||
| Fig. 5 N2 sorption isotherms of (a) pure hemp fibers, (b) zeolite–hemp composite, and (c) zeolite L nanocrystals. | ||
| Samples | S BET | V micro | S meso | V meso | d micro a | d meso b |
|---|---|---|---|---|---|---|
| S BET: surface area (m2 g−1), Vmicro: micropore volume (cm3 g−1), Smeso: mesopore area (m2 g−1), Vmeso: mesopore volume (cm3 g−1), dmicro: micropore size (nm), dmeso: average mesopore size (nm), nd: not identified. a determined by DFT method. b determined by BJH method. | ||||||
| Hemp | 5 | nd | nd | nd | nd | nd |
| LTL | 409 | 0.138 | 121 | 0.504 | 1.201 | 15 |
| LTL–Hemp | 93 | 0.027 | 36 | 0.220 | 1.273 | 31 |
Separation of organic contaminants from water
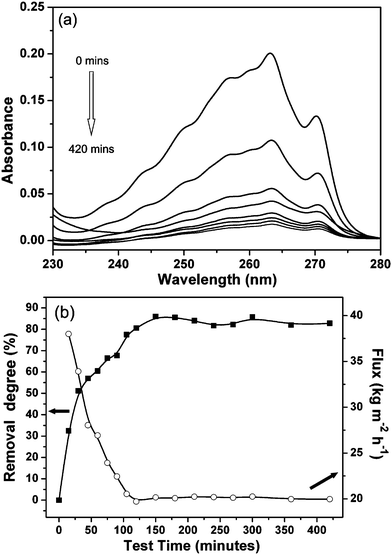
The as-prepared zeolite–hemp composite was used to separate the organic contaminants from aqueous solutions. The characteristics of the employed organic molecules are given in Table 2. The three compounds have similar sizes but different solubilities in water.The zeolite–hemp composite was exposed to water with 200 ppm contaminants. The UV spectra of benzene in the permeated solutions taken over the testing time are shown in Fig. 6a. The intensity of the benzene band in the UV region decreases gradually which indicates a reduced concentration of benzene in the permeated solution. Removal degrees of benzene from the feed solution and water flux through the composite are shown in Fig. 6b. A rapid increase in the removal degree and a decrease in the flux with time were measured. A high steady-state removal degree, about 84% for benzene, was reached after 100 min, and the solution flux was stabilized at 19.3 kg m−2 h−1. The removal degree (84%) of the zeolite–hemp composite was higher than that reported in the literature (from 15% to 80%).14,15,24 Also, the removal rate to reach the steady state (100 min) was faster than for the sorbents working in static mode (more than 10 h). It was found that the removal degree of the zeolite–hemp composite (84%) was much higher in respect to only hemp fibers (48%). Moreover, this value was higher than that in the static absorption mode (40%), where the zeolite–hemp composite was immersed and stirred in the same feed solution overnight.
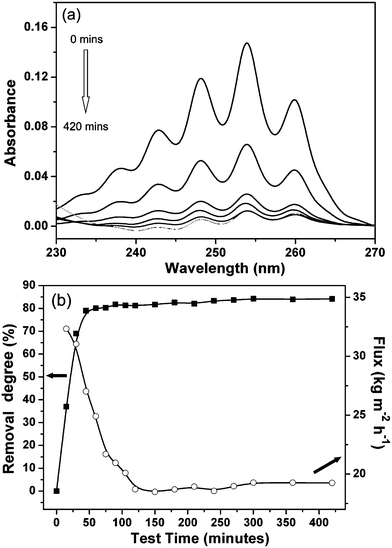 | ||
| Fig. 6 (a) UV spectra of benzene in feed solution (0 min) and permeated solutions collected at different time (15, 30, 45, 60, 420 min), and (b) removal degree (square) and flux (open circle) as a function of time. | ||
Based on these observations, the performance of the zeolite–hemp composite is explained with an improved absorption ability coupled with higher dynamic separation efficiency. It is worth noting that the zeolite–hemp composite showed a water flux of 19.3 kg m−2 h−1, which is substantially higher than that of pure zeolite.37–39,48
Furthermore, the performance of the zeolite–hemp composite in toluene separation was studied. In Fig. 7, the UV spectra of toluene in the permeated solutions, removal degrees and flux are shown. A gradual decrease of toluene peak with time was observed (Fig. 7a). About 80% of the toluene was removed by the zeolite–hemp composite with a flux of 20.9 kg m−2 h−1. Additionally, the removal degree increased slowly, and then reached a steady state. The time taken to reach the steady state for toluene was slightly longer in respect to that for benzene. In the same manner, the concentrations of chlorobenzene in the permeated solutions were studied by UV spectroscopy. The evolution of chlorobenzene band in the UV spectra, removal degrees and water flux are shown in Fig. 8 a,b. A removal degree of 86% for chlorobenzene and flux of 19.9 kg m−2 h−1 were measured (Fig. 8b). The removal degree for chlorobenzene was similar to that of benzene and slightly higher in respect to toluene (see Fig. 7b). Also, the performance of the zeolite–hemp composite after regeneration did not change, which is another proof of the stability.
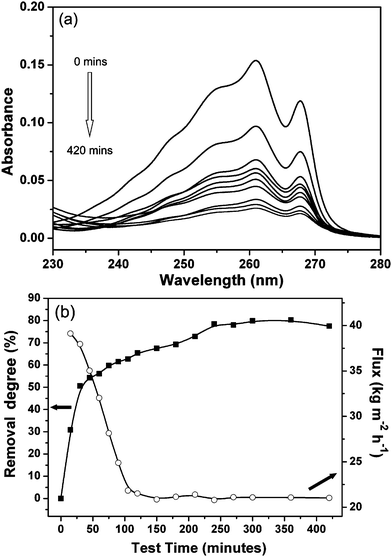 | ||
| Fig. 7 (a) UV spectra of toluene in feed solution (0 min) and permeated solutions collected at different times (15, 30, 45, 60, 90, 120, 180, 270, 420 min), and (b) removal degree (square) and flux (open circle) as a function of time. | ||
 | ||
| Fig. 8 (a) UV spectra of chlorobenzene in feed solution (0 min) and permeated solutions collected at different times (15, 30, 45, 60,120, 240, 420), and (b) removal degree (square) and flux (open circle) as a function of time. | ||
The separation kinetics were deduced by analyzing the data presented in Fig. 6b, 7b and 8b. The optimal fitting profiles of removal degree are presented in Fig. 9. The experimental data were fitted using the following eqn:
 | (3) |
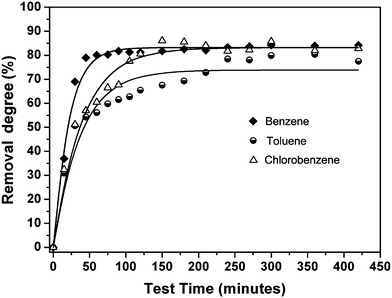 | ||
| Fig. 9 Optimal fitting profiles of removal degree for benzene, toluene and chlorobenzene as a function of time according to eqn (3). | ||
The Rm (maximum removal degree) and τ (rate constant, minutes) by monitoring the effects of test time (t, minutes), concentrations of feed (Cfeed) and permeated (Cpermeate) solutions through the composite are summarized in Table 4.
| Contaminants | R m | τ/min |
|---|---|---|
| Benzene | 83.2 | 20 |
| Toluene | 73.9 | 37 |
| Chlorobenzene | 83.2 | 39 |
The τ value for benzene is half that of toluene and chlorobenzene. Notably, the calculated specific rate constants for the three contaminants correlate well with the water solubility of the three organic contaminants (see Table 2). The good relationship between the rate constant (τ) and water solubility of the contaminants explains the trend observed for their removal degrees (see Fig. 6b, 7b and 8b).
Complementary IR analyses of benzene, toluene and chlorobenzene on pure zeolite L, the zeolite–hemp composite and pure hemp fibers were performed, and the results are shown in Fig. 10. The absorbed amounts of benzene, toluene and chlorobenzene on the zeolite–hemp composite were 0.46 mmol g−1, 0.42 mmol g−1 and 0.48 mmol g−1, respectively. For pure zeolite L, 1.27 mmol g−1 of benzene, 1.16 mmol g−1 of toluene and 1.31 mmol g−1 of chlorobenzene were absorbed. For pure hemp fibers, the absorbed amounts for benzene, toluene and chlorobenzene were 0.005 mmol g−1, 0.01 mmol g−1, 0.01 mmol g−1, respectively. These results show that the absorption ability of the zeolite–hemp composite for the three molecules is in good accordance with the maximum removal degree (Rm).
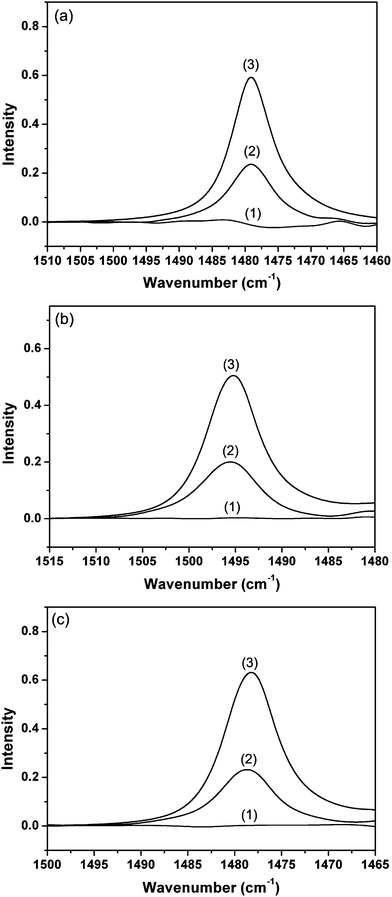 | ||
| Fig. 10 IR spectra of (a) benzene, (b) toluene and (c) chlorobenzene absorbed on pure hemp fibers (1), zeolite–hemp composite (2) and pure zeolite L (3). | ||
Conclusions
In conclusion, a zeolite–hemp composite was prepared via in situ crystallization of zeolite L nanocrystals on hemp fibers. The sequestration ability of the composite towards benzene, toluene and chlorobenzene from aqueous solutions was studied. The composite exhibits high removal degrees of 84%, 80% and 86% and high flux of 19.3 kg m−2 h−1, 20.9 kg m−2 h−1 and 19.9 kg m−2 h−1 for benzene, toluene and chlorobenzene, respectively. The removal degree of the zeolite–hemp composite (84%) is much higher in respect to only hemp fibers (48%). Moreover, this value is higher than that in the static absorption mode (40%). The separation behavior of the composite is described and a kinetic model is proposed using the maximum removal degree and the rate constant on the basis of sorption ability of the composite and solubility of the contaminants in water.Both materials (zeolite L and hemp) employed for preparation of the composite are environmental friendly, and thus make the materials particularly appropriate for water purification.
Acknowledgements
Xiaoqin Zou acknowledges the China Scholarship Council (CSC) for the financial support.References
- C. H. Tate and K. F. Arnold, Water Quality and Treatment: A Handbook of Community Water Supplies, ed. R. D. Letterman, McGraw-Hill, New York, 5th edn, 1999 Search PubMed.
- S. H. Swan, E. P. Elkin and L. Fenster, Environ. Health Perspect., 2000, 108, 961–966 CrossRef CAS.
- C. E. Purdom, P. A. Hardiman, V. J. Bye, N. C. Eno, C. R. Tyler and J. P. Sumpter, Chem. Ecol., 1994, 8, 275–285 CrossRef CAS.
- E. J. Routledge, D. Sheahan, C. Desbrow, G. C. Brightly, M. Waldock and J. P. Sumpter, Environ. Sci. Technol., 1998, 32, 1559–1565 CrossRef CAS.
- S. Budavari, M. O’Neil, A. Smith, P. Heckelman and J. Obenchain, The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, ed. S. Budavari, Merck, NJ, 12th edn, 1996 Search PubMed.
- National Primary Drinking Water Regulations, Environmental Protection Agency, 2009, website: http://water.epa.gov/drink/contaminants/index.cfm Search PubMed.
- Proposal for a Directive amending the WFD and EQSD (COM(2011)876) and Report (COM(2011)875), European Commission Environment, 2011, website: http://ec.europa.eu/environment/water/water-dangersub/lib_pri_substances.htm Search PubMed.
- E. K. Quagraine, H. G. Peterson and J. V. Headley, J. Environ. Sci. Health A, 2005, 40, 685–722 CAS.
- E. W. Allen, J. Environ. Eng. Sci., 2008, 7, 499–524 CrossRef CAS.
- J. F. Garcia-Araya, F. J. Beltran, P. Alvarez and F. J. Lasa, Adsorption, 2003, 9, 107–115 CrossRef CAS.
- C. Long, P. Liu, Y. Li, A. M. Li and Q. X. Zhang, Environ. Sci. Technol., 2011, 45, 4506–4512 CrossRef CAS.
- Y. Jia, R. Wang, A. G. Fane and W. B. Krantz, Sep. Purif. Technol., 2005, 46, 79–87 CrossRef CAS.
- A. Rossner, S. A. Snyder and D. R. U. Knappe, Water Res., 2009, 43, 3787–3796 CrossRef CAS.
- P. Huttenloch, K. E. Roehl and K. Czurda, Environ. Sci. Technol., 2001, 35, 4260–4264 CrossRef CAS.
- L. L. Ji, F. L. Liu, Z. Y. Xu, S. R. Zheng and D. Q. Zhu, Environ. Sci. Technol., 2009, 43, 7870–7876 CrossRef CAS.
- W. L. IJdo and T. J. Pinnavaia, Green Chem., 2001, 3, 10–12 RSC.
- C. L. Warner, R. S. Addleman, A. D. Cinson, T. C. Droubay, M. H. Engelhard, M. A. Nash, W. Yantasee and M. G. Warner, ChemSusChem, 2010, 3, 749–757 CrossRef CAS.
- M. Pera-Titus, V. Garcia-Molina, M. Banos, J. Gimenez and S. Esplugas, Appl. Catal., B, 2004, 47, 219–256 CrossRef CAS.
- A. A. Alturki, N. Tadkaew, J. A. McDonald, S. J. Khan, W. E. Price and L. D. Nghiem, J. Membr. Sci., 2010, 365, 206–215 CrossRef CAS.
- H. Ganjidoust, K. Tatsumi, S. Wada and M. Kawase, Water Sci. Technol., 1996, 34, 151–159 CrossRef CAS.
- Y. L. Zhang, S. Wei, W. Zhang, Y. J. Xu, S. Liu, D. S. Su and F. S. Xiao, ChemSusChem, 2009, 2, 867–872 CrossRef CAS.
- J. H. Huang, K. N. Ding, Y. D. Hou, X. C. Wang and X. Z. Fu, ChemSusChem, 2008, 1, 1011–1019 CrossRef CAS.
- L. F. Liotta, M. Grattadauria, G. Dicarlo, G. Perrini and V. Librando, J. Hazard. Mater., 2009, 162, 588–606 CrossRef CAS.
- M. A. Keane, Green Chem., 2003, 5, 309–317 RSC.
- J. D. Pless, J. Krumhansl, J. Voigt, D. Moore, M. Axness, M. L. F. Phillips and T. M. Nenoff, Ind. Eng. Chem. Res., 2006, 45, 4752–4756 CrossRef CAS.
- T. Suzuki, Y. Y. Lu, W. Zhang, J. S. Moore and B. J. Marinas, Environ. Sci. Technol., 2007, 41, 6246–6252 CrossRef CAS.
- H. Wu, L. Liu, F. S. Pan, C. L. Hu and Z. Y. Jiang, Sep. Purif. Technol., 2006, 51, 352–358 CrossRef CAS.
- D. Panek and K. Konieczny, Sep. Purif. Technol., 2007, 57, 507–512 CrossRef CAS.
- S. Das, A. K. Banthia and B. Adhikari, Chem. Eng. Sci., 2006, 61, 6454–6467 CrossRef CAS.
- S. A. Tarafder, C. McDermott and C. Schüth, J. Membr. Sci., 2007, 292, 9–16 CrossRef CAS.
- P. Izák, M. Köckerling and U. Kragl, Green Chem., 2006, 8, 947–948 RSC.
- T. Ohshima, T. Miyata, T. Uragami and H. Berghmens, J. Mol. Struct., 2005, 739, 47–55 CrossRef CAS.
- C. Brazinha, D. S. Barbosa and J. G. Crespo, Green Chem., 2011, 13, 2197–2203 RSC.
- C. L. Kong, T. Shintani and T. Tsuru, New J. Chem., 2010, 34, 2101–2104 RSC.
- G. Ciobanu, G. Carja and O. Ciobanu, Desalination, 2008, 222, 197–201 CrossRef CAS.
- Y. Yoon and R. M. Lueptow, J. Membr. Sci., 2005, 261, 76–86 CrossRef CAS.
- N. Liu, L. X. Li, B. McPherson and R. Lee, J. Membr. Sci., 2008, 325, 357–361 CrossRef CAS.
- L. Li, J. Dong, T. M. Nenoff and R. Lee, J. Membr. Sci., 2004, 243, 401–404 CrossRef CAS.
- C. Covarrubias, R. García, R. Arriagada, J. Yánez, H. Ramanan, Z. P. Lai and M. Tsapatsis, J. Membr. Sci., 2008, 312, 163–173 CrossRef CAS.
- A. Ben-David, R. Bernstein, Y. Oren, S. Belfer, C. Dosoretz and V. Freger, J. Membr. Sci., 2010, 357, 152–159 CrossRef CAS.
- M. A. Zazouli, H. Susanto, S. Nasseri and M. Ulbricht, Water Res., 2009, 43, 3270–3280 CrossRef CAS.
- V. Yangali-Quintanilla, S. K. Maeng, T. Fujioka, M. Kennedy and G. Amy, J. Membr. Sci., 2010, 362, 334–345 CrossRef CAS.
- E. Steinle-Darling, E. Litwiller and M. Reinhard, Environ. Sci. Technol., 2010, 44, 2592–2598 CrossRef CAS.
- K. Majewska-Nowak, M. Kabsch-Korbutowicz and T. Winnicki, Desalination, 2008, 221, 358–369 CrossRef CAS.
- A. Deriszadeh, M. M. Husein and T. G. Harding, Environ. Sci. Technol., 2010, 44, 1767–1772 CrossRef CAS.
- K. Materna, E. Goralska, A. Sobczynska and J. Szymanowski, Green Chem., 2004, 6, 176–182 RSC.
- M. M. Zhang, C. Li, M. M. Benjamin and Y. J. Chang, Environ. Sci. Technol., 2003, 37, 1663–1669 CrossRef CAS.
- J. Y. Cui, X. F. Zhang, H. O. Liu, S. Q. Liu and K. L. Yeung, J. Membr. Sci., 2008, 325, 420–426 CrossRef CAS.
- Y. T. Tsai, A. Y. Lin, Y. H. Weng and K. C. Li, Environ. Sci. Technol., 2010, 44, 7914–7920 CrossRef CAS.
- B. Pejic, M. Vukcevic, M. Kostic and P. Skundric, J. Hazard. Mater., 2009, 164, 146–153 CrossRef CAS.
- S. G. Wang, W. X. Gong, X. W. Liu, B. Y. Gao and Q. Y. Yue, Sep. Purif. Technol., 2006, 51, 367–373 CrossRef CAS.
- Y. Dong, D. Y. Wu, X. C. Chen and Y. Lin, J. Colloid Interface Sci., 2010, 348, 585–590 CrossRef CAS.
- L. Abu-Lail, J. A. Bergendahl and R. W. Thompson, J. Hazard. Mater., 2010, 178, 363–369 CrossRef CAS.
- J. W. Choi, K. S. Yang, D. J. Kim and C. E. Lee, Curr. Appl. Phys., 2009, 9, 694–697 CrossRef.
- M. Ghiaci, A. Abbaspur, R. Kia and F. Seyedeyn-Azad, Sep. Purif. Technol., 2004, 40, 217–229 CrossRef CAS.
- B. Ersoy and M. S. Çelik, Environ. Technol., 2004, 25, 341–348 CrossRef CAS.
- J. Caro and M. Noack, Microporous Mesoporous Mater., 2008, 115, 215–233 CrossRef CAS.
- G. Calzaferri, S. Huber, H. Maas and C. Minkowski, Angew. Chem., Int. Ed., 2003, 42, 3732–3758 CrossRef CAS.
- O. Bossart and G. Calzaferri, Microporous Mesoporous Mater., 2008, 109, 392–397 CrossRef CAS.
- M. Hölzl, S. Mintova and T. Bein, Stud. Surf. Sci. Catal., 2005, 158, 11–18 CrossRef.
- R. Kubinec, J. Adamuščin, H. Jurdáková, M. Foltin, I. Ostrovský, A. Kraus and L. Soják, J. Chromatogr., A, 2005, 1084, 90–94 CrossRef CAS.
- J. X. Wang, D. Q. Jiang and X. P. Yan, Talanta, 2006, 68, 945–950 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
